Osthole Suppresses the Migratory Ability of Human Glioblastoma Multiforme Cells via Inhibition of Focal Adhesion Kinase-Mediated Matrix Metalloproteinase-13 Expression
Abstract
:1. Introduction
2. Results
2.1. Osthole Inhibits the Proliferation of Human Glioma Cells
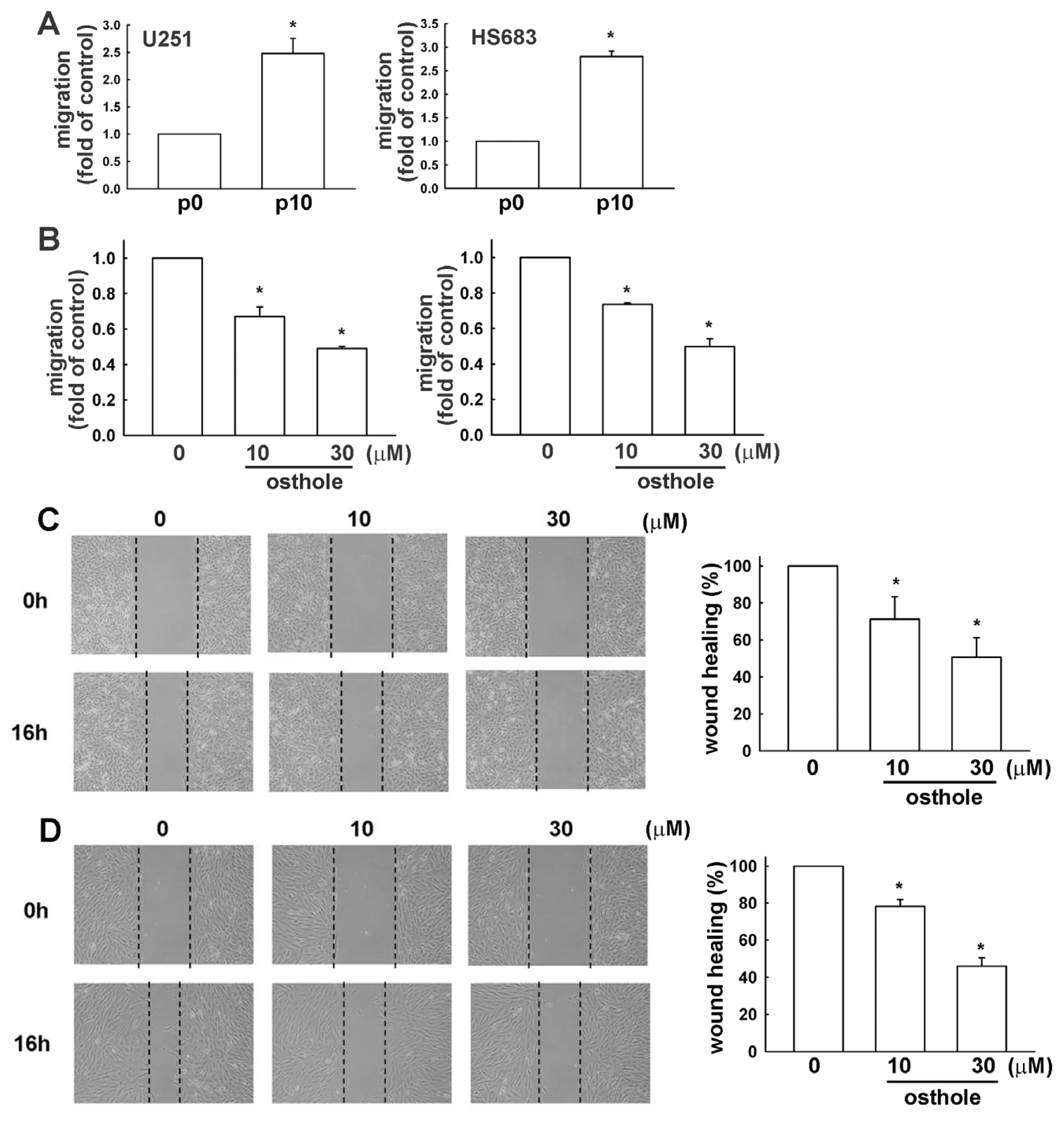
2.2. Osthole Inhibits Migration of Human Glioma Cells
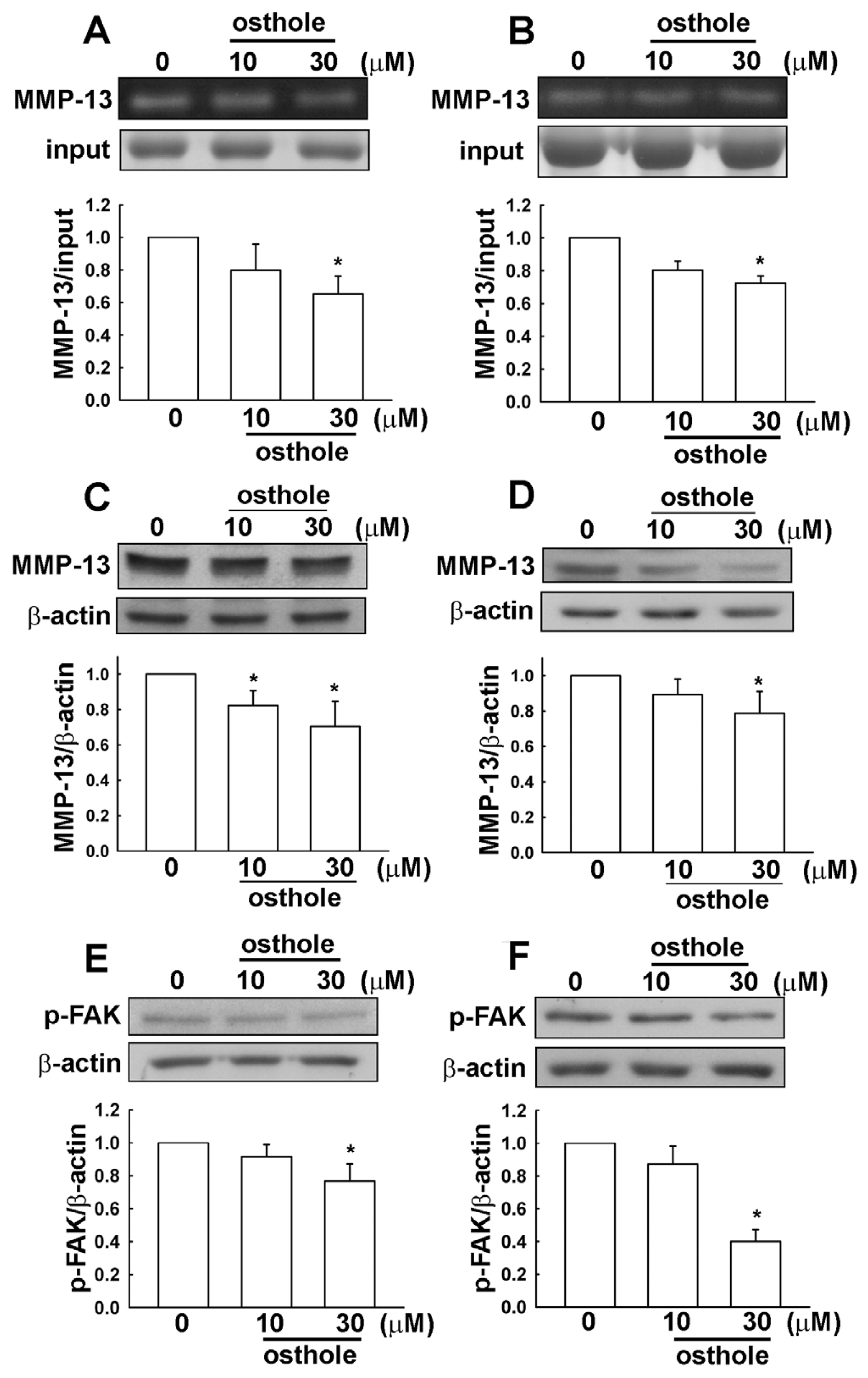
2.3. Osthole-Induced Inhibition of Human Glioma Cell Migration Involves MMP-13 and FAK Expression
2.4. Down-Regulation of Osthole in Migration-Prone Cells
2.5. The Osthole Effects on Migration-Prone Human Glioma Cells Involve a Modulation of MMP-13 and FAK Expression
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture
4.3. MTT Assay
4.4. Sulforhodamine B Assay (SRB)
4.5. Western Blot Analysis
4.6. Migration Assay
4.7. Wound-Healing Assay
4.8. Gelatin Zymography
4.9. Establishment of Migration-Prone Sublines
4.10. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsC.-F. Tsai, C.Y.-J. Wu and D.-Y. Lu designed the research and wrote the paper; W.-L. Yeh, J.-H. Chen, C. Lin, and S.-S. Huang performed experiments and analyzed results.
