Dexamethasone Improves Heat Stroke-Induced Multiorgan Dysfunction and Damage in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. DXM Improves Survival during Heat Stroke in a Dose-Dependent Manner
| Treatment Groups | Survival Time (min) |
|---|---|
| 1. Normal saline-treated (1 mL/kg, iv) normothermic control rats | >480 |
| 2. Normal saline-treated (1 mL/kg, iv) heat stroke rats | 24 ± 3 †,‡ |
| 3. Dexamethasone (4 mg/kg, iv)-treated heat stroke rats | 104 ± 9 *,‡ |
| 4. Dexamethasone (6 mg/kg, iv)-treated heat stroke rats | 204 ± 25 *,† |
| 5. Dexamethasone (8 mg/kg, iv)-treated heat stroke rats | 268 ± 27 *,†,‡ |
2.2. DXM Ameliorates Arterial Hypotension, Cerebral Ischemia and Damage during Heat Stroke
2.3. DXM Attenuates Heat Stroke-Induced Hypercoagulable State


2.4. DXM Protects from Hepatic and Renal Dysfunction during Heat Stroke

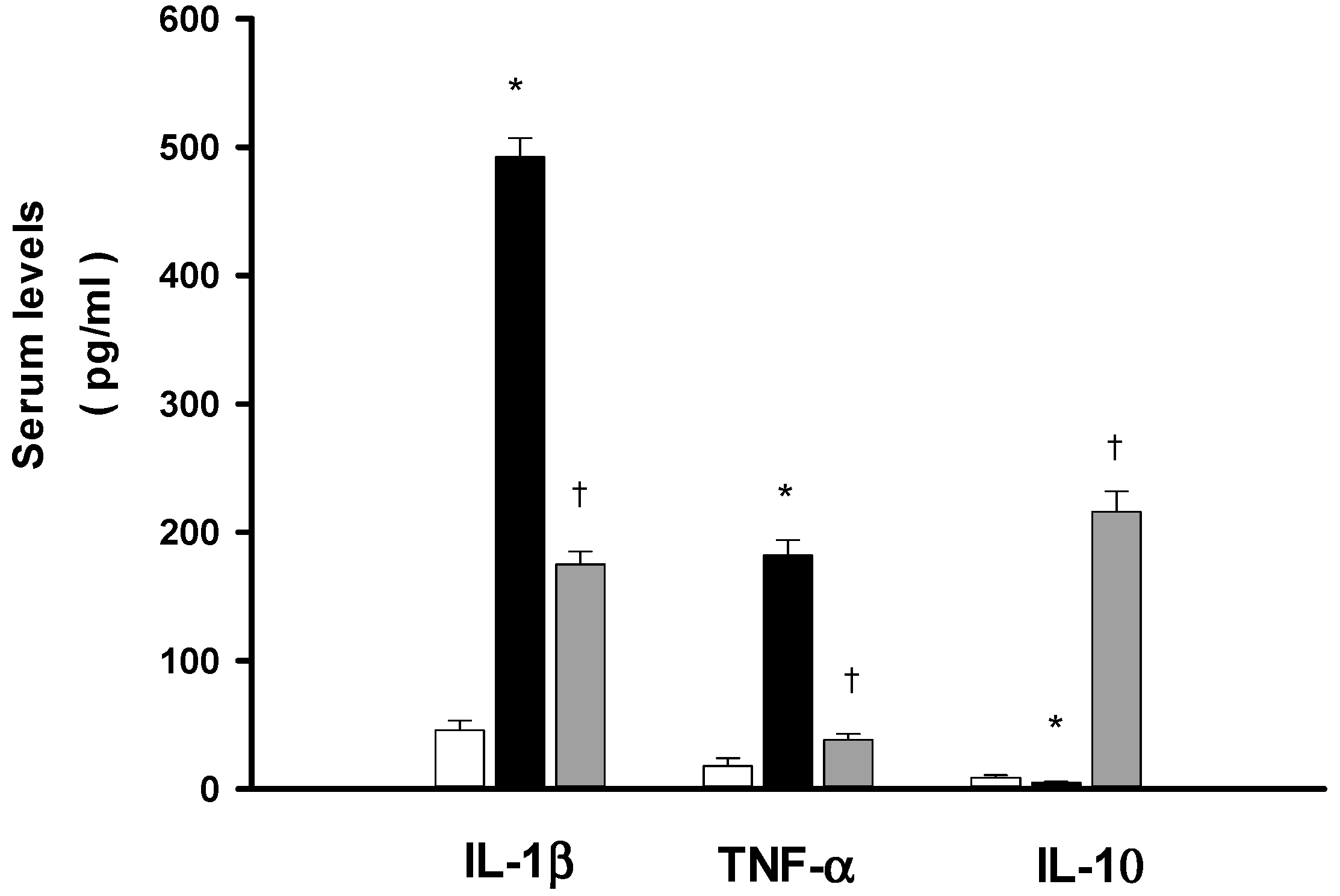
2.5. DXM Reduces both IL-1β and Tumor Necrosis Factor-α (TNF-α) Increase but Enhances Interleukin-10 (IL-10) during Heat Stroke
2.6. Discussion
3. Materials and Methods
3.1. Experimental Animals
3.2. Surgery and Physiological Parameter Monitoring
3.3. Induction of Heat Stroke and Experimental Design
3.4. Measurement of CBF
3.5. Measurements of Extracellular Ischemia and Damage Markers in Brain
3.6. Biochemical Measurements
3.7. Measurement for Serum Cytokines
3.8. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bouchama, A.; Knochel, J.P. Heat stroke. N. Engl. J. Med. 2002, 346, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Liu, Y.C.; Yen, D.H.; Wang, L.M.; Huang, C.I.; Lee, C.H.; Lin, M.T. l-arginine causes amelioration of cerebrovascular dysfunction and brain inflammation during experimental heat stroke. Shock 2008, 29, 212–216. [Google Scholar]
- Yang, T.H.; Ho, W.Y.; Shih, M.F.; Leu, K.L.; Wen, Y.S.; Liu, C.C. Effects of combination treatment with dexamethasone and mannitol on neuronal damage and survival in experimental heat stroke. Biol. Pharm. Bull. 2010, 33, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Shih, M.F.; Wen, Y.S.; Ho, W.Y.; Leu, K.L.; Wang, M.Y.; Liu, C.C. Attenuation of circulatory shock and cerebral ischemia injury in heat stroke by combination treatment with dexamethasone and hydroxyethyl starch. Exp. Transl. Stroke Med. 2010, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Chen, T.H.; Sue, Y.M.; Chen, T.J.; Cheng, C.Y. High-volume plasma exchange in a patient with acute liver failure due to non-exertional heat stroke in a sauna. J. Clin. Apher. 2014, 29, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.F.; Lin, C.H.; Hsu, S.F.; Lin, M.T. Melatonin improves outcomes of heat stroke in mice by reducing brain inflammation and oxidative damage and multiple organ dysfunction. Mediat. Inflamm. 