Effect of Glucans from Caripia montagnei Mushroom on TNBS-Induced Colitis
Abstract
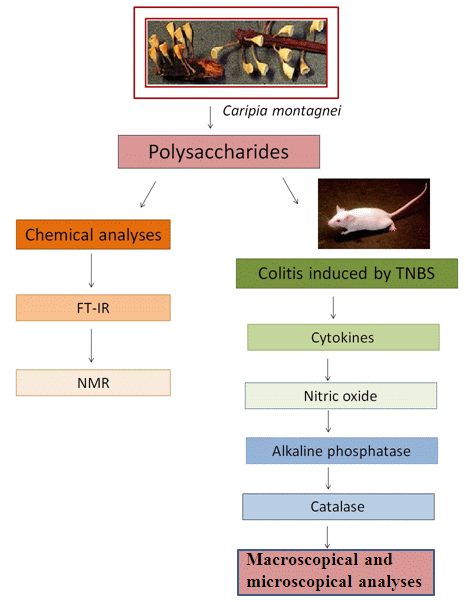
:1. Introduction
2. Results
2.1. Chemical Analysis
2.2. FT-IR Spectrum
2.3. NMR
2.4. Macroscopic Analysis
2.5. Activity of Myeloperoxidase (MPO)
2.6. Activity of Alkaline Phosphatase
2.7. Nitric Oxide
2.8. Activity of Catalase
2.9. Cytokine Analysis
2.10. Histological Analyses
3. Discussion
4. Experimental Section
4.1. Mushrooms
4.2. Animals
4.3. Materials
4.4. Polysaccharide Extraction and Fractionation
4.5. Chemical Analysis
4.6. Infrared Analysis
4.7. Nuclear Magnetic Resonance (NMR)
4.8. Anti-Inflammatory Activity in the Ulcerative Colitis Model Induced by 2,4,6-Trinitrobenzene Sulfonic Acid (TNBS)
4.9. Treatment of Colitis with Polysaccharides
4.10. Macroscopic Analysis
- Score 0: No injuries.
- Score 1: Hyperemia without ulceration.
- Score 2: Linear ulceration without inflammation.
- Score 3: Linear ulceration with inflammation.
- Score 4: Two or more ulcerations and inflammation.
- Score 5: Two or more ulcerations and inflammation or ulceration longer than 1 cm along the colon.
- Score 6–10: If the lesions are greater than 2 cm in length longitudinally, 1 point for every extra inch will be awarded.
4.11. Assessment of Myeloperoxidase Activity
4.12. Evaluation of Alkaline Phosphatase Activity
4.13. Nitric Oxide
4.14. Evaluation of Catalase Activity
4.15. Analysis of Cytokines
4.16. Histological Analyses
4.17. Statistical Analyses
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev 2002, 15, 79–85. [Google Scholar]
- Magalhães, A.F.N. Crohn’s disease (CH). In Clinical Gastroenterology, 3rd ed.; Dani, R., Castro, L.P., Eds.; Guanabara Koogan: Rio de Janeiro, Brazil, 1993; Volume 2, pp. 765–776. [Google Scholar]
- Danese, S.; Fiocchi, C. Etiopathogenesis of inflammatory bowel diseases. World J. Gastroenterol 2006, 12, 4807–4812. [Google Scholar]
- Singleton, J.W. Progressin inflammatory bowel disease. Chin. J. Dig. Dis 2005, 6, 59–61. [Google Scholar]
- Head, K.A.; Jurenka, J.S. Inflammatory bowel disease. Part 1: Ulcerative colitis pathophysiology and conventional and alternative treatment options. Altern. Med. Rev 2003, 8, 247–283. [Google Scholar]
- Ardizzone, S.; Bianchi-Porro, G. Biologic therapy for inflammatory bowel disease. Drugs 2005, 65, 2253–2286. [Google Scholar]
- Waldner, M.J.; Neurath, M.F. Novel cytokine-targeted therapies and intestinal inflammation. Curr. Opin. Pharmacol 2009, 9, 702–707. [Google Scholar]
- Gautam, R.; Jachak, S. Recent developments in anti-inflammatory natural products. Med. Res. Rev 2009, 29, 767–820. [Google Scholar]
- Ruthes, A.C.; Carbonero, E.R.; Córdova, M.M.; Baggio, C.H.; Santos, A.R.S.; Sassaki, G.L.; Cipriani, C.R.; Gorin, P.A.J.; Iacomini, M. Lactariusrufus (1→3),(1→6)-β-d-glucans: Structure, antinociceptive and anti-inflammatory effects. Carbohydr. Polym 2013, 94, 129–136. [Google Scholar]
- Queiroz, L.S.; Nascimento, M.S.; Cruz, A.K.M.; Castro, A.J.G.; Moura, M.F.V.; Baseia, I.G.; Araujo, R.M.; Benevides, N.M.; Lima, L.F.; Leite, E.L. Glucans from the mushroom Caripia montagnei present anti-inflammatory activity. Int. Immunopharmacol 2010, 10, 34–42. [Google Scholar]
- Dore, C.M.P.G.; Azevedo, T.C.G.; Souza, M.C.R.; Rego, L.A.; Dantas, J.C.M.; Silva, F.R.F.; Rocha, H.A.O.; Baseia, I.G.; Leite, E.L. Anti-inflammatory, antioxidant and cytotoxic actions of β-glucan-rich extract from Geastrum saccatum mushroom. Int. Immunopharmacol 2007, 7, 1160–1169. [Google Scholar]
- Finimundy, T.C.; Gambato, G.; Fontana, R.; Camassola, M.; Salvador, M.; Moura, S.; Hess, J.; Henriques, J.A.P.; Dillon, A.J.P.; Roesch-Ely, M. Aqueous extracts of Lentinulaedodes and Pleurotussajor-caju exhibit high antioxidant capability and promising in vitro antitumor activity. Nutr. Res 2013, 33, 76–84. [Google Scholar]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem 2011, 129, 1667–1675. [Google Scholar]