References
- Fidler, I.J. Critical factors in the biology of human cancer metastasis. Am. Surg. 1995, 61, 1065–1066. [Google Scholar]
- Kleihues, P.; Soylemezoglu, F.; Schauble, B.; Scheithauer, B.W.; Burger, P.C. Histopathology classification and grading of gliomas. Glia 1995, 15, 211–221. [Google Scholar]
- Griscelli, F.; Li, H.; Cheong, C.; Opolon, P.; Bennaceur-Griscelli, A.; Vassal, G.; Soria, J.; Soria, C.; Lu, H.; Perricaudet, M.; et al. Combined effects of radiotherapy and angiostatin gene therapy in glioma tumor model. Proc. Natl. Acad. Sci. USA 2000, 97, 6698–6703. [Google Scholar]
- Amberger, V.R.; Hensel, T.; Ogata, N.; Schwab, M.E. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998, 58, 149–158. [Google Scholar]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar]
- Koul, D.; Shen, R.; Bergh, S.; Sheng, X.; Shishodia, S.; Lafortune, T.A.; Lu, Y.; de Groot, J.F.; Mills, G.B.; Yung, W.K. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol. Cancer Ther. 2006, 5, 637–644. [Google Scholar]
- Boiardi, A.; Silvani, A.; Milanesi, I.; Botturi, M.; Broggi, G. Primary glial tumor patients treated by combining cis-platin and etoposide. J. Neurooncol. 1991, 11, 165–170. [Google Scholar]
- Kondo, Y.; Hollingsworth, E.F.; Kondo, S. Molecular targeting for malignant gliomas. Int. J. Oncol. 2004, 24, 1101–1109. [Google Scholar]
- Tang, C.H. Molecular mechanisms of chondrosarcoma metastasis. Biomedicine 2012, 2, 92–98. [Google Scholar]
- Ala-aho, R.; Kahari, V.M. Collagenases in cancer. Biochimie 2005, 87, 273–286. [Google Scholar]
- Hsu, S.C.; Lin, J.H.; Weng, S.W.; Chueh, F.S.; Yu, C.C.; Lu, K.W.; Wood, W.G.; Chung, J.G. Crude extract of Rheum palmatum inhibits migration and invasion of U-2 OS human osteosarcoma cells by suppression of matrix metalloproteinase-2 and -9. Biomedicine 2013, 3, 120–129. [Google Scholar]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar]
- Yang, B.; Gao, J.; Rao, Z.; Shen, Q. Clinicopathological significance and prognostic value of MMP-13 expression in colorectal cancer. Scand. J. Clin. Lab. Investig. 2012, 72, 501–505. [Google Scholar]
- Zhang, B.; Cao, X.; Liu, Y.; Cao, W.; Zhang, F.; Zhang, S.; Li, H.; Ning, L.; Fu, L.; Niu, Y.; et al. Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer 2008, 8, 83. [Google Scholar]
- Fukuda, H.; Mochizuki, S.; Abe, H.; Okano, H.J.; Hara-Miyauchi, C.; Okano, H.; Yamaguchi, N.; Nakayama, M.; D’Armiento, J.; Okada, Y. Host-derived MMP-13 exhibits a protective role in lung metastasis of melanoma cells by local endostatin production. Br. J. Cancer 2011, 105, 1615–1624. [Google Scholar]
- Lu, D.Y.; Yu, W.H.; Yeh, W.L.; Tang, C.H.; Leung, Y.M.; Wong, K.L.; Chen, Y.F.; Lai, C.H.; Fu, W.M. Hypoxia-induced matrix metalloproteinase-13 expression in astrocytes enhances permeability of brain endothelial cells. J. Cell. Physiol. 2009, 220, 163–173. [Google Scholar]
