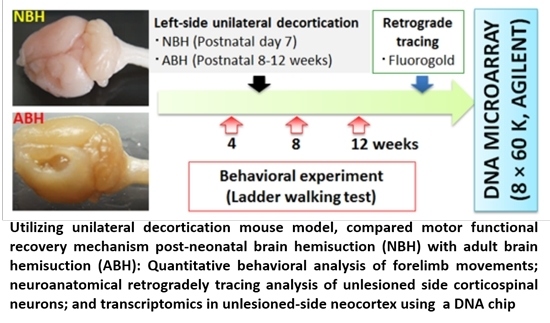
Comprehensive Analysis of Neonatal versus Adult Unilateral Decortication in a Mouse Model Using Behavioral, Neuroanatomical, and DNA Microarray Approaches
Abstract
:1. Introduction
2. Results
2.1. Cortical Hemisuction


2.2. Ladder Rung Walking Test Reveals Difference of Motor Functional Recovery between NBH and ABH
2.3. Differences in Retrogradely Fluorogold (FG) Labeled Corticospinal Neurons in Unlesioned Cerebral Cortex




2.4. Total RNA Extraction, cDNA Synthesis, RT-PCR, and Experimental Strategy for DNA Microarray Analysis

2.5. DNA Microarray Analysis Reveals Predominantly Down-Regulated Differentially Expressed Genes in NBH and ABH

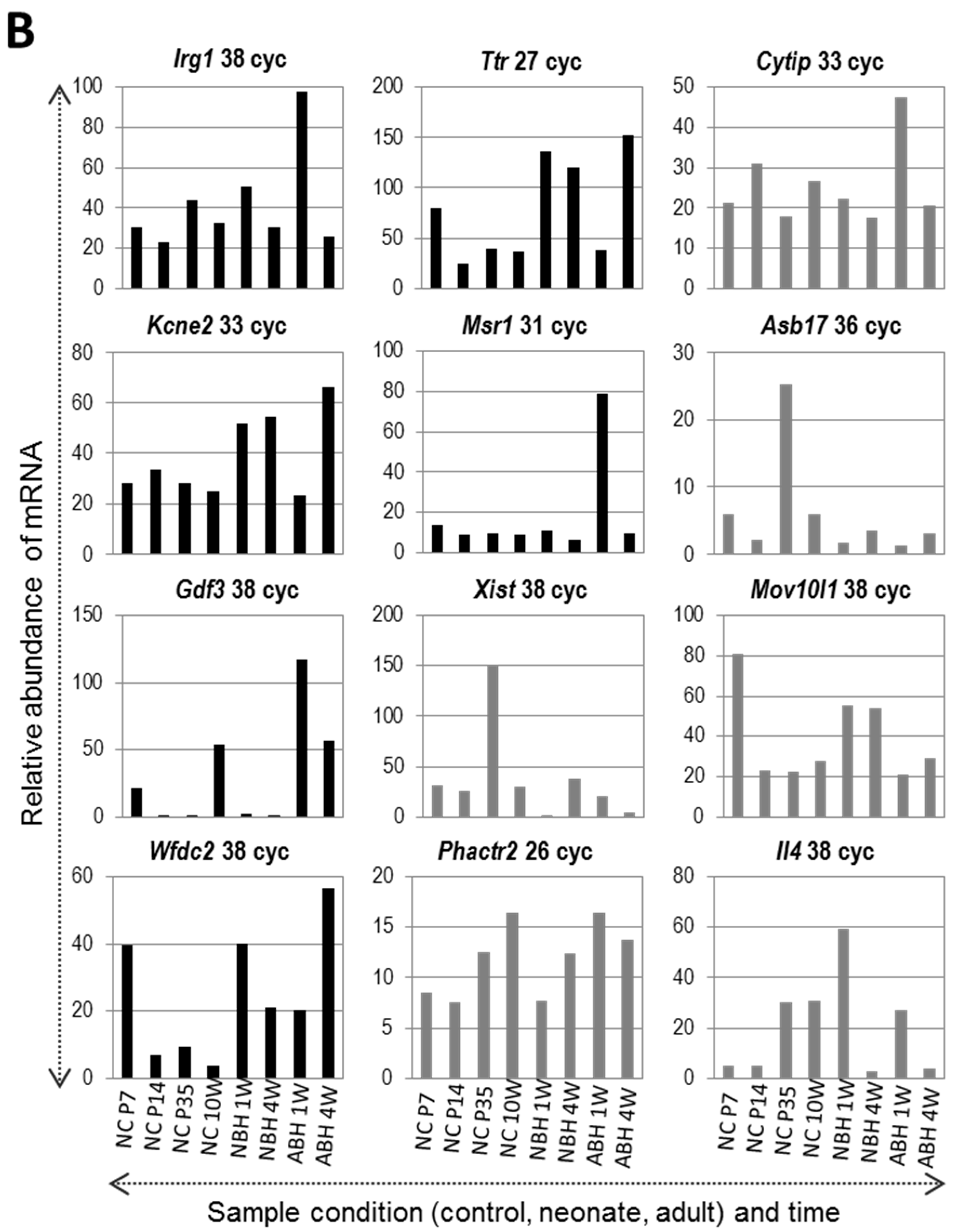
2.6. Confirmatory RT-PCR Analysis of Gene Expression Changes after DNA Microarray
2.7. Functional Categorization of Differentially Expressed Genes Reveals Up-Expression and Down-Expression of Diverse Functions
3. Discussion
3.1. Behavior and Histological Analyses of Corticospinal Neurons in Non-Lesioned Hemisphere
| Accession (Gene) | Gene Symbol | Description | Nucleotide Sequence (5'–3'): Forward * | Nucleotide Sequence (5'–3'): Reverse * | Product Size (bp) |
|---|---|---|---|---|---|
| NM_008084 | Gapdh | glyceraldehyde-3-phosphate dehydrogenase | gctacactgaggaccaggttgt | ctcctgttattatgggggtctg | 306 |
| NM_008392 | Irg1 | immunoresponsive gene 1 | accttctatggtcactggagga | tgcaacgtcgtttagatattgg | 266 |
| NM_013697 | Ttr | transthyretin | caccaaatcgtactggaagaca | ataagaatgcttcacggcatct | 285 |
| NM_139200 | Cytip | cytohesin 1 interacting protein | gagtcgtccttgtttggaaatc | aagggagaaaaggacccactac | 290 |
| NM_134110 | Kcne2 | potassium voltage-gated channel, Isk-related subfamily, gene 2 | tacgtcatcctgtacctcatgg | tgtcttctgagcaagcacaagt | 314 |
| NR_001463 | Xist | X-inactive specific transcript | aaggtggcttgctatggtaaaa | ggaactgcattaaagtcccaac | 275 |
| NM_025758 | Asb17 | ankyrin repeat and SOCS box-containing 17 | tacgtggctcagacaagacagt | tgatggttattctgcaaaggtg | 337 |
| NM_008108 | Gdf3 | growth differentiation factor 3 | aagaacgtcattctccgacatt | acaagaagcccagctactatgc | 264 |
| NM_031195 | Msr1 | macrophage scavenger receptor 1 | cgtgaatctacagcaaagcaac | gtaagccctctgtctccctttt | 257 |
| NM_031260 | Mov10l1 | Moloney leukemia virus 10-like 1 | acataaaggttggctcggtaga | ccctgtgtagacaccattgcta | 260 |
| NM_026323 | Wfdc2 | WAP four-disulfide core domain 2 | ctctgtctgctccaagcctaat | catttcatgttgccagaacact | 276 |
| NM_001195065 | Phactr2 | phosphatase and actin regulator 2 | ccaacaacggaagaattagagc | tcatgaacttccatttctgtgc | 296 |
3.2. Genome-Wide Gene Expression Analysis of NBH versus ABH Mouse
4. Conclusions
4.1. Clinical Issues
4.2. Results
4.3. Implications and Future Directions
5. Experimental Section
5.1. Animals and Husbandry
5.2. Left Unilateral Decortication Model
5.3. Behavioral Test
5.4. Retrograde Neuronal Tracing Study
5.5. Staining
5.6. PKCγ Immunohistochemistry
5.7. Neuronal Counts

5.8. Total RNA Extraction, cDNA Synthesis, and RT-PCR