- Bellini, M.F.; Angel, J.P.; Matuo, R.; Terezan, A.P.; Ribeiro, L.R.; Mantovani, M.S. Antigenotoxicity of Agaricus blazei mushroom organic and aqueous extracts in chromosomal aberration and cytokinesis block micronucleus assays in CHO-k1 and HTC cells. Toxicol. in Vitro 2006, 20, 355–360. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med 1967, 70, 158–169. [Google Scholar]
- Worthington, D.J.; Rosemeyer, M.A. Human glutathione reductase: Purification of the crystalline enzyme from erythrocytes. Eur. J. Biochem 1974, 48, 167–177. [Google Scholar]
- Mathlouthi, M.; Koenig, J.L. Vibrational spectra of carbohydrates. Adv. Carbohydr. Chem. Biochem 1986, 44, 87–89. [Google Scholar]
- Stone, B.A.; Clarke, A.E. Chemistry and Biology of (1,3)-β-Glucans; La Trobe University Press: Boondora, Australia, 1992; p. 808. [Google Scholar]
- Fang, J.; Wang, Y.; Lv, X.; Shen, X.; Ni, X.; Ding, K. Structure of the β- glucan from Grifolafrondosa and its antitumor effect by activating dectin- 1/ Syk/ NF-κB signaling. Glycoconj. J 2012, 29, 365–377. [Google Scholar]
- Roy, S.K.; Das, D.; Mondal, S.; Maiti, D.; Bhunia, B.; Maiti, T.; Islam, S.S. Structural studies of an immunoenhancing water-soluble glucan isolated from hot water extract of an edible mushroom, Pleurotus florida, cultivar Assam Florida. Carbohydr. Res 2009, 344, 2596–2601. [Google Scholar]
- Perlin, A.S.; Casu, B. Carbon-13 and proton magnetic resonance spectra of d-13C-glucose. Tetrahedron Lett 1969, 34, 2919–2924. [Google Scholar]
- Ghosh, K.; Chandra, K.; Ojha, A.K.; Islam, S.S. NMR and MALDI-TOF analysis of the water-soluble glucan from an edible mushroom. Volvarielladiplasia Carbohydr. Res 2008, 343, 2834–2840. [Google Scholar]
- Huang, Z.P.; Zhang, L.N. Chemical structures of water-soluble polysaccharides from Rhizoma Panacis Japonici. Carbohydr. Res 2009, 344, 1136–1140. [Google Scholar]
- Sadovskaya, I.; Evgeny, V.; Jianjun, L.; Abderrahman, H.; Kowalska, K.; Filloux, A. High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: The ndvB gene is Involved in the production of highly phosphorylated glycerol-beta-(1->3)-glucans, which bind aminoglycosides. Glycobiology 2010, 20, 895–904. [Google Scholar]
- Hoffmann, J.C.; Pawlowski, N.N.; Kuhl, A.A.; Hohne, W.; Zeitz, M. Animal models of inflammatory bowel disease: an overview. Pathobiology 2002, 70, 121–130. [Google Scholar]
- Carlson, M.; Raab, Y.; Seveus, L.; Xu, S.; Hallgren, R.; Venge, P. Human neutrophil lipocalin is a unique marker of neutrophil inflammation in ulcerative colitis and proctitis. Gut 2002, 50, 501–506. [Google Scholar]
- Talero, E. Sánchez- Fidalgo, S., de la Lastra, C.A., llanes, M., Calvo, J.R., Motilva, V. Acute and chronic responses associated with adrenomedullin administration in experimental colitis. Peptides 2008, 29, 2001–2012. [Google Scholar]
- Martín, A.R.; Villegas, I. Sánchez- Hidalgo, M., de la Lastra, C.A. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br. J. Pharmacol 2006, 147, 873–885. [Google Scholar]
- Sanchez de Medina, F.; Martinez-Augustin, O.; Gonzalez, R.; Ballester, I.; Nieto, A.; Galvez, J.; Zarzuelo, A. Induction of alkaline phosphatase in the inflamed intestine: A novel pharmacological target for inflammatory bowel disease. Biochem. Pharmacol 2004, 68, 2317–2326. [Google Scholar]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med 2002, 33, 311–332. [Google Scholar]
- Buffinton, G.D.; Doe, W.F. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic. Biol. Med 1995, 19, 911–918. [Google Scholar]