2013, 2013, 349280. [Google Scholar] [CrossRef]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, G.; Ergungor, M.F.; Sezer, M.; Albayrak, L.; Daglioglu, E.; Kilinc, K. Therapeutic efficacy of intraventricular cyclosporine A and methylprednisolone on a global cerebral ischemia model in rats. Neurol. Res. 2005, 27, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D. The neuroprotective pharmacology of methylprednisolone. J. Neurosurg. 1992, 76, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Chien, C.H.; Lin, M.T. Glucocorticoids reduce interleukin-1 concentration and result in neuroprotective effects in rat heat stroke. J. Physiol. 2000, 527, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Chang, C.P.; Liu, S.Y.; Lin, M.T. Oxidative stress and ischemic injuries in heat stroke. Prog. Brain Res. 2007, 162, 525–546. [Google Scholar] [PubMed]
- Liu, C.C.; Chen, Z.C.; Cheng, B.C.; Lin, M.T. Prior antagonism of endothelin-1A receptors alleviates circulatory shock and cerebral ischemia during rat heat stroke. J. Pharmacol. Sci. 2004, 96, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Cheng, B.C.; Lin, M.T.; Lin, H.J. Small volume resuscitation in a rat model of heat stroke. Am. J. Med. Sci. 2009, 337, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Huang, W.T.; Cheng, B.C.; Hsu, C.C.; Lin, M.T. The flavonoid baicalin protects against cerebrovascular dysfunction and brain inflammation in experimental heat stroke. Neuropharmacology 2007, 52, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Krau, S.D. Heat-related illness: A hot topic in critical care. Crit. Care Nurs. Clin. N. Am. 2013, 25, 251–262. [Google Scholar] [CrossRef]
- Chen, S.H.; Niu, K.C.; Lin, M.T. Cerebrovascular dysfunction is an attractive target for therapy in heat stroke. Clin. Exp. Pharmacol. Physiol. 2006, 33, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.J.; Mei, G.P.; Liu, J.; Li, Y.L.; Zuo, D.; Liu, S.J.; Zhao, T.B.; Lin, M.T. Therapeutic effects of melatonin on heat stroke-induced multiple organ dysfunction syndrome in rats. J. Pineal Res. 2011, 50, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Alzeer, A.H.; el-Hazmi, M.A.; Warsy, A.S.; Ansari, Z.A.; Yrkendi, M.S. Serum enzymes in heat stroke: prognostic implication. Clin. Chem. 1997, 43, 1182–1187. [Google Scholar] [PubMed]
- Bouchama, A.; de Vol, E.B. Acid-base alterations in heat stroke. Intensive Care Med. 2001, 27, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.; Bersohn, I.; Seftel, H.; Kent, G. Liver damage in heat stroke. Am. J. Med. 1970, 49, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C.; Abrahams, C.; Seftel, H.C. Chronic interstitial nephritis as a consequence of heat stroke. Q. J. Med. 1970, 39, 189–199. [Google Scholar] [PubMed]
- Leon, L.R. Heat stroke and cytokines. Prog. Brain Res. 2007, 162, 481–524. [Google Scholar] [PubMed]