5.9. Global Gene Expression Analysis
5.10. Access to Gene Array Data
5.11. Functional Categorization
5.12. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Abbreviations
| ABH | Adult Brain Hemisuction |
| CFA | Caudal Forelimb Area |
| CST | Corticospinal Tract |
| CCL | Chemokine (C-C) Ligand |
| CXCL | Chemokine (C-X-C) Ligand |
| FG | Fluorogold |
| GAPDH | Glyceraldehyde 3-Phosphate Dehydrogenase |
| GEO | Gene Expression Omnibus |
| IL6 | Interleukin 6 |
| IRG1 | Immunoresponsive Gene 1 |
| KO | Knockout |
| lncRNA | Long Noncoding RNAs |
| NBH | Neonatal Brain Hemisuction |
| NCBI | National Center for Biotechnology Information |
| NHS | Normal Horse Serum |
| PKCγ | Protein Kinase-C gamma |
| RFA | Rostral Caudal Forelimb |
| SMC | Sensorimotor Cortex |
| TTR | transthyretin |
| XIST | X-Inactive Specific Transcript |
Conflicts of Interest
References
- Devlin, A.M.; Cross, J.H.; Harkness, W.; Chong, W.K.; Harding, B.; Vargha-Khadem, F.; Neville, B.G. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain 2003, 126, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Peacock, W.J.; Wehby-Grant, M.C.; Shields, W.D.; Shewmon, D.A.; Chugani, H.T.; Sankar, R.; Vinters, H.V. Hemispherectomy for intractable seizures in children: A report of 58 cases. Childs Nerv. Syst. 1996, 12, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Van Empelen, R.; Jennekens-Schinkel, A.; Buskens, E.; Helders, P.J.; van Nieuwenhuizen, O. Functional consequences of hemispherectomy. Brain 2004, 127, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Van Empelen, R.; Jennekens-Schinkel, A.; Gorter, J.W.; Volman, M.J.; van Nieuwenhuizen, O.; Helders, P.J. Epilepsy surgery does not harm motor performance of children and adolescents. Brain 2005, 128, 1536–1545. [Google Scholar]
- Wilson, P.J. Cerebral hemispherectomy for infantile hemiplegia: A report of 50 cases. Brain 1970, 93, 147–180. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Spencer-Smith, M.; Leventer, R.; Coleman, L.; Anderson, P.; Williams, J.; Greenham, M.; Jcobs, R. Childhood brain insult: Can age at insult help us predict outcome? Brain 2009, 132, 45–56. [Google Scholar] [PubMed]
- Benecke, R.; Meyer, B.U.; Freund, H.J. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp. Brain Res. 1991, 83, 419–426. [Google Scholar] [CrossRef] [PubMed]
- McClelland, S., III; Maxwell, R.E. Hemispherectomy for intractable epilepsy in adults: The first reported series. Ann. Neurol. 2007, 61, 372–376. [Google Scholar]
- Nemati, F.; Kolb, B. Recovery from medial prefrontal cortex injury during adolescence: Implications for age-dependent plasticity. Behav. Brain Res. 2012, 229, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.Y.; Wright, S.; Armstrong, E.A.; Jahraus, C.M.; Saucier, D.M. The influence of aging on recovery following ischemic brain damage. Behav. Brain Res. 2006, 173, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Gianino, S.; Stein, S.A.; Li, H.; Lu, X.; Biesiada, E.; Ulas, J.; Xu, X.M. Postnatal growth of corticospinal axons in the spinal cord of developing mice. Brain Res. Dev. Brain Res. 1999, 112, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Gribnau, A.A.; de Kort, E.J.; Dederen, P.J.; Nieuwenhuys, R. On the development of the pyramidal tract in the rat: II. An anterograde tracer study of the outgrowth of the corticospinal fibers. Anat. Embryol. (Berl.) 1986, 175, 101–110. [Google Scholar] [CrossRef]
