Recent Developments in β-Cell Differentiation of Pluripotent Stem Cells Induced by Small and Large Molecules
Abstract
:1. Introduction
2. Importance of β-Cell Differentiation
| References | Initial stage | DE Induction | Pancreas Induction | Differentiation | ||
|---|---|---|---|---|---|---|
| ** Mesoendoderm/Definitive Endoderm | Primitive Gut Tube | Posterior for Gut | Pancreatic Endoderm | Hormone Expressing | ||
| STAGE 1 | STAGE 2 | STAGE 3 | STAGE 4 | STAGE 5 | ||
| [32] | OCT4, NANOG, SOX2, ECAD | ** [BRA, FGF4, WNT3, NCAD] SOX17, CER, FOXA2, CXCR4 (DE) | HNF1β, HNF4α | PDX1, HNF6, HLXB9 | NKX6-1, NGN3, PAX4, NKX2-2 | INS, CGL, GHRL, SST, PPY |
| [33] (No Serum) | – | SOX17, FOXA2, HNF4α, GATA4, CXCR4 | PDX1, FOXA2, SOX17, CXCR4, HLXB9, PTF1α, NGN3, NKX6.1 | PDX1, PTF1α, NGN3, ISL1, NKX6-1 | PDX1, CK-19, INS, Glucagon, GLU2, ISL1, NKX6-1 | – |
| [34] | FOXA2, SOX17 | PDX1, PTF1α, NGN3, INS, Somatostatin, Glucagon, Amylase | PAX4, NKX2.2, NKX6.1, ISL1, INS, Somatostatin, Glucagon, Amylase | PAX4, NKX2.2, NKX6.1, ISL1, INS, Somatostatin, Glucagon, Amylase | PAX4, NKX2.2, NKX6.1, ISL1, INS, Somatostatin, Glucagon, Amylase | – |
| [35] | – | BRACHURRY, SOX17, FOXA2, HNF4α | HNF4α | HNF4α, PDX1 | NGN3, PDX1 | INS, C-peptide and glucagon |
| [36] | OCT4, NANOG, SOX2, ECAD | ** [ BRA, FGF4, WNT3, NCAD (1–2 days)] SOX17, CER, FOXA2, CXCR4 | HNF1B, HNF4A | PDX1, HNF6, PROX1, SOX9 | NKX6-1, NGN3, PTF1A, NKX2-2 | – |
| [37] | – | CXCR4, SOX17, FOXA2 | PDX1 | – | PDX1 | - |
| [38] | – | FOXA2, CXCR4, SOX 17 | PDX1, HNF6, PAX6 | PDX1, FOXA2, SOX9, HNF1B, MAFA, INS, GLU2, NKX6-1, GLUCOKINASE, TCF1 | – | PDX1, NKX6-1, GLUT2, MAFA, ISL-1, NEUROD |
| [39] | – | SOX17, GSC, FOXA2, CXCR4 | HNF1β, HNF6 | HNF1b, HNF6, SOX9, HLXB9, PDX1 | NKX6.1, NGN3, PAX4, PDX1, FOXA2 | PDX1 |
| [40] | OCT4 | FOXA2, SOX 17 | HNF1β, HNF4α | PDX1 | AMY | – |
| [41] | – | CDX2, SOX2, SOX9 | – | – | NGN3, ISL1, NEUROD1, PAX6, MAFB, PROX1 | INS, GCG, SST, ARX1, MAF, INSM1 |
| [42] | – | SOX17, FOXA2 | – | PDX1, HNF6, HLXB, NGN3, NEUROD1, SOX9 | INS, C-Peptide, PDX1, NEUROD1, ISLET-1, PAX6, and NKX2.2, glucagon ghrelin, or somatostatin | – |
| [43] | – | SOX17, GSC, FOXA2, CXCR4 | FOXA1, HNF1β, HNF4α | PDX1, HNF6, PROX1, SOX9 | NKX6-1, PTF1α, NGN3, NKX2-2 | CHGA, INS, GCG, SST |
| [44] | – | SOX17, GSC, FOXA2, CXCR4 | FOXA1, HNF1β, HNF4α | PDX1, HNF6, PROX1, SOX9 | NKX6-1, PTF1α, NGN3, NKX2-2 | CHGA, INS, GCG, SST |

3. Signal Transduction Pathways

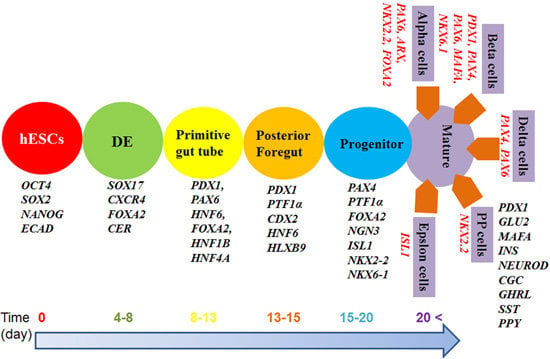
4. Timelines for β-Cell Differentiation

| Advantage and Disadvantage of Clinical Conditions | hMSCs | hESCs | hiPSCs |
|---|---|---|---|
| Ethical concern | no | yes | no |
| Xeno-free, feeder free culture | easy | difficult | difficult |
| Preparation of pluripotent (multipotent) stem cells | easy | relatively difficult | relatively difficult |
| Long-term expansion | difficult | easy | easy |
| Differentiation ability into β-cells | low | high | high |
| Tumor generation possibility | no | yes | yes |
| Mass production for clinical usage | no | yes | yes |
5. Small and Large Molecules
| References | DE Induction | Pancreas Induction | Differentiation | ||||
|---|---|---|---|---|---|---|---|
| ** Mesoendoderm/Definitive Endoderm | Primitive Gut Tube | Posterior for Gut | Pancreatic Endoderm | Hormone Expressing | |||
| STAGE 1 | STAGE 2 | STAGE 3 | STAGE 4 | STAGE 5 | |||
| [32] | ** [Activin A + WNT3A (RPMI) (1–2 days)] Activin A (RPMI) (1–2 days) | FGF10 + CYC (RPMI + FBS) (2–4 days) | RA + CYC + FGF10 (DMEM/B27) (2–4 days) | +/− DAPT + EX4 (DMEM/B27) (2–3 days) | +/− EX4 + IGF1 + HGF (CMRL/B27) (3+ days) | ||
| [71] | Activin A + Sodium butyrate (1 day) | – | EGF + FGF-2 + Noggin (7–14 days) (RPMI/B27) | EGF + Noggin (7 days) (RPMI/B27) | RPMI/bovine serum albumin) (Nicotinamide + IGF-II) (5 days) & without IGF-II for 2 days | ||
| (No Serum) | (RPMI/B27) | ||||||
| [83] | Activin A + BMP4 (10 days) | FGF18 + B27 (DMEM-F12/B27) (7 days) | FGF18 + B27, (EGF + TGFα + IGFI + IGFII + VEGF) DMEM F12/B27) (7 days) | Forskolin + FBS (HGF + PYY) (10 days) | – | ||
| [84] | Activin A + WNT3A (RPMI) (1 day) | FGF10 + sKAAD-Cyclopamine | All-trans retinoic acid | Betacellulin + Nicotinamide | Betacellulin + Nicotinamide | ||
| Activin A + FBS (RPMI) (2 days) | (RPMI) (3 days) | FGF10, KAAD-cyclopamine (DMEM/B27) | (DMEM/B27, Gamma SIX + EX-4) (2 days) | (CMRL/B27) (6 days) | |||
| [39] | ** [Activin A, WNT3A (RPMI) (1 day)] | KGF (RPMI + FBS) (3 days) | RA, CYC, NOG (DMEM/B27) (3 days) | No factors (DMEM/B27) (3 days) | – | ||
| Activin A (RPMI, FBS) (2 days) | |||||||
| [65] | Activin A; Activin A + ITS (2 days) | DE cells were dissociated and replated on mitomycin treated 3T3 Cells in Matrigel plate FGF7 + RA (6 days) (DF12/B27) | (DF12/B27) (KGF + BMP2 + RA + Noggin) (2 days) | Basal medium without KGF, HGF, EX4, Nicotinamide) (6 days) | – | ||
| [70] | Activin A, Wortmannin (DF12) (4 days) | RA + NOGGIN + FGF7 (DF12/IMDM) (4 days) | EGF (5 days) | Nico + FGF-2 + EX4 + BMP4 (DF12) (7 days) | – | ||
| [68] | ** [Activin A, WNT3A]; Activin A + FBS (RPMI) | FGF10, KAAD, Cyclopamine (3 days) | FGF10, KAAD, Cyclopamine, RA, Noggin | NA, EX4, IGF1, HGF | – | ||
| EX4 + Gamma secretase inhibitor compound E (4 days) | |||||||
| [85] | Activin A, BMP4, FGF-2; Matrigel (3–4 days) | – | FGF-2 + ITS (14 days) | Serum free-ITS, FINE, FGF7, Nicotinamid, EX-4; Matrigel (14 to 28 days) | Nicotinamide + Matrigel (4–14 days) | ||