- Abbas, A.K.; Janeway, C.A.J.R. Immunology: Improving on nature in the twenty-first century. Cell 2000, 100, 129–138. [Google Scholar]
- Gordon, S. Macrophage Function Disorders. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd: Chichester, UK, 2001; Volume 1, pp. 1–11. [Google Scholar]
- Hermann, G.E.; Rogers, R.C.; Bresnahan, J.C.; Beattie, M.S. Tumor necrosis factor-alpha induces cfos and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol. Dis 2001, 8, 590–599. [Google Scholar]
- Chang, A.Y.W.; Chan, J.Y.H.; Chan, S.H.H. Differential distribution of nitric oxide synthase isoforms in the rostral ventrolateral medulla of the rat. J. Biomed. Sci 2003, 10, 285–291. [Google Scholar]
- Koutroubakis, I.E.; Malliaraki, N.; Dimoulios, P.D.; Karmiris, K.; Castanas, E.; Kouroumalis, E.A. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig. Dis. Sci 2004, 49, 1433–1437. [Google Scholar]
- Bouzid, D.; Gargouri, B.; Mansour, R.B.; Amouri, A.; Tahri, N.; Lassoued, S.; Masmoudi, H. Oxidative stress markers in intestinal mucosa of Tunisian inflammatory bowel disease patients. Saudi J. Gastroenterol 2013, 19, 131–135. [Google Scholar]
- Lull, C.; Wichers, H.J.; Savelkoul, H.F.J. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediat. Inflamm 2005, 2, 63–80. [Google Scholar]
- Stallmach, A.; Hagel, S.; Bruns, T. Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol 2010, 24, 167–182. [Google Scholar]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 1984, 87, 1344–1350. [Google Scholar]
- Roupas, P.; Keogh, J.; Noakes, M.; Margetts, C.; Taylor, P. The role of edible mushrooms in health: Evaluation of the evidence. J. Funct. Foods 2012, 4, 687–709. [Google Scholar]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem 2011, 3, 674–678. [Google Scholar]
- Dudhgaonkar, S.; Thyagarajan, A.; Sliva, D. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int. Immunopharmacol 2009, 11, 1272–1280. [Google Scholar]
- Fangkrathok, N.; Junlatat, J.; Sripanidkulchai, B. In vivo and in vitro anti-inflammatory activity of Lentinuspolychrous extract. J. Ethnopharmacol 2013, 3, 631–637. [Google Scholar]
- Lavi, I.; Friesem, D.; Geresh, S.; Hadar, Y.; Schwartz, B. An aqueous polysaccharide extract from the edible mushroomPleurotusostreatus induces anti-proliferative and pro-apoptotic effects on HT-29 colon cancer cells. Cancer Lett 2006, 244, 61–70. [Google Scholar]
- Suseem, S.R.; Mary, S.A.; Neelakanda, R.P.; Gregory, M. Evaluation of the analgesic activity of ethyl acetate, methanol and aqueous extracts of Pleurotuseous mushroom. Asian Pac. J. Trop. Med 2011, 2, 117–120. [Google Scholar]
- Nascimento, M.S.; Magalhães, J.E.M.; Pinheiro, T.S.; Silva, T.A.; Coutinho, L.G.; Baseia, I.G.; Lima, L.F.A.; Leite, E.L. Polysaccharides from the fungus Scleroderma nitidum with anti-inflammatory potential modulate cytokine levels and the expression of nuclear factor κB. Rev. Bras. Farmacogn 2012, 22, 60–68. [Google Scholar]
- Battle, J.; Ha, T.; Li, C.; Della Beffa, V.; Rice, P.; Kalbfleisch, J.; Browder, W.; Williams, D. Ligand binding to the (1→3)-β-d-glucan receptor stimulates NFkappaB activation, but not apoptosis in U937 cells. Biochem. Biophys. Commun 1998, 249, 499–504. [Google Scholar]
- Yoon, W.J.; Ham, Y.M.; Kim, S.S.; Yoo, B.S.; Moon, J.Y.; Baik, J.S.; Lee, N.H.; Hyun, C.-G. Suppression of pro-inflammatory cytokines, iNOS, and COX-2 expression bybrown algae Sargassum micracanthumin RAW 264.7 macrophages. EurAsian J. BioSci 2009, 3, 130–143. [Google Scholar]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol 2003, 3, 23–35. [Google Scholar]