- Joosten, E.A.; van Eden, C.G. An anterograde tracer study on the development of corticospinal projections from the medial prefrontal cortex in the rat. Brain Res. Dev. Brain Res. 1989, 45, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.A.; Schuitman, R.L.; Vermelis, M.E.; Dederen, P.J. Postnatal development of the ipsilateral corticospinal component in rat spinal cord: a light and electron microscopic anterograde HRP study. J. Comp. Neurol. 1992, 326, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Gorgels, T.G. A quantitative analysis of axon outgrowth, axon loss, and myelination in the rat pyramidal tract. Brain Res. Dev. Brain Res. 1990, 54, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Stein, S.A.; Xu, X.M. Development of the corticospinal tract in the mouse spinal cord: A quantitative ultrastructural analysis. Brain Res. 2006, 1084, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Oudega, M.; Varon, S.; Hagg, T. Distribution of corticospinal motor neurons in the postnatal rat: Quantitative evidence for massive collateral elimination and modest cell death. J. Comp. Neurol. 1994, 347, 115–126. [Google Scholar] [CrossRef]
- Stanfield, B.B.; O’Leary, D.D.; Fricks, C. Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature 1982, 298, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, T.; Yoshioka, N.; Sakurai, M. Synapse elimination in the corticospinal projection during the early postnatal period. J. Neurophysiol. 2006, 95, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Murabe, N.; Sakurai, M. Regressive events in rat corticospinal axons during development in in vitro slice cocultures: Retraction, amputation, and degeneration. J. Comp. Neurol. 2009, 513, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Canty, A.J.; Murphy, M. Molecular mechanisms of axon guidance in the developing corticospinal tract. Prog. Neurobiol. 2008, 85, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Semeralul, M.O.; Boutros, P.C.; Likhodi, O.; Okey, A.B.; van Tol, H.H.; Wong, A.H. Microarray analysis of the developing cortex. J. Neurobiol. 2006, 66, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Harel, N.Y.; Strittmatter, S.M. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat. Rev. Neurosci. 2006, 7, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Vattanajun, A.; Umeda, T.; Isa, K.; Isa, T. Large-scale reorganization of corticofugal fibers after neonatal hemidecortication for functional restoration of forelimb movements. Eur. J. Neurosci. 2009, 30, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Takahashi, M.; Isa, K.; Isa, T. Formation of descending pathways mediating cortical command to forelimb motoneurons in neonatally hemidecorticated rats. J. Neurophysiol. 2010, 104, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Z’Graggen, W.J.; Fouad, K.; Raineteau, O.; Metz, G.A.; Schwab, M.E.; Kartje, G.L. Compensatory sprouting and impulse rerouting after unilateral pyramidal tract lesion in neonatal rats. J. Neurosci. 2000, 20, 6561–6569. [Google Scholar] [PubMed]