| [86] | Activin A (EB) (6 days) | RA (EB) (1 day) | FGF7 (DMEM/B27) (3 days) | FGF7 + GLP-1+ Nicotinamide) (DMEM/B27) (4 days) | – | ||
| [87] | Activin A, WNT3A, BMP4, VEGF, FGF-2) (RPMI) (2 days) | SFD + FGF10 + WNT3A ± DM (3 days) | Noggin + CYC + RA + FGF10 (DMEM) (3 days) | SB + Noggin (DMEM) (4 days) | SFD, SB, Noggin, Gamma SIX (9 days) | ||
| [42] | Activin A | Activin A + FBS (RPMI) (3 days) | Dorsomorphin | Forskolin | – | ||
| CHIR99021) | Retinoic acid | Dexamethasone | |||||
| (RPMI) (FBS) | SB431542 (7 days) | Alk5 inhibitor II | |||||
| (1 day) | – | Nicotinamide (11 days) | |||||
| [88] | Activin A, Wnt 3A | Activin A (RPMI + FBS + ITS) (3 days) | KGF + TGF-β RI kinase Inhibitor IV | TT + CYC + Noggin (DMEM/B27) | Noggin + KGF + EGF | ||
| (RPMI + FBS + ITS) | (RPMI + FBS + ITS) (3 days) | (3 days) | (4+ days) | ||||
| [81] | GDF8 | FGF7 | FGF7, VitC, RA, SANT | FGF7, VitC, RA | Stage 5 | Stage 6 | Stage 7 |
| GSK3β inh | VitC | TPB, LDN (2 days) | SANT, TPB | SANT, RA | ALK5 inh II | ALK5 inh II | |
| (3 days) | (2 days) | – | LDN (3 days) | ALK5 inh II | T3, LDN | T3, N-Cys | |
| – | – | – | – | T3 LDN | GS inh XX | AXL inh | |
| – | – | – | – | (3 days) | (7–15 days) | (7–15 days) | |
5.1. Activin A
5.2. Fibroblast Growth Factor (FGF)
5.3. Retinoic Acid
5.4. KAAD-Cyclopamine (CYC)
5.5. Wortmannin
5.6. Sodium Butyrate
5.7. Betacellulin
5.8. Noggin
5.9. EGF, HGF, KGF, and IGF
6. Clinical Trials
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes—Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of metabolic syndrome—Report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 997–986. [Google Scholar]
- Fioretto, P.; Steffes, M.W.; Sutherland, D.E.; Goetz, F.C.; Mauer, M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N. Engl. J. Med. 1998, 339, 69–75. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Ling, Q.D.; Hsu, S.T.; Umezawa, A. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem. Rev. 2012, 112, 4507–4540. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Ling, Q.D.; Ko, Y.A.; Chang, Y.; Umezawa, A. Biomaterials for the feeder-free culture of human embryonic stem cells and induced pluripotent stem cells. Chem. Rev. 2011, 111, 3021–3035. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilic, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Eminli, S.; Foudi, A.; Stadtfeld, M.; Maherali, N.; Ahfeldt, T.; Mostoslavsky, G.; Hock, H.; Hochedlinger, K. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat. Genet. 2009, 41, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Zhou, J.M.; Shi, G.L.; Ma, Y.; Yang, Y.; Yu, H.; Jin, S.; Wei, Z.; Chen, F.; Jin, Y. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum. Mol. Genet. 2009, 18, 4340–4349. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Ohnishi, H.; Oda, Y.; Tadokoro, M.; Sasa, M.; Kato, H.; Hattori, K.; Ohgushi, H. Generation of induced pluripotent stem cells from human adipose-derived stem cells without c-MYC. Tissue Eng. Part A 2010, 16, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, Z.; Kim, Y.; Sharkis, S.; Jang, Y.Y. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology 2010, 51, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.Y.; Trounson, A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar] [CrossRef]
- Semb, H. Definitive endoderm: A key step in coaxing human embryonic stem cells into transplantable β-cells. Biochem. Soc. Trans. 2008, 36, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Van Hoof, D.; D’Amour, K.A.; German, M.S. Derivation of insulin-producing cells from human embryonic stem cells. Stem Cell Res. 2009, 3, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Jiang, W.; Shi, Y.; Deng, H. Generation of pancreatic islet cells from human embryonic stem cells. Sci. China Ser. C 2009, 52, 615–621. [Google Scholar] [CrossRef]
- Oh, S.K.W.; Choo, A.B.H. Human embryonic stem cells: Technological challenges towards therapy. Clin. Exp. Pharmacol. Physiol. 2006, 33, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.X.; Hebrok, M. Stem cells to pancreatic β-cells: New sources for diabetes cell therapy. Endocr. Rev. 2009, 30, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Bruin, J.E.; Erener, S.; Vela, J.; Hu, X.; Johnson, D.J.; Kurata, T.H.; Lynn, C.F.; Piret, M.J.; Asadi, A.; Rezania, A.; et al. Characterization of polyhormonal insulin-producing cells derived in vitro from human embryonic stem cells. Stem Cell Res. 2014, 12, 194–208. [Google Scholar] [CrossRef]
- Hrvatin, S.; O’Donnell, C.W.; Deng, F.; Millman, J.R.; Pagliuca, W.F.; Dilorio, P.; Rezania, A.; Gifford, K.D.; Melton, D.A. Differentiated human stem cells resemble fetal, not adult, β-cells. Proc. Natl. Acad. Sci. USA 2014, 111, 3038–3043. [Google Scholar] [CrossRef] [PubMed]
- Calne, R. Cell transplantation for diabetes. Philos. Trans. R. Soc. B 2005, 360, 1769–1774. [Google Scholar] [CrossRef]
- Robertson, R.P. Consequences on β-cell function and reserve after long-term pancreas transplantation. Diabetes 2004, 53, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Lakey, J.R.T.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Keymeulen, B.; Gillard, P.; Mathieu, C.; Movahedi, B.; Delvaux, G.; Ysebaert, D.; Roep, B.; Vandemeulebroucke, E.; Marichal, M.; Veldt, P.; et al. Correlation between β cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc. Natl. Acad. Sci. USA 2006, 103, 17444–17449. [Google Scholar] [CrossRef]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [PubMed]
