3,4,5-Trichloroaniline Nephrotoxicity in Vitro: Potential Role of Free Radicals and Renal Biotransformation
Abstract
:1. Introduction
2. Results
2.1. Time and Concentration Cytotoxicity Studies

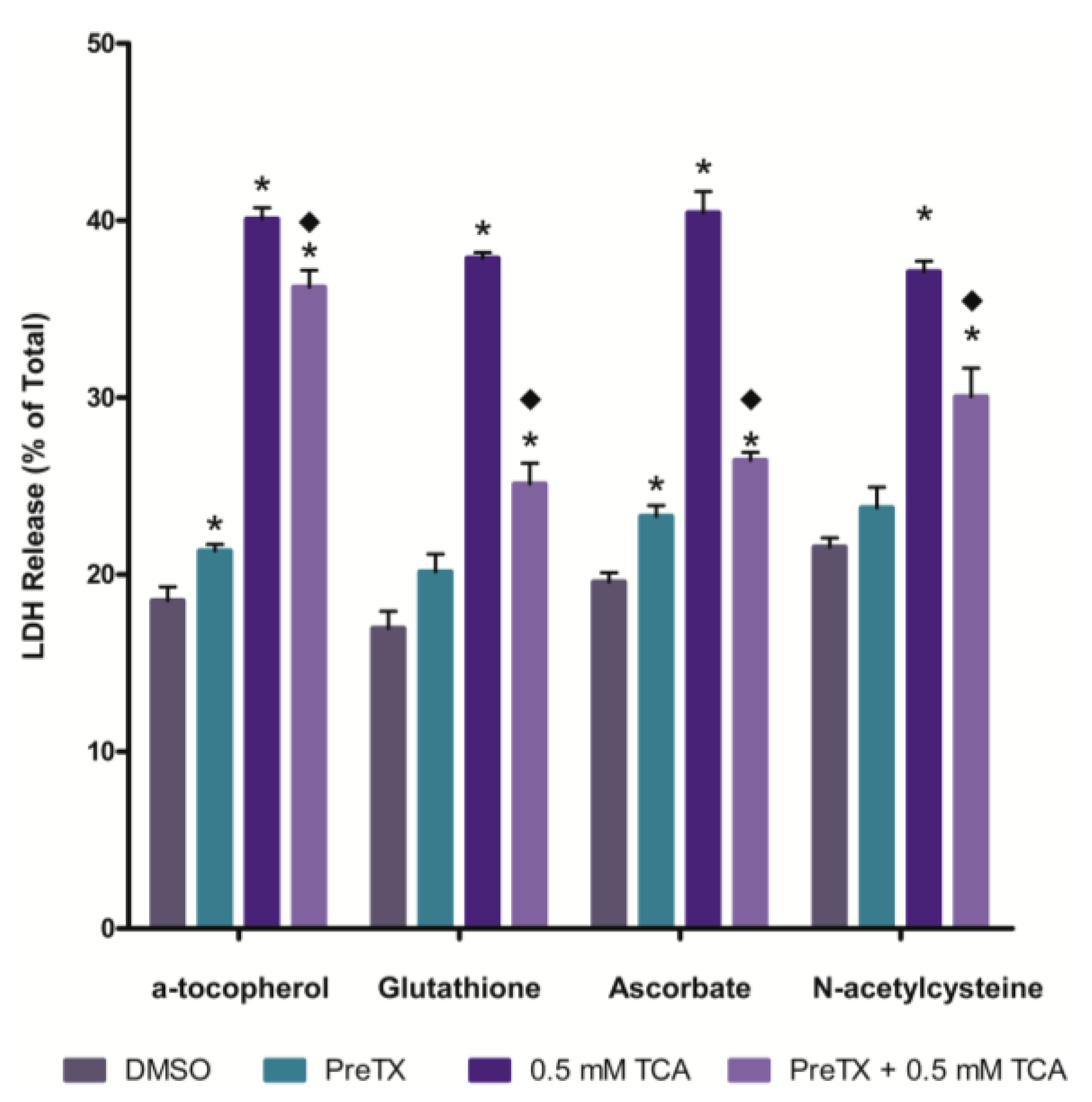
2.2. Effects of Antioxidants on 3,4,5-Trichloroaniline (TCA) Cytotoxicity
2.3. Effects of Cytochrome P450 (CYP) and Flavin-containing Monooxygenase (FMO) Inhibition

2.4. Effects of Cyclooxygenase and Peroxidase Inhibition

3. Discussion

4. Experimental Section
4.1. Experimental Animals
4.2. Chemicals
4.3. Isolated Renal Cortical Cell (IRCC) Preparation and Treatment
4.4. Statistics
| Pretreatment | Concentration (mM) | Pretreatment Time (min) | Mechanism of Action |
|---|---|---|---|
| N-Acetyl-l-cysteine | 2.0 | 30 | Antioxidant |
| α-Tocopherol | 1.0 | 5 | Antioxidant |
| Glutathione | 1.0 | 30 | Antioxidant |
| Ascorbate | 2.0 | 5 | Antioxidant |
| Methimazole | 1.0 | 30 | FMO Inhibitor |
| N-Octylamine | 2.0 | 5 | FMO Inhibitor |
| Indomethacin | 1.0 | 15 | Cyclooxygenase Inhibitor |
| Piperonyl Butoxide | 1.0 | 15 | Non-specific CYP Inhibitor |
| Metyrapone | 1.0 | 5 | Non-specific CYP Inhibitor |
| Mercaptosuccinate | 0.1 | 15 | Peroxidase Inhibitor |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aizawa, H. Metabolic Maps of Pesticides; Academic Press, Inc.: London, UK, 1989; Volume 2, pp. 27–42. [Google Scholar]
- Ehlhardt, W.J. Metabolism and disposition of the anticancer agent sulfenur in mouse, rat, monkey, and human. Drug Metab. Dispos. 1991, 19, 370–375. [Google Scholar] [PubMed]
- Rickert, D.E.; Held, S.D. Metabolism of chloronitrobenzenes by isolated rat hepatocytes. Drug Metab. Dispos. 1990, 18, 5–9. [Google Scholar] [PubMed]
- Lee, J.B.; Sohn, H.Y.; Shin, K.S.; Jo, M.S.; Jeon, C.P.; Jang, J.O.; Kim, J.E.; Kwon, G.S. Microbial biodegradation and toxicity of vinclozolin and its toxic metabolite 3,5-dichloroaniline. J. Microbiol. Biotechnol. 2008, 18, 343–349. [Google Scholar]
- Santos, T.C.R.; Rocha, J.C.; Alonsa, R.M.; Martinez, E.; Ibanez, C.; Barcelo, D. Rapid degradation of propanil in rice field crops. Environ. Sci. Technol. 1998, 32, 3479–3484. [Google Scholar] [CrossRef]
- Mercadier, C.; Vega, D.; Bastide, J. Chemical and biological transformation of the fungicide vinclozolin. J. Agric. Food Chem. 1998, 46, 3817–3822. [Google Scholar] [CrossRef]
- Lindh, C.H.; Littorin, M.; Amilon, A.; Jonsson, B.A. Analysis of 3,5-dichloroaniline as a biomarker of vinclozolin an iprodione in human urine using liquid chromatography/triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Kutting, B.; Goen, T.; Schwegler, U.; Fromme, H.; Uter, W.; Angerer, J.; Drexler, H. Monoarylamines in the general population—A cross-sectional population-based study including 1004 Bavarian subjects. Int. J. Hyg. Environ. Health 2009, 212, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Turci, R.; Barisano, A.; Baldducci, C.; Colosio, C.; Minoia, C. Determination of dichloroanilines in human urine by gas chromatography/mass spectrometry: Validation protocol and establishment of reference values in a population group living in central Italy. Rapid Commun. Mass Spectrom. 2006, 20, 2621–2625. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, N.; Chiodini, A.; Colosio, C.; de Paschale, G.; Somaruga, C.; Turci, R.; Minoia, C.; Brambilla, G.; Colombi, A. Occupational and environmental exposure to anilide and dicarboximide pesticides. G. Ital. Med. Lav. Ergon. 2007, 29, 276–277. [Google Scholar] [PubMed]