- Yoshikawa, A.; Atobe, Y.; Takeda, A.; Kamiya, Y.; Takiguchi, M.; Funakoshi, K. A retrograde tracing study of compensatory corticospinal projections in rats with neonatal hemidecortication. Dev. Neurosci. 2011, 33, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Metz, G.A.; Whishaw, I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: A new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods 2002, 115, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Starkey, M.L.; Barritt, A.W.; Yip, P.K.; Davies, M.; Hamers, F.P.; McMahon, S.B.; Bradbury, E.J. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp. Neurol. 2005, 195, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sydekum, E.; Haiss, F.; Peduzzi, S.; Zörner, B.; Schneider, R.; Baltes, C.; Rudin, M.; Weber, B.; Schwab, M.E. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 2009, 29, 12210–12219. [Google Scholar] [CrossRef]
- Tennant, K.A.; Adkins, D.L.; Donlan, N.A.; Asay, A.L.; Thomas, N.; Kleim, J.A.; Jones, T.A. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb. Cortex 2011, 21, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Isa, T. Differential contributions of rostral and caudal frontal forelimb areas to compensatory process after neonatal hemidecortication in rats. Eur. J. Neurosci. 2011, 34, 1453–1460. [Google Scholar] [CrossRef]
- Santos, S.D.; Lambertsen, K.L.; Clausen, B.H.; Akinc, A.; Alvarez, R.; Finsen, B.; Saraiva, M.J. CSF transthyretin neuroprotection in a mouse model of brain ischemia. J. Neurochem. 2010, 115, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Davis, F.B.; Luidens, M.K.; Mousa, S.A.; Cao, J.H.; Zhou, M.; Davis, P.J. Molecular basis for certain neuroprotective effects of thyroid hormone. Front. Mol. Neurosci. 2011, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Ballabio, A.; Rupert, J.L.; Lafreniere, R.G.; Grompe, M.; Tonlorenzi, R.; Willard, H.F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 1991, 349, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Hayano, Y.; Nakagawa, H.; Yamashita, T. Intraspinal rewiring of the corticospinal tract requires target-derived brain-derived neurotrophic factor and compensates lost function after brain injury. Brain 2012, 135, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, R.L.; Li, Y.; Cui, Y.; Chopp, M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke 2009, 40, 2546–2551. [Google Scholar]
- Nishibe, M.; Barbay, S.; Guggenmos, D.; Nudo, R.J. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J. Neurotrauma 2010, 27, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-responsive gene 1 proteins links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 2013, 110, 7820–7825. [Google Scholar]