- Bouchama, A.; al Sedairy, S.; Siddiqui, S.; Shail, E.; Rezeig, M. Elevated pyrogenic cytokines in heat stroke. Chest 1993, 104, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Hammami, M.M.; Bouchama, A.; al Sedairy, S.; Shail, E.; AlOhaly, Y.; Mohamed, G.E. Concentrations of soluble tumor necrosis factor and interleukin-6 receptors in heat stroke and heatstress. Crit. Care Med. 1997, 25, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T. Heat stroke-induced cerebral ischemia and neuronal damage. Involvement of cytokines and monoamines. Ann. N. Y. Acad. Sci. 1997, 813, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.H.; Chang, C.K.; Lin, M.T.; Chang, C.P. Interleukin-1 receptor antagonist restores homeostatic function and limits multiorgan damage in heat stroke. Eur. J. Appl. Physiol. 2008, 103, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Liu, H.H.; Yang, Y.L. Involvement of interleukin-1 receptor mechanisms in development of arterial hypotension in rat heat stroke. Am. J. Physiol. 1997, 273, H2072–H2077. [Google Scholar] [PubMed]
- Oberholzer, A.; Oberholzer, C.; Moldawer, L.L. Cytokine signaling—Regulation of the immune response in normal and critically ill states. Crit. Care Med. 2000, 28, N3–N12. [Google Scholar] [CrossRef] [PubMed]
- Standiford, T.J.; Strieter, R.M.; Lukacs, N.W.; Kunkel, S.L. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J. Immunol. 1995, 155, 2222–2229. [Google Scholar] [PubMed]
- Hillered, L.; Persson, L. Neurochemical monitoring of the acutely injured human brain. Scand. J. Clin. Lab. Investig. Suppl. 1999, 229, 9–18. [Google Scholar] [CrossRef]
- Hillered, L.; Persson, L.; Ponten, U.; Ungerstedt, U. Neurometabolic monitoring of the ischaemic human brain using microdialysis. Acta Neurochir. 1990, 102, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.C.; Valadka, A.B.; Gopinath, S.P.; Uzura, M.; Robertson, C.S. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit. Care Med. 1999, 27, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Hillered, L.; Valtysson, J.; Enblad, P.; Persson, L. Interstitial glycerol as a marker for membrane phospholipid degradation in the acutely injured human brain. J. Neurol. Neurosurg. Psychiatry 1998, 64, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.G.; Saveland, H.; Boris-Moller, F.; Brandt, L.; Wieloch, T. Increased levels of glutamate in patients with subarachnoid haemorrhage as measured by intracerebral microdialysis. Acta Neurochir. Suppl. 1996, 67, 45–47. [Google Scholar] [PubMed]
- Persson, L.; Hillered, L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J. Neurosurg. 1992, 76, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Pan, W.H.; Chiu, T.H.; Lin, M.T. Striatal glutamate release is important for development of ischemic damage to striatal neurons during rat heat stroke. Brain Res. 1998, 795, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.Y.; Lin, M.T. Brain serotonin depletion attenuates heat stroke-induced cerebral ischemia and cell death in rats. J. Appl. Physiol. 1996, 80, 680–684. [Google Scholar] [PubMed]