- Chhabra, R.S.; Thompson, M.; Elwell, M.R.; Greken, D.K. Toxicity of p-chloroaniline in rats and mice. Food Chem. Toxicol. 1990, 28, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Guilhermino, L.; Soares, A.M.V.M.; Carvalho, A.P.; Lopes, M.C. Acute effects of 3,4-dichloroaniline on blood of male Wistar rats. Chemosphere 1998, 37, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M.A.; Rogers, B.A.; Meadows, M.K.; Conner, J.T.; Williams, E.; Hong, S.K.; Rankin, G.O. Characterization of methemoglobin formation induced by 3,5-dichloroaniline, 4-amino-2,6-dichlorophenol and 3,5-dichlorophenylhydroxylamine. Toxicology 1997, 118, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Reznik, G.; Garner, F.M. Proliferative lesions of the spleen in male F344 rats fed diets containing p-chloroaniline. Vet. Pathol. 1980, 17, 200–205. [Google Scholar]
- Valentovic, M.A.; Ball, J.G.; Anestis, D.K.; Beers, K.W.; Madan, E.; Hubbard, J.L.; Rankin, G.O. Acute renal and hepatic toxicity of 2-haloanilines in Fischer 344 rats. Toxicology 1992, 75, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M.A.; Ball, J.G.; Anestis, D.K.; Rankin, G.O. Comparison of the in vitro toxicity of dichloroaniline structural isomers. Toxicol. In Vitro 1995, 9, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M.A.; Lo, H.H.; Brown, P.I.; Rankin, G.O. 3,5-Dichloroaniline toxicity in Fischer 344 rats pretreated with inhibitors and inducers of cytochrome P450. Toxicol. Lett. 1995, 78, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Anestis, D.K.; Henderson, T.T.; Rankin, G.O. Haloaniline-induced in vitro nephrotoxicity: Effects of 4-haloanilines and 3,5-dihaloanilines. Toxicol. Lett. 2000, 14, 125–133. [Google Scholar] [CrossRef]
- Lo, H.H.; Brown, P.I.; Rankin, G.O. Acute nephrotoxicity induced by isomeric dichloroanilines in Fischer 344 rats. Toxicology 1990, 63, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, A.; Kielhorn, J.; Konnecker, G.; Pohlenz-Michel, C.; Mangelsdorfer, I. Concise International Chemical Assessment Document 48—4-Chloroaniline; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Vangnai, A.S.; Kataoka, N.; Soonglerdsongpha, S.; Kalambaheti, C.; Tajima, T.; Kato, J. Construction and application of an Escherichia coli bioreporter for aniline and chloroaniline detection. J. Ind. Microbiol. Biotechnol. 2012, 39, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, D.G.; Furlani, A.; Scarcia, V.; Ghirvu, C.; Doadrio, A. On the synthesis, cytostatic and antitumor properties of new Pt(II) and Pt(IV) complexes with chloroanilines. Chem. Biol. Interact. 1985, 53, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takahashi, O.; Watanabe, K.; Nakazawa, S.; Yamanaka, H. The effects of antiprostatic agents on the accessory sex organs of rats treated with adrenal androgens. Hinyokika Kiyo 1991, 37, 1669–1676. [Google Scholar] [PubMed]
- Limban, C.; Marutescu, L.; Chifiriuc, M.C. Synthesis, spectroscopic properties and antipathogenic activity of new thiourea derivatives. Molecules 2011, 16, 7593–7607. [Google Scholar] [CrossRef]