- Chuang, J.Y.; Tsai, C.F.; Chang, S.W.; Chiang, I.P.; Huang, S.M.; Lin, H.Y.; Yeh, W.L.; Lu, D.Y. Glial cell line-derived neurotrophic factor induces cell migration in human oral squamous cell carcinoma. Oral Oncol. 2013, 49, 1103–1112. [Google Scholar]
- Lu, D.Y.; Leung, Y.M.; Cheung, C.W.; Chen, Y.R.; Wong, K.L. Glial cell line-derived neurotrophic factor induces cell migration and matrix metalloproteinase-13 expression in glioma cells. Biochem. Pharmacol. 2010, 80, 1201–1209. [Google Scholar]
- Yeh, W.L.; Lu, D.Y.; Lee, M.J.; Fu, W.M. Leptin induces migration and invasion of glioma cells through MMP-13 production. Glia 2009, 57, 454–464. [Google Scholar]
- Wang, J.; Li, Y.; Li, C.; Yu, K.; Wang, Q. Increased expression of matrix metalloproteinase-13 in glioma is associated with poor overall survival of patients. Med. Oncol. 2012, 29, 2432–2437. [Google Scholar]
- Inoue, A.; Takahashi, H.; Harada, H.; Kohno, S.; Ohue, S.; Kobayashi, K.; Yano, H.; Tanaka, J.; Ohnishi, T. Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int. J. Oncol. 2010, 37, 1121–1131. [Google Scholar]
- Lu, D.Y.; Chang, C.S.; Yeh, W.L.; Tang, C.H.; Cheung, C.W.; Leung, Y.M.; Liu, J.F.; Wong, K.L. The novel phloroglucinol derivative BFP induces apoptosis of glioma cancer through reactive oxygen species and endoplasmic reticulum stress pathways. Phytomedicine 2012, 19, 1093–1100. [Google Scholar]
- Tsai, C.F.; Yeh, W.L.; Huang, S.M.; Tan, T.W.; Lu, D.Y. Wogonin induces reactive oxygen species production and cell apoptosis in human glioma cancer cells. Int. J. Mol. Sci. 2012, 13, 9877–9892. [Google Scholar]
- Huang, S.M.; Cheung, C.W.; Chang, C.S.; Tang, C.H.; Liu, J.F.; Lin, Y.H.; Chen, J.H.; Ko, S.H.; Wong, K.L.; Lu, D.Y. Phloroglucinol derivative MCPP induces cell apoptosis in human colon cancer. J. Cell. Biochem. 2011, 112, 643–652. [Google Scholar]
- Dinicola, S.; Pasqualato, A.; Cucina, A.; Coluccia, P.; Ferranti, F.; Canipari, R.; Catizone, A.; Proietti, S.; D’Anselmi, F.; Ricci, G.; et al. Grape seed extract suppresses MDA-MB231 breast cancer cell migration and invasion. Eur. J. Nutr. 2014, 53, 421–431. [Google Scholar]
- Yin, M.C. Development of natural antitumor agents. Biomedicine 2013, 3, 105. [Google Scholar]
- Sadraei, H.; Shokoohinia, Y.; Sajjadi, S.E.; Mozafari, M. Antispasmodic effects of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Res. Pharm. Sci. 2013, 8, 137–144. [Google Scholar]
- Liu, J.; Zhang, W.; Zhou, L.; Wang, X.; Lian, Q. Anti-inflammatory effect and mechanism of osthole in rats. Zhong Yao Cai 2005, 28, 1002–1006. [Google Scholar]
- Zhang, Q.; Qin, L.; He, W.; van Puyvelde, L.; Maes, D.; Adams, A.; Zheng, H.; de Kimpe, N. Coumarins from Cnidium monnieri and their antiosteoporotic activity. Planta. Med. 2007, 73, 13–19. [Google Scholar]
- Luszczki, J.J.; Andres-Mach, M.; Cisowski, W.; Mazol, I.; Glowniak, K.; Czuczwar, S.J. Osthole suppresses seizures in the mouse maximal electroshock seizure model. Eur. J. Pharmacol. 2009, 607, 107–109. [Google Scholar]