- Lin, M.T.; Kao, T.Y.; Chio, C.C.; Jin, Y.T. Dopamine depletion protects striatal neurons from heat stroke-induced ischemia and cell death in rats. Am. J. Physiol. 1995, 269, H487–H490. [Google Scholar] [PubMed]
- Chen, S.H.; Lin, M.T.; Chang, C.P. Ischemic and oxidative damage to the hypothalamus may be responsible for heat stroke. Curr. Neuropharmacol. 2013, 11, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.C.; Lin, K.C.; Yang, C.Y.; Lin, M.T. Protective effects of alpha-tocopherol and mannitol in both circulatory shock and cerebral ischaemia injury in rat heat stroke. Clin. Exp. Pharmacol. Physiol. 2003, 30, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Bouchama, A.; Kwaasi, A.; Dehbi, M.; Al, M.F.; Eldali, A.; El-Sayed, R.; Tbdelghani, T.; Alzahrani, A.S.; Roberts, G. Glucocorticoids do not protect against the lethal effects of experimental heatstroke in baboons. Shock 2007, 27, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.A.; Soane, T. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst. Rev. 2011, 9, CD000064. [Google Scholar] [CrossRef] [PubMed]
- Lukas, R.; Zykova, I.; Barsa, P.; Sram, J. Current role of methylprednisolone in the treatment of acute spinal cord injury. Acta Chir. Orthop. Traumatol. Cech. 2011, 78, 305–313. [Google Scholar] [PubMed]
- Xue, Q.; Patterson, A.J.; Xiao, D.; Zhang, L. Glucocorticoid Modulates Angiotensin II Receptor Expression Patterns and Protects the Heart from Ischemia and Reperfusion Injury. PLoS One 2014, 9, e106827. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Peinnequin, A.; Alonso, A.; Buguet, A.; Cespuglio, R.; Canini, F. Effect of glucocorticoid depletion on heat-induced Hsp70, IL-1beta and TNF-alpha gene expression. Brain Res. 2007, 1164, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic press: New York, NY, USA, 1982. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-C.; Shih, M.-F.; Wen, Y.-S.; Lai, Y.-H.; Yang, T.-H. Dexamethasone Improves Heat Stroke-Induced Multiorgan Dysfunction and Damage in Rats. Int. J. Mol. Sci. 2014, 15, 21299-21313. https://doi.org/10.3390/ijms151121299
Liu C-C, Shih M-F, Wen Y-S, Lai Y-H, Yang T-H. Dexamethasone Improves Heat Stroke-Induced Multiorgan Dysfunction and Damage in Rats. International Journal of Molecular Sciences. 2014; 15(11):21299-21313. https://doi.org/10.3390/ijms151121299
Chicago/Turabian StyleLiu, Chia-Chyuan, Mei-Fen Shih, Yi-Szu Wen, Ying-Hsiu Lai, and Tsai-Hsiu Yang. 2014. "Dexamethasone Improves Heat Stroke-Induced Multiorgan Dysfunction and Damage in Rats" International Journal of Molecular Sciences 15, no. 11: 21299-21313. https://doi.org/10.3390/ijms151121299