- Peters, A.T.; Yang, S.S. Monoazo disperse dyes derived from mononitro-dichloro-2-aminobenzothiazoles. Dyes Pigments 1996, 30, 291–299. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kumar, R.; Dureja, P.; Rawat, D.S. Schiff bases as potential fungicides and nitrification inhibitors. J. Agric. Food Chem. 2009, 57, 8520–8525. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.H.; Brown, P.I.; Rankin, G.O. Trichloroaniline effects on renal function in vivo and in vitro. Toxicol. Lett. 1991, 57, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Valentovic, M.A.; Anestis, D.K.; Ball, J.G.; Brown, P.I.; Rankin, G.O. Nephrotoxicity of 4-amino-2-chlorophenol and 2-amino-4-chlorophenol in the Fischer 344 rat. Toxicology 1996, 110, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Anestis, D.K.; Ball, J.G.; Valentovic, M.A.; Brown, P.I.; Rankin, G.O. 4-Amino-2,6-Dichlorophenol nephrotoxicity in the Fischer 344 rat: Protection by ascorbic acid, AT-125, and aminooxyacetic acid. Toxicol. Appl. Pharmacol. 1997, 147, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Rankin, G.O.; Racine, C.; Sweeney, A.; Kraynie, A.; Anestis, D.K.; Barnett, J.B. In vitro nephrotoxicity induced by propanil. Environ. Toxicol. 2008, 23, 435–442. [Google Scholar]
- Valentovic, M.; Ball, J.G.; Stoll, S.; Rankin, G.O. 3,4-Dichlorophenylhydroxylamine cytotoxicity in renal cortical slices from Fischer 344 rats. Toxicology 2001, 162, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.A.; Cross, T.J.; Schnellmann, R.G. Studies on the mechanism of 4-aminophenol-induced toxicity to renal proximal tubules. Hum. Exp. Toxicol. 1993, 12, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Baliga, R.; Zhang, Z.; Baliga, M.; Ueda, N.; Shah, S.V. Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998, 54, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; Acosta, D., Jr. N-Deacetyl ketoconazole-induced hepatotoxicity in a primary culture system of rat hepatocytes. Toxicology 1997, 117, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M.; Meadows, M.K.; Harmon, R.C.; Ball, J.G.; Hong, S.K.; Rankin, G.O. 2-Amino-5-chlorophenol toxicity in renal cortical slices from Fischer 344 rats: Effects of antioxidants and sulfhydryl agents. Toxicol. Appl. Pharmacol. 1999, 161, 1–9. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Siraki, A.G. Accelerated cytotoxicity mechanism screening using drug metabolizing enzyme modulators. Curr. Drug Metab. 2005, 6, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.S.; Monks, T.J. Co-oxidation of 2-bromohydroquinone by renal prostaglandin synthase: Modulation of prostaglandin synthesis by 2-bromohrdroquinone and glutathione. Drug Metab. Dispos. 1987, 15, 801–807. [Google Scholar] [PubMed]
- Katsuda, H.; Yamashita, M.; Katsura, H.; Yu, J.; Waki, Y.; Nagata, N.; Sai, Y.; Miyamoto, K. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect tumor activity. Biol. Pharm. Bull. 2010, 33, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sudo, J. Lipid peroxidation and generations of oxygen radicals induced by cephalosporin in renal cortical microsomes of rats. Jpn. J. Pharmacol. 1990, 52, 233–243. [Google Scholar] [CrossRef] [PubMed]