- Mitsuyama, K.; Sata, M.; Rose-John, S. Interleukin-6 trans-signaling in inflammatory bowel disease. Cytokine Growth Factor Rev 2006, 17, 451–461. [Google Scholar]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol 2006, 72, 1493–1505. [Google Scholar]
- Aupperle, K.R.; Bennett, B.L.; Boyle, D.L.; Tak, P.P.; Manning, A.M.; Firestein, G.S. NF-κB regulation by IkB kinase in primary fibroblast-like synoviocytes. J. Immunol 1999, 163, 427–433. [Google Scholar]
- Molavi, B.; Mehta, J.L. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr. Opin. Cardiol 2004, 19, 488–493. [Google Scholar]
- Jayasooriya, R.G.P.T.; Kang, C.; Seo, M.; Choi, Y.H.; Jeong, Y.; Kim, G. Exopolysaccharide of Laetiporus sulphureus var. miniatus downregulates LPS-induced production of NO, PGE2, and TNF-α in BV2 microglia cells via suppression of the NF-κB pathway. Food Chem. Toxicol 2011, 49, 2758–2764. [Google Scholar]
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; Lim, M.H.; Chang, W.; Lim, S.; Choi, S.; et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol 2007, 114, 307–315. [Google Scholar]
- Van, Q.; Nayak, B.N.; Reimer, M.; Jones, P.J.H.R.; Fulcher, G.; Rempel, C.B. Anti- inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in acell screening assay. J. Ethnopharmacol 2009, 125, 487–493. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Simth, F. Colorimetric method for the determination of sugars, and related substances. Anal. Chem 1956, 28, 350–356. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantization of microgram quanties of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 7, 248–254. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult 1965, 20, 144–158. [Google Scholar]
- Liu, L.; Guo, Z.J.; Lv, Z.G.; Sun, Y.; Cao, W.; Zhang, R.; Liu, Z.; Li, C.; Cao, S.; Mei, Q. The beneficial effect of polysaccharide on Rheum tanguticum protecting against diarrhea, colonic inflammation and ulceration in rats with TNBS-induced colitis: The Role of macrophage mannose receptor in inflammation and immune response. Int. Immunopharmacol 2008, 8, 1481–1492. [Google Scholar]
- Rodriguez-Cabezas, M.E.; Gálvez, J.; Lorente, M.D.; Concha, A.; Camuesco, D.; Azzouz, S.; Osuna, A.; Redondo, L.; Zarzuelo, A. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulforic acid induced colitic rats. J. Nutr 2002, 132, 3263–3271. [Google Scholar]
- Grisham, M.B.; Beniot, J.N.; Granger, D.N. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol 1990, 186, 729–742. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem 1985, 150, 76–85. [Google Scholar]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 1987, 84, 450–455. [Google Scholar]







© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Da S. Nascimento Santos, M.; De M. Magalhães, J.E.; Castro, L.S.E.P.W.; De Sousa Pinheiro, T.; Sabry, D.A.; Nobre, L.T.D.B.; Lima, J.P.M.S.; Baseia, I.G.; Leite, E.L. Effect of Glucans from Caripia montagnei Mushroom on TNBS-Induced Colitis. Int. J. Mol. Sci. 2014, 15, 2368-2385. https://doi.org/10.3390/ijms15022368
Da S. Nascimento Santos M, De M. Magalhães JE, Castro LSEPW, De Sousa Pinheiro T, Sabry DA, Nobre LTDB, Lima JPMS, Baseia IG, Leite EL. Effect of Glucans from Caripia montagnei Mushroom on TNBS-Induced Colitis. International Journal of Molecular Sciences. 2014; 15(2):2368-2385. https://doi.org/10.3390/ijms15022368
Chicago/Turabian StyleDa S. Nascimento Santos, Marilia, Joedyson Emmanuel De M. Magalhães, Luiza Sheyla Evenni P. Will Castro, Thuane De Sousa Pinheiro, Diego Araujo Sabry, Leonardo Thiago Duarte B. Nobre, João Paulo Matos Santos Lima, Iuri Goulart Baseia, and Edda Lisboa Leite. 2014. "Effect of Glucans from Caripia montagnei Mushroom on TNBS-Induced Colitis" International Journal of Molecular Sciences 15, no. 2: 2368-2385. https://doi.org/10.3390/ijms15022368