- Soria, B.; Roche, E.; Berna, G.; Leon-Quinto, T.; Reig, J.A.; Martin, F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 2000, 49, 157–162. [Google Scholar] [CrossRef]
- Jiang, W.; Shi, Y.; Zhao, D.X.; Chen, S.; Yong, J.; Zhang, J.; Qing, T.; Sun, X.; Zhang, P.; Ding, M.; et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Riedel, M.J.; Wideman, R.D.; Karanu, F.; Ao, Z.; Warnock, G.L.; Kieffer, T.J. Productionoffunctional glucagon-secreting α-cells from human embryonic stem cells. Diabetes 2011, 60, 239–247. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Bang, A.G.; Eliazer, S.; Kelly, O.G.; Agulnick, A.D.; Smart, N.; Moorman, M.A.; Kroon, E.; Carpenter, M.K.; Baetge, E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006, 24, 1392–1401. [Google Scholar] [CrossRef]
- Marson, A.; Foreman, R.; Chevalier, B.; Bilodeau, S.; kahn, M.; Young, R.A.; Jaenisch, R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell 2008, 3, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Quesada, I.; Soria, B. Homologous and heterologous asynchronicity between identified α-, β- and δ-cells within intact islets of Langerhans in the mouse. J. Physiol. 1999, 517, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Riese, D.J.; Bermingham, Y.; van Raaij, T.M.; Buckley, S.; Plowman, G.D.; Stern, D.F. Betacellulin activates the epidermal growth factor receptor and erbB-4, and induces cellular response patterns distinct from those stimulated by epidermal growth factor or neuregulin-β. Oncogene 1996, 12, 345–353. [Google Scholar] [PubMed]
- Lyashenko, N.; Winter, M.; Migliorini, D.; Biechele, T.; Moon, R.T.; Hartmann, C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 2011, 13, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Habener, J.F. Wnt signaling in pancreatic islets. Adv. Exp. Med. Biol. 2010, 654, 391–419. [Google Scholar] [PubMed]
- Lluis, F.; Pedone, E.; Pepe, S.; Cosma, M.P. Periodic activation of Wnt/β-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell 2008, 3, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar]
- Lee, S.H.; Hao, E.; Savinov, A.Y.; Geron, I.; Strongin, A.Y.; Itkin-Ansari, P. Human β-cell precursors mature into functional insulin-producing cells in an immunoisolation device: Implications for diabetes cell therapies. Transplantation 2009, 87, 983–991. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.S.; Vegas, A.; Anderson, D.G.; Weir, G.C. Islets transplanted in immunoisolation devices: A review of the progress and the challenges that remain. Endocr. Rev. 2011, 32, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jordan, N.; Melton, D.; Grapin-Botton, A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 2003, 259, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Fiorina, P.; Voltarelli, J.; Zavazava, N. Immunological applications of stem cells in type 1 diabetes. Endocr. Rev. 2011, 32, 725–754. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; Segel, M.; Dervort, A.V.; Ryu, J.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic β-cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.C.; Chao, K.F.; Fu, Y.S.; Liu, S.H. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One 2008, 3, e1451. [Google Scholar] [CrossRef] [PubMed]
- Alipio, Z.; Liao, W.B.; Roemer, E.J.; Waner, M.; Fink, L.M.; Ward, D.C.; Ma, Y. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic β-like cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13426–13431. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Lim, H.; Kim, J.H.; Thuan, N.V.; Park, S.H.; Lim, Y.M.; Coi, H.Y.; Lee, E.R.; Kim, J.H.; Lee, M.S.; et al. Differentiation and transplantation of functional pancreatic β cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem Cells Dev. 2012, 21, 2642–2655. [Google Scholar] [CrossRef] [PubMed]
- Grapin-Botton, A.; Heimberg, H.; Lemaigre, F. The genetic programme of pancreatic β-cells: Basic science for the development of β-cell therapy. EMBO Rep. 2007, 8, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Chen, Y.G. Controlling TGF-β signaling. Gene Dev. 2000, 14, 627–644. [Google Scholar] [PubMed]
- Frandsen, U.; Porneki, A.D.; Floridon, C.; Abdallah, B.M.; Kassem, M. Activin B mediated induction of Pdx1 in human embryonic stem cell derived embryoid bodies. Biochem. Biophys. Res. Commun. 2007, 362, 568–574. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.B.; D’Amour, K.A.; Jones, K.L.; Krishnamoorthy, M.; Kulik, M.J.; Reynolds, D.M.; Sheppard, A.M.; Liu, H.; Xu, Y.; Baetge, E.E.; et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 2007, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Rulifson, I.C.; Tsai, B.C.; Heit, J.J.; Cahoy, J.D.; Kim, S.K. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16105–16110. [Google Scholar] [CrossRef] [PubMed]
- Powis, G.; Bonjouklian, R.; Berggren, M.M.; Gallegos, A.; Abraham, R.; Ashendel, C.; Zalkow, L.; Matter, W.F.; Dodge, J.; Grindey, G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994, 54, 2419–2423. [Google Scholar] [PubMed]
- Vlahos, C.J.; Matter, W.F.; Brown, R.F. A Specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 1994, 269, 5241–5248. [Google Scholar] [PubMed]