- De Wolf, W.; Seinen, W.; Hermens, J.L.M. Biotransformation and toxicokinetics of trichloroanilines in fish in relation to their hydrophobicity. Arch. Environ. Contam. Toxicol. 1993, 25, 110–117. [Google Scholar] [CrossRef]
- Hong, S.K.; Rankin, G.O. Biotransformation of 2-chloroaniline in the Fischer 344 rat: Identification of urinary metabolites. Xenobiotica 1998, 10, 985–994. [Google Scholar] [CrossRef]
- Ehlhardt, W.J.; Howbert, J.J. Metabolism and disposition of p-chloroaniline in rat, mouse and monkey. Drug Metab. Dispos. 1991, 19, 366–369. [Google Scholar] [PubMed]
- McMillan, D.C.; Freeman, J.P.; Hinson, J.A. Metabolism of the arylamide herbicide propanil. I. Microsomal metabolism and in vitro methemoglobinemia. Toxicol. Appl. Pharmacol. 1990, 103, 90–101. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; Leakey, J.E.; Arlotto, M.P.; McMillan, J.M.; Hinson, J.A. Metabolism of the arylamide herbicide propanil. II. Effects of propanil and its derivatives on hepatic microsomal drug metabolizing enzymes in the rat. Toxicol. Appl. Pharmacol. 1990, 103, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Rankin, G.O.; Valentovic, M.A.; Beers, K.W.; Nicoll, D.W.; Ball, J.G.; Anestis, D.K.; Brown, P.I.; Hubbard, J.L. Renal and hepatic toxicity of monochloroacetanilides in the Fischer 344 rat. Toxicology 1993, 79, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Rankin, G.O.; Valentovic, M.A.; Nicoll, D.W.; Ball, J.G.; Anestis, D.K.; Wang, R.T.; Brown, P.I. In vivo and in vitro 4-amino-2,6-dichlorophenol nephrotoxicity and hepatotoxicity in the Fischer 344 rat. Toxicology 1994, 90, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Rankin, G.O.; Hong, S.K.; Anestis, D.K.; Ball, J.G.; Valentovic, M.A. Mechanistic aspects of 4-amino-2,6-dichlorophenol-induced in vitro nephrotoxicity. Toxicology 2008, 245, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M.A.; Ball, J.G.; Sun, H.; Rankin, G.O. Characterization of 2-amino-4,5-dichlorophenol (2A45CP) in vitro toxicity in renal cortical slices from male Fischer 344 rats. Toxicology 2002, 172, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, Y.; Sakurai, E.; Nomura, A.; Itoh, K.; Tanaka, Y. Metabolism of nicotine in rat lung microvascular endothelial cells. J. Pharm. Pharmacol. 2006, 58, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ehlhardt, W.J.; Kulanthaivel, P.; Lanza, D.L.; Reilly, A.; Yost, G.S. Dehydrogenation of indoline by cytochrome P450 enzymes: A novel “aromatase” process. J. Pharmacol. Exp. Ther. 2007, 322, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Yanni, S.B.; Annaert, P.P.; Augustijns, P.; Ibrahim, J.G.; Benjamin, D.K., Jr.; Thakker, D.R. In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: Role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab. Dispos. 2010, 38, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Golly, I.; Hlavica, P. N-Oxidation of 4-chloroaniline by prostaglandin synthase. Biochem. J. 1985, 260, 803–809. [Google Scholar]
- Corbett, M.D.; Chipko, B.R.; Batchelor, A.O. The action of chloride peroxidase on 4-chloroaniline. Biochem. J. 1980, 187, 893–903. [Google Scholar] [PubMed]
- Bakkenist, A.R.J.; Plat, H.; Wever, R. Oxidation of 4-chloroaniline catalyzed by human myeloperoxidase. Bioorg. Chem. 1981, 10, 324–328. [Google Scholar] [CrossRef]