- Xu, X.; Zhang, Y.; Qu, D.; Jiang, T.; Li, S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011, 30. [Google Scholar] [CrossRef]
- Riviere, C.; Goossens, L.; Pommery, N.; Fourneau, C.; Delelis, A.; Henichart, J.P. Antiproliferative effects of isopentenylated coumarins isolated from Phellolophium madagascariense Baker. Nat. Prod. Res. 2006, 20, 909–916. [Google Scholar]
- Chou, S.Y.; Hsu, C.S.; Wang, K.T.; Wang, M.C.; Wang, C.C. Antitumor effects of Osthol from Cnidium monnieri: An in vitro and in vivo study. Phytother. Res. 2007, 21, 226–230. [Google Scholar]
- Van Meir, E.G.; Hadjipanayis, C.G.; Norden, A.D.; Shu, H.K.; Wen, P.Y.; Olson, J.J. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J. Clin. 2010, 60, 166–193. [Google Scholar]
- Ohgaki, H.; Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005, 109, 93–108. [Google Scholar]
- Lu, D.Y.; Yeh, W.L.; Huang, S.M.; Tang, C.H.; Lin, H.Y.; Chou, S.J. Osteopontin increases heme oxygenase-1 expression and subsequently induces cell migration and invasion in glioma cells. Neuro Oncol. 2012, 14, 1367–1378. [Google Scholar]
- Li, F.; Gong, Q.; Wang, L.; Shi, J. Osthole attenuates focal inflammatory reaction following permanent middle cerebral artery occlusion in rats. Biol. Pharm. Bull. 2012, 35, 1686–1690. [Google Scholar]
- He, Y.; Qu, S.; Wang, J.; He, X.; Lin, W.; Zhen, H.; Zhang, X. Neuroprotective effects of osthole pretreatment against traumatic brain injury in rats. Brain Res. 2012, 1433, 127–136. [Google Scholar]
- Mao, X.; Yin, W.; Liu, M.; Ye, M.; Liu, P.; Liu, J.; Lian, Q.; Xu, S.; Pi, R. Osthole a natural coumarin improves neurobehavioral functions and reduces infarct volume and matrix metalloproteinase-9 activity after transient focal cerebral ischemia in rats. Brain Res. 2011, 1385, 275–280. [Google Scholar]
- Chao, X.; Zhou, J.; Chen, T.; Liu, W.; Dong, W.; Qu, Y.; Jiang, X.; Ji, X.; Zhen, H.; Fei, Z. Neuroprotective effect of osthole against acute ischemic stroke on middle cerebral ischemia occlusion in rats. Brain Res. 2010, 1363, 206–211. [Google Scholar]
- Liotta, L.A.; Tryggvason, K.; Garbisa, S.; Hart, I.; Foltz, C.M.; Shafie, S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980, 284, 67–68. [Google Scholar]
- Parsons, S.L.; Watson, S.A.; Brown, P.D.; Collins, H.M.; Steele, R.J. Matrix metalloproteinases. Br. J. Surg. 1997, 84, 160–166. [Google Scholar]
- Curran, S.; Murray, G.I. Matrix metalloproteinases: Molecular aspects of their roles in tumour invasion and metastasis. Eur. J. Cancer 2000, 36, 1621–1630. [Google Scholar]
- Parsons, J.T.; Martin, K.H.; Slack, J.K.; Taylor, J.M.; Weed, S.A. Focal adhesion kinase: A regulator of focal adhesion dynamics and cell movement. Oncogene 2000, 19, 5606–5613. [Google Scholar]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell. Biol. 2005, 6, 56–68. [Google Scholar]
- Maa, M