- Vallier, L.; Touboul, T.; Chng, Z.Z.; Brimpari, M.; Hannan, N.; Millan, E.; Smithers, L.; Trotter, M.; Ragg-Gunn, P.; Weber, A.; et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS One 2009, 4, e6082. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Wang, Q.; Lin, S.L.; Chang, C.D.; Chuang, J.; Ying, S.Y. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp. Biol. Med. 2006, 231, 534–544. [Google Scholar]
- Moriya, N.; Komazaki, S.; Takahashi, S.; Yokota, C.; Asashima, M. In vitro pancreas formation from Xenopus ectoderm treated with activin and retinoic acid. Dev. Growth Differ. 2000, 42, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, T.S.; Kadison, A.S.; Crisera, C.A.; Grau, J.B.; Alkasab, S.L.; Longaker, M.T.; Gittes, G.K. Ontogeny of activin B and follistatin in developing embryonic mouse pancreas: Implications for lineage selection. J. Gastrointest. Surg. 2000, 4, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hebrok, M.; Li, E.; Oh, S.P.; Schrewe, H.; Harmon, E.B.; Lee, J.S.; Melton, D.A. Activin receptor patterning of foregut organogenesis. Gene Dev. 2000, 14, 1866–1871. [Google Scholar] [PubMed]
- Miralles, F.; Czernichow, P.; Scharfmann, R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development 1998, 125, 1017–1024. [Google Scholar] [PubMed]
- Shiozaki, S.; Tajima, T.; Zhang, Y.Q.; Furukawa, M.; Nakazato, Y.; Kojima, I. Impaired differentiation of endocrine and exocrine cells of the pancreas in transgenic mouse expressing the truncated type II activin receptor. Biochim. Biophys. Acta 1999, 1450, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Idehara, C.; Yano, M.; Matsushita, T.; Yamada, T.; Li, S.; Moritani, M.; Hata, J.; Sugino, H.; Noji, S.; et al. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J. Clin. Investig. 1998, 102, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Zhang, H.; Maeshima, A.; Kurihara, H.; Miyagawa, J.-I.; Takeuchi, T.; Kojima, I. Up-regulation of the expression of activins in the pancreatic duct by reduction of the β-cell mass. Endocrinology 2002, 143, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.C.; Adams, A.M.; Goodson, J.M.; McDonald, C.E.; Potter, J.C.; Berndt, J.D.; Biechele, T.L.; Taylor, R.J.; Moon, R.T. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. USA 2012, 109, 4485–4490. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yu, C.; Liu, Y.; Chen, S.; Guo, Y.; Yong, J.; Lu, W.; Ding, M.; Deng, H. Generation of homogeneous PDX1+ pancreatic progenitors from human ES cell-derived endoderm cells. J. Mol. Cell Biol. 2010, 2, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.T.; Xu, X.; Yi, F.; Merrill, B.J.; Cooney, A.J. Canonical Wnt/β-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells 2010, 28, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, M.; Stahlberg, A.; Ameri, J.; Sand, F.W.; Norman, K.; Semb, H. FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner. PLoS One 2009, 4, e4794. [Google Scholar] [CrossRef] [PubMed]
- Mfopou, J.K.; Chen, B.; Mateizel, I. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology 2010, 138, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotencyin human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Jiang, W.; Liu, M.; Sui, X.; Yin, X.; Chen, S.; Shi, Y.; Deng, H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009, 19, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Au, M.; Lu, K.H.; Eshpeter, A.; Korbutt, G.; Fisk, G.; Majumdar, A.S. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007, 25, 1940–1953. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Cooper, B.; Gannon, M.; Ray, M.; McDonald, R.J.; Wright, C.V. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 2002, 32, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Melton, D.A. Pancreas development is promoted by cyclopamine, a Hedgehog signaling inhibitor. Proc. Natl. Acad. Sci. USA 1998, 95, 13036–13041. [Google Scholar] [CrossRef] [PubMed]
- Hebrok, M.; Kim, S.K.; St-Jacques, B.; McMahon, A.P.; Melton, D.A. Regulation of pancreas development by hedgehog signaling. Development 2000, 127, 4905–4913. [Google Scholar] [PubMed]
- Kawahira, H.; Scheel, D.W.; Smith, S.B.; German, M.S.; hebrok, M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev. Biol. 2005, 280, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, E.H.; Walterhouse, D.O.; Iannaccone, P.M. The sonic hedgehog-patched-gli pathway in human development and disease. Am. J. Hum. Genet. 2000, 67, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Chen, J.K.; Cooper, M.K.; Wang, B.; Mann, R.K.; Milenkovic, L.; Scott, M.P.; Beachy, P.A. Effects of oncogenic mutations in smoothened and patched can be reversed by cyclopamine. Nature 2000, 406, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Melton, D. Pancreas specification: A budding question. Curr. Opin. Genet. Dev. 2003, 13, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hou, L.L.; Tang, F.C.; Jiang, W.; Wang, P.; Ding, M.; Deng, H. Inducing embryonic stem cells to differentiate into pancreatic β cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells 2005, 23, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Kunisada, Y.; Tsubooka-Yamazoe, N.; Shoji, M.; Hosoya, M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012, 8, 274–284. [Google Scholar] [PubMed]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, I.; Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Attali, M.; Stetsyuk, V.; Basmaciogullari, A.; Aiello, V.; Boussif, M.; Duvillie, B.; Scharfmann, R. Control of β-cell differentiation by the pancreatic mesenchyme. Diabetes 2007, 56, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Otonkoski, T.; Cirulli, V.; Beattie, G.M.; Mally, M.I.; Soto, G.; Rubin, J.S.; Hayek, A. A role for hepatocyte growth factor/scatter factor in fetal mesenchyme-induced pancreatic β-cell growth. Endocrinology 1996, 137, 3131–3139. [Google Scholar] [PubMed]