- Umbreit, J. Methemoglobin—It’s not just blue: A concise review. Am. J. Hematol. 2007, 82, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kiese, M.; Taeger, K. The fate of phenylhydroxylamine in human red cells. Naunyn Schmiedeberg’s Arch. Pharmacol. 1976, 292, 59–66. [Google Scholar] [CrossRef]
- Eyer, P.; Ascherl, M. Reactions of para-substituted nitrosobenzenes with human hemoglobin. Biol. Chem. 1987, 368, 285–294. [Google Scholar]
- Stiborova, M.; Frei, E.; Schmeiser, H.H.; Wiessler, M.; Anzenbacher, P. Peroxidase oxidizes N-nitrosomethylaniline to ultimate carcinogens: Binding to DNA and transfer RNA in vitro. Cancer Lett. 1992, 63, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Loew, G.H.; Goldblum, A. Metabolic activation and toxicity of acetaminophen and related analogs: A theoretical study. Mol. Pharmacol. 1985, 27, 375–386. [Google Scholar] [PubMed]
- Harmon, R.C.; Kiningham, K.K.; Valentovic, M.A. Pyruvate reduces 4-aminophenol in vitro toxicity. Toxicol. Appl. Pharmacol. 2006, 213, 179–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, C.S.; Mun, G.H.; Kim, J.H.; Chung, Y.H.; Hwang, Y.I.; Shin, D.H.; Lee, W.J. Immunohistochemical localization of sodium-dependent l-ascorbic acid transporter 1 protein in rat kidney. Histochem. Cell Biol. 2006, 126, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H. Renal membrane transport of glutathione in toxicology and disease. Vet. Path. 2011, 48, 408–419. [Google Scholar] [CrossRef]
- Koh, A.S.; Simmons-Willis, T.A.; Pritchard, J.B.; Grassl, S.M.; Ballatori, N. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: Transport by the renal organic anion transporter. Mol. Pharmacol. 2002, 62, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Machlin, L.J.; Bendich, A. Free radical tissue damage: Protective role of antioxidant nutrients. FASEB J. 1987, 1, 441–445. [Google Scholar] [PubMed]
- American Association for Laboratory Animal Science. Humane Care and Use of Laboratory Animals. Available online: https://www.aalas.org/about-aalas/position-papers/humane-care-and-use#.VGDjlE10yfA (accessed on 1 February 2013).
- Jones, D.P.; Sundby, G-B.; Ormsted, K.; Orrenius, S. Use of isolated kidney cells for study of drug metabolism. Biochem. Pharmacol. 1979, 28, 929–935. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racine, C.; Ward, D.; Anestis, D.K.; Ferguson, T.; Preston, D.; Rankin, G.O. 3,4,5-Trichloroaniline Nephrotoxicity in Vitro: Potential Role of Free Radicals and Renal Biotransformation. Int. J. Mol. Sci. 2014, 15, 20900-20912. https://doi.org/10.3390/ijms151120900
Racine C, Ward D, Anestis DK, Ferguson T, Preston D, Rankin GO. 3,4,5-Trichloroaniline Nephrotoxicity in Vitro: Potential Role of Free Radicals and Renal Biotransformation. International Journal of Molecular Sciences. 2014; 15(11):20900-20912. https://doi.org/10.3390/ijms151120900
Chicago/Turabian StyleRacine, Christopher, Dakota Ward, Dianne K. Anestis, Travis Ferguson, Deborah Preston, and Gary O. Rankin. 2014. "3,4,5-Trichloroaniline Nephrotoxicity in Vitro: Potential Role of Free Radicals and Renal Biotransformation" International Journal of Molecular Sciences 15, no. 11: 20900-20912. https://doi.org/10.3390/ijms151120900