- Wu, P.; Zuo, X.; Deng, H.; Liu, X.; Liu, L.; Ji, A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res. Bull. 2013, 97, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, I.A.; Mehler, M.F. Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Prog. Brain Res. 2010, 186, 77–95. [Google Scholar] [PubMed]
- Qureshi, I.A.; Mehler, M.F. The emerging role of epigenetics in stroke: II. RNA regulatory circuitry. Arch. Neurol. 2010, 67, 1435–1441. [Google Scholar] [PubMed]
- Pronichev, I.V.; Lenkov, D.N. Functional mapping of the motor cortex of the white mouse by a microstimulation method. Neurosci. Behav. Physiol. 1998, 28, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Paxonos, G.; Franklin, K.B.J. The Mouse Bain in Sterotaxic Coordinates, 1st ed.; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Hori, M.; Nakamachi, T.; Rakwal, R.; Shibato, J.; Nakamura, K.; Wada, Y. Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis. Model Mech. 2012, 5, 270–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, M.; Nakamachi, T.; Rakwal, R.; Shibato, J.; Ogawa, T.; Aiuchi, T.; Tsuruyama, T.; Tamaki, K.; Shioda, S. Transcriptomics and proteomics analyses of the PACAP38 influenced ischemic brain in permanent middle cerebral artery occlusion model mice. J. Neuroinflamm. 2012, 9, 256. [Google Scholar] [CrossRef]
- Ogawa, T.; Rakwal, R.; Shibato, J.; Sawa, C.; Saito, T.; Murayama, A.; Kuwagata, M.; Kageyama, H.; Yagi, M.; Satoh, K.; et al. Seeking gene candidates responsible for developmental origins of health and disease. Congenit. Anom. (Kyoto) 2011, 51, 110–125. [Google Scholar] [CrossRef]
- Ogawa, T.; Shibato, J.; Rakwal, R.; Saito, T.; Tamura, G.; Kuwagata, M.; Shioda, S. Seeking genes responsible for developmental origins of health and disease from the fetal mouse liver following maternal food restriction. Congenit. Anom. (Kyoto) 2014, 54, 195–219. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, A.; Nakamachi, T.; Shibato, J.; Rakwal, R.; Shioda, S. Comprehensive Analysis of Neonatal versus Adult Unilateral Decortication in a Mouse Model Using Behavioral, Neuroanatomical, and DNA Microarray Approaches. Int. J. Mol. Sci. 2014, 15, 22492-22517. https://doi.org/10.3390/ijms151222492
Yoshikawa A, Nakamachi T, Shibato J, Rakwal R, Shioda S. Comprehensive Analysis of Neonatal versus Adult Unilateral Decortication in a Mouse Model Using Behavioral, Neuroanatomical, and DNA Microarray Approaches. International Journal of Molecular Sciences. 2014; 15(12):22492-22517. https://doi.org/10.3390/ijms151222492
Chicago/Turabian StyleYoshikawa, Akira, Tomoya Nakamachi, Junko Shibato, Randeep Rakwal, and Seiji Shioda. 2014. "Comprehensive Analysis of Neonatal versus Adult Unilateral Decortication in a Mouse Model Using Behavioral, Neuroanatomical, and DNA Microarray Approaches" International Journal of Molecular Sciences 15, no. 12: 22492-22517. https://doi.org/10.3390/ijms151222492