- Cho, Y.M.; Lim, J.M.; Yoo, D.H.; Kim, J.H.; Chung, S.S.; Park, S.G.; Kim, T.H.; Oh, S.K.; Choi, Y.M.; Moon, S.Y.; et al. Betacellulin and nicotinamide sustain PDX1 expression and induce pancreatic β-cell differentiation in human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 366, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Browning, V.L.; Odorico, J.S. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech. Dev. 2011, 128, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, S.; Yoshie, S.; Yokoyama, T.; Tomotsune, D.; Yue, F.; Sasaki, K. A novel stepwise differentiation of functional pancreatic exocrine cells from embryonic stem cells. Stem Cells Dev. 2011, 20, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Basford, C.L.; Prentice, K.J.; Hardy, A.B.; Sarangi, F.; Micallef, S.J.; Li., X.; Guo, Q.; Elefanty, E.; Keller, G.; Allister, E.M. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic β cells. Diabetologia 2012, 55, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.C.; Young, H.Y.; Agulnick, A.D.; Babin, M.J.; Baetge, E.; Bang, A.; Bhoumik, A.; Cepa, I.; Cesario, R.; Haakmeester, C.; et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One 2012, 7, e37004. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K.; Mather, J.P. Inhibin, Activin and the female reproductive axis. Annu. Rev. Physiol. 1995, 57, 219–244. [Google Scholar] [CrossRef] [PubMed]
- Risbridger, G.P.; Schmitt, J.F.; Robertson, D.M. Activins and inhibins in endocrine and other tumors. Endocr. Rev. 2001, 22, 836–858. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, S.; Wankell, M.; Alzheimer, C.; Werner, S. Activin: An important regulator of wound repair, fibrosis, and neuroprotection. Mol. Cell Endocrinol. 2004, 225, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, Y.; Tabuchi, M.; Kojima, I.; Shibai, H.; Ogata, E. A novel action of activin A: Stimulation of insulin secretion in rat pancreatic islets. Biochem. Biophys. Res. Commun. 1988, 156, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kondo, S.; Sakurai, T.; Etoh, Y.; Shibai, H.; Muramatsu, M. Activin/EDF as an inhibitor of neural differentiation. Biochem. Biophys. Res. Commun. 1990, 173, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Albano, R.M.; Godsave, S.F.; Huylebroeck, D.; van Nimmen, K.; Isaacs, H.V.; Slack, J.M.; Smith, J.C. A mesoderm-inducing factor produced by Wehi-3 murine myelomonocytic leukemia-cells is activin-A. Development 1990, 110, 435–443. [Google Scholar] [PubMed]
- Eto, Y.; Tsuji, T.; Takezawa, M.; Takano, S.; Yokogawa, Y.; Shibai, H. Purification and characterization of erythroid differentiation factor (EDF) isolated from human leukemia cell line THP-1. Biochem. Biophys. Res. Commun. 1987, 142, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Bilezikjian, L.M.; Corrigan, A.Z.; Vale, W. Activin a modulates growth hormone secretion from cultures of rat anterior pituitary cells. Endocrinology 1990, 126, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Tada, S.; Era, T.; Furusawa, C.; Sakurai, H.; Nishikawa, S.; Kioshita, M.; Nakao, K.; Chiba, T.; Nishikawa, S. Characterization of mesendoderm: A diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 2005, 132, 4363–4374. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Rodriguez, R.T.; Wang, J.; Ghodasara, A.; Kim, S.K. Targeting Sox17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell 2011, 8, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M.; Tada, S.; Nishikawa, S.T.; Nakano, Y.; Okada, M.; Jakt, L.M.; Nishikawa, S.; Chiba, T.; Era, T.; Nishikawa, S. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat. Biotechnol. 2005, 23, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Sui, X.; Zhang, D.H.; Liu, M.; Ding, M.; Shi, Y.; Deng, H. CD24: A novel surface marker for PDX1-positive pancreatic progenitors derived from human embryonic stem cells. Stem Cells 2011, 29, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, S.; Yoshie, S.; Yue, F.M.; Ichikawa, H.; Yokoyama, H.; Nagai, M.; Tomotsune, D.; Hirayama, M.; Sasaki, K. Pancreatic exocrine enzyme-producing cell differentiation via embryoid bodies from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2011, 410, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Van Hoof, D.; Mendelsohn, A.D.; Seerke, R.; Desai, T.A.; German, M.S. Differentiation of human embryonic stem cells into pancreatic endoderm in patterned size-controlled clusters. Stem Cell Res. 2011, 6, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Norrman, K.; Strombeck, A.; Semb, H.; Stahlberg, A. Distinct gene expression signatures in human embryonic stem cells differentiated towards definitive endoderm at single-cell level. Methods 2013, 59, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, R.T.; Niehrs, C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005, 26, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Xu, J.S.; Colvin, J.S.; McEwen, D.G.; MacArthur, C.A.; Coulier, F.; Gao, G.; Goldfarb, M. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 1996, 271, 15292–15297. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, A.; Itoh, N.; Kato, S.; Thiery, J.P.; Czernichow, P.; Bellusci, S.; Scharfmann, R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 2001, 128, 5109–5117. [Google Scholar] [PubMed]
- Dichmann, D.S.; Miller, C.P.; Jensen, J.; Heller, R.S. Expression and misexpression of members of the FGF and TGF β families of growth factors in the developing mouse pancreas. Dev. Dynam. 2003, 226, 663–674. [Google Scholar] [CrossRef]
- Hart, A.W.; Baeza, N.; Apelqvist, A.; Edlund, H. Attenuation of FGF signalling in mouse β-cells leads to diabetes. Nature 2000, 408, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Roszell, B.; Mondrinos, M.J.; Seaton, A.; Simons, D.M.; Koutzaki, S.H.; Fong, G.H.; Lelkes, P.I.; Flinck, C.M. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng. Part A 2009, 15, 3351–3365. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Yoshino, K.; Yamada, T.; Yano, M.; Matsui, T.; Yamaguchi, T.; Moritani, M.; Hata, J.; Noji, S.; Itakura, M. Transgenic expression of FGF8 and FGF10 induces transdifferentiation of pancreatic islet cells into hepatocytes and exocrine cells. Biochem. Biophys. Res. Commun. 2002, 292, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Papadopoulou, S.; Edlund, H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev. Dyn. 2003, 228, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Dabernat, S.; Kritzik, M.; Kayali, A.G.; Zhang, Y.Q.; Liu, G.; Ungles, C.; Sarvetnick, N. FGFR3 is a negative regulator of the expansion of pancreatic epithelial cells. Diabetes 2007, 56, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ameri, J.; Stahlberg, A.; Pedersen, J.; Johansson, J.K.; Johannesson, M.M.; Artner, I.; Semb, H. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells 2010, 28, 45–56. [Google Scholar] [PubMed]
- Yamaguchi, T.P.; Harpal, K.; Henkemeyer, M.; Rossant, J. FGFR-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Gene Dev. 1994, 8, 3032–3044. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.X.; Wynshawboris, A.; Shen, M.M.; Daugherty, C.; Ornitz, D.M.; Leder, P. Murine Fgfr-1 is required for early postimplantation growth and axial organization. Gene Dev. 1994, 8, 3045–3057. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, F.J.; Heath, J.K. Developmentally-regulated expression of fibroblast growth-factor receptor genes and splice variants by murine embryonic stem and embryonal carcinoma-cells. Dev. Genet. 1994, 15, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Arman, E.; Haffner-Krausz, R.; Chen, Y.; Heath, J.K.; Lonai, P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc. Natl. Acad. Sci. USA 1998, 95, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.K.; Melton, D.A. Single-cell transcript analysis of pancreas development. Dev. Cell 2003, 4, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family—The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef] [PubMed]
- Miralles, F.; Czernichow, P.; Ozaki, K.; Itoh, N.; Scharfmann, R. Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc. Natl. Acad. Sci. USA 1999, 96, 6267–6272. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, G.A.; Jensen, J.N.; Jensen, J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev. Biol. 2003, 264, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, J.; Carlsson, L.; Edlund, T.; Edlund, H. Insulin-promoter-factor-1 is required for pancreas development in mice. Nature 1994, 371, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hebrok, M.; Melton, D.A. Notochord to endoderm signaling is required for pancreas development. Development 1997, 124, 4243–4252. [Google Scholar] [PubMed]
- Harrison, K.A.; Thaler, J.; Pfaff, S.L.; Gu, H. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nat. Genet. 1999, 23, 71–75. [Google Scholar] [PubMed]
- Li, H.; Arber, S.; Jessell, T.M.; Edlund, H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat. Genet. 1999, 23, 67–70. [Google Scholar] [PubMed]
- Jacquemin, P.; Lemaigre, F.P.; Rousseau, G.G. The onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev. Biol. 2003, 258, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, G.; Jung, J.N.; Zheng, M.H.; Lora, J.; Zaret, K.S. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 2001, 128, 871–881. [Google Scholar] [PubMed]
- Zaret, K.S.; Grompe, M. Generation and regeneration of cells of the liver and pancreas. Science 2008, 322, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Sekine, K.; Ohuchi, H.; Fujiwara, M.; Yamasaki, M.; Yoshizawa, T.; Sato, T.; Yagishita, N.; Matsui, D.; Koga, Y.; Itoh, N.; et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999, 21, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Solloway, M.J.; Robertson, E.J. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 1999, 126, 1753–1768. [Google Scholar] [PubMed]
- Nakanishi, M.; Hamazaki, T.S.; Komazaki, S.; Okochi, H.; Asashima, M. Pancreatic tissue formation from murine embryonic stem cells in vitro. Differentiation 2007, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Nakamura, K.; Mizukami, Y.; Ii, M.; Sasajima, J.; Sugiyama, Y.; Nishikawa, T.; Nakano, Y.; Yangawa, N.; Sato, K.; et al. Sonic hedgehog derived from human pancreatic cancer cells augments angiogenic function of endothelial progenitor cells. Cancer Sci. 2008, 99, 1131–1138. [Google Scholar]
- Thatava, T.; Nelson, T.J.; Edukulla, R.; Sakuma, T.; Okmine, S.; Tonne, J.M.; Yamada, S.; Kudva, Y.; Terzic, A.; Ikeda, Y. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther. 2011, 18, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.; Banerjee, I. Endothelial cell co-culture mediates maturation of human embryonic stem cell to pancreatic insulin producing cells in a directed differentiation approach. J. Vis. Exp. 2012, 61, 3759. [Google Scholar] [PubMed]
- Cooper, M.K.; Porter, J.A.; Young, K.E.; Beachy, P.A. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science 1998, 280, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Taipale, J.; Young, K.E.; Maiti, T. Small molecule modulation of smoothened activity. Proc. Natl. Acad. Sci. USA 2002, 99, 14071–14076. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hilcove, S.; Ding, S. Exploring stem cell biology with small molecules. Mol. Biosyst. 2006, 2, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Walker, J.; Zhang, J.; Ding, S.; Schultz, P.G. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem. Biol. 2004, 11, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Green, M.D.; Chen, A.; Nostro, M.C.; D’Souza, S.L.; Schaniel, C.; Lemischka, I.; Evans, V.; Gordon, K.; Snoeck, H. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Best, M.; Carroll, M.; Hanley, N.A.; Hanley, K.P. Embryonic stem cells to β-cells by understanding pancreas development. Mol. Cell Endocrinol. 2008, 288, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Wang, Y.F.; Carpino, G.; Mendel, G.; Alpini, G.; Gaudio, E.; Reid, L.M.; Alvaro, D. The biliary tree-a reservoir of multipotent stem cells. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, G.R.; Bleuming, S.A.; Hardwick, J.C.H.; Schepman, B.L.; Offerhaus, G.; Keller, J.J.; Nielson, C.; Gaffield, W.; Deventer, S.J.; Roberts, D.J.; et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 2004, 36, 277–282. [Google Scholar]
- Ptasznik, A.; Beattie, G.M.; Mally, M.I.; Cirulli, V.; Lopez, A.; Hayek, A. Phosphatidylinositol 3-kinase is a negative regulator of cellular differentiation. J. Cell Biol. 1997, 137, 1127–1136. [Google Scholar]
- Rooman, I.; Heremans, Y.; Heimberg, H.; Bouwens, L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia 2000, 43, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Haumaitre, C.; Lenoir, O.; Scharfmann, R. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Mol. Cell Biol. 2008, 28, 6373–6383. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, L.; Bouwens, L. Can β-cells be derived from exocrine pancreas? Diabetes Obes. Metab. 2008, 10, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.C.; Philippe, J.; Hermann, H.; Habener, J.F. Sodium butyrate increases glucagon and insulin gene expression by recruiting immunocytochemically negative cells to produce hormone. Diabetes 1988, 37, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.G.S.; Phadnis, S.; Nair, P.D.; Bhonde, R.R. Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells. Stem Cells 2009, 27, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Goicoa, S.; Alvarez, S.; Ricordi, C.; Inverardi, L.; Dominquez-Bendala, J. Sodium butyrate activates genes of early pancreatic development in embryonic stem cells. Cloning Stem Cells 2006, 8, 140–149. [Google Scholar] [CrossRef] [PubMed]
- De Aizpurua, H.J.; Cram, D.S.; Naselli, G.; Devereux, L.; Dorow, D.S. Expression of mixed lineage kinase-1 in pancreatic β-cell lines at different stages of maturation and during embryonic pancreas development. J. Biol. Chem. 1997, 272, 16364–16373. [Google Scholar] [CrossRef] [PubMed]
- Eshpeter, A.; Jiang, J.; Au, M.; Rajotte, R.V.; Lu, K.; Lebkowski, J.S.; Majumdar, A.S.; Korbutt, G.S. In vivo characterization of transplanted human embryonic stem cell-derived pancreatic endocrine islet cells. Cell Proliferat. 2008, 41, 843–858. [Google Scholar] [CrossRef]
- Salehi, M.; Aulinger, B.A.; D’Alessio, D.A. Targeting β-cell mass in type 2 diabetes: Promise and limitations of new drugs based on incretins. Endocr. Rev. 2008, 29, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, L.; Qi, H.; Wang, M.; Xue, W.; Xin, S.; Jing, L.; Yan, L.; Yegiang, L.; Fenrong, H.; et al. Combination of GLP-1 and sodium butyrate promote differentiation of pancreatic progenitor cells into insulin-producing cells. Tissue Cell 2008, 40, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Yan, L.; Shang, C.Z.; Cao, J.; Lu, L.H.; Min, J.; Cheng, H. Effects of sodium butyrate on the differentiation of pancreatic and hepatic progenitor cells from mouse embryonic stem cells. J. Cell. Biochem. 2010, 109, 236–244. [Google Scholar] [PubMed]
- Dunbar, A.J.; Goddard, C. Structure-function and biological role of betacellulin. Int. J. Biochem. Cell B 2000, 32, 805–815. [Google Scholar] [CrossRef]
- Huotari, M.A.; Palgi, J.; Otonkoski, T. Growth factor-mediated proliferation and differentiation of insulin-producing INS-1 and RINm5F cells: Identification of betacellulin as a novel β-cell mitogen. Endocrinology 1998, 139, 1494–1499. [Google Scholar] [PubMed]
- Li, L.; Seno, M.; Yamada, H.; Kojima, I. Betacellulin improves glucose metabolism by promoting conversion of intraislet precursor cells to β-cells in streptozotocin-treated mice. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E577–E583. [Google Scholar] [PubMed]
- Yechoor, V.; Liu, V.; Espiritu, C.; Paul, A.; Oka, K.; Kojima, H.; Chan, L. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets, in vivo but not transdifferentiation of hepatocytes. Dev. Cell 2009, 16, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Mashima, H.; Ohnishi, H.; Wakabayashi, K.; Mine, T.; Miyagawa, J.; Hanafusa, T.; Seno, M.; Yamada, H.; Kojima, I. Betacellulin and activin A coordinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J. Clin. Investig. 1996, 97, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Seno, M.; Yamada, H.; Kojima, I. Promotion of β-cell regeneration by betacellulin in ninety percent-pancreatectomized rats. Endocrinology 2001, 142, 5379–5385. [Google Scholar] [PubMed]
- Watada, H.; Kajimoto, Y.; Miyagawa, J.; Hanafusa, T.; Hamaguchi, K.; Matsuoka, T.; Yamamoto, K.; Matsuzawa, Y.; Kawamori, R.; Yamasaki, Y. PDX-1 induces insulin and glucokinase gene expressions in α TC1 clone 6 cells in the presence of betacellulin. Diabetes 1996, 45, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, F.M.; Brivanlou, A.H. The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm. Development 2008, 135, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Ahnfelt-Ronne, J.; Ravassard, P.; Pardanaud-Glavieux, C.; Scharfmann, R.; Serup, P. Mesenchymal bone morphogenetic protein signaling is required for normal pancreas development. Diabetes 2010, 59, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.M.; Dunn, N.R.; Hogan, B.L.M.; Zaret, K.S. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Gene Dev. 2001, 15, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Bruin, J.E.; Riedel, M.J.; Mojibian, M.; Asadi, A.; Xu, J.; Gauvin, R.; Narayan, K.; Karanu, F.; O’Neil, J.J.; et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012, 61, 2016–2029. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Sarvetnick, N. Expression of Id1 in adult, regenerating and developing pancreas. Endocrine 2007, 32, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Mashima, H.; Shibata, H.; Mine, T.; Kojima, I. Formation of insulin-producing cells from pancreatic acinar AR42J cells by hepatocyte growth factor. Endocrinology 1996, 137, 3969–7396. [Google Scholar] [PubMed]
- Gilon, P.; Henquin, J.C. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr. Rev. 2001, 22, 565–604. [Google Scholar] [PubMed]
- Wang, H.S.; Shyu, J.F.; Shen, W.S.; Hsu, H.C.; Chi, T.C.; Chen, C.P.; Huang, S.W.; Shyr, Y.M.; Tang, K.T.; Chen, T.H. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transpl. 2011, 20, 455–466. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, Y.S.; Ko, E.S.; Lim, S.M.; Lee, C.W.; Kim, D.I. Glucose-stimulated insulin secretion of various mesenchymal stem cells after insulin-producing cell differentiation. J. Biosci. Bioeng. 2012, 113, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, H.L.; Vanikar, A.V.; Thakker, U.; Firoze, A.; Dave, S.D.; Patel, C.N.; Patel, J.V.; Bhargava, A.B.; Shankar, V. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transpl. Proc. 2008, 40, 1135–1139. [Google Scholar] [CrossRef]
- Drucker, D.J. Glucagon-like peptides. Diabetes 1998, 47, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.W.; Hentze, H.; Rust, W.L.; Chen, Q.P.; Chipperfield, H.; Tan, E.; Abraham, S.; Sadasivam, A.; Soong, P.L.; Ang, S.T.; et al. Directed differentiation of human embryonic stem cells into the pancreatic endocrine lineage. Stem Cells Dev. 2007, 16, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Schiesser, J.V.; Wells, J.M. Generation of β cells from human pluripotent stem cells: Are we there yet? Ann. N. Y. Acad. Sci. 2014, 1311, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Blum, B.; Roose, A.N.; Barrandon, O.; Maehr, R.; Arvanites, A.; Davidow, L.; Davis, J.; Peterson, Q.P.; Rubin, L.; Melton, D.A. Reversal of β cell de-differentiation by a small molecule inhibitor of the TGF-β pathway. eLife 2014, 3, e02809. [Google Scholar] [PubMed]
- Sakano, D.; Shiraki, N.; Kikawa, K.; Yamazoe, T.; Kataoka, M.; Umeda, K.; Araki, K.; Mao, D.; Matsumoto, S.; Nakagata, N.; et al. VMAT2 identified as a regulator of late-stage β-cell differentiation. Nat. Chem. Biol. 2014, 10, 141–148. [Google Scholar]
- Golestaneh, N.; Kokkinaki, M.; Pant, D.; Jiang, J.; DeStefano, D.; Fernandez-Bueno, C.; Rone, J.D.; Haddad, B.R.; Gallicano, G.I.; Dym, M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009, 18, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Bruin, J.E.; Rezania, A.; Xu, J.; Narayan, K.; Fox, J.K.; O’Neil, J.J.; Kieffer, T.J. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 2013, 56, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.S.; Alarfaj, A.A.; Munusamy, M.A.; Singh, A.J.A.R.; Peng, I.-C.; Priya, S.P.; Hamat, R.A.; Higuchi, A. Recent Developments in β-Cell Differentiation of Pluripotent Stem Cells Induced by Small and Large Molecules. Int. J. Mol. Sci. 2014, 15, 23418-23447. https://doi.org/10.3390/ijms151223418
Kumar SS, Alarfaj AA, Munusamy MA, Singh AJAR, Peng I-C, Priya SP, Hamat RA, Higuchi A. Recent Developments in β-Cell Differentiation of Pluripotent Stem Cells Induced by Small and Large Molecules. International Journal of Molecular Sciences. 2014; 15(12):23418-23447. https://doi.org/10.3390/ijms151223418
Chicago/Turabian StyleKumar, S. Suresh, Abdullah A. Alarfaj, Murugan A. Munusamy, A. J. A. Ranjith Singh, I-Chia Peng, Sivan Padma Priya, Rukman Awang Hamat, and Akon Higuchi. 2014. "Recent Developments in β-Cell Differentiation of Pluripotent Stem Cells Induced by Small and Large Molecules" International Journal of Molecular Sciences 15, no. 12: 23418-23447. https://doi.org/10.3390/ijms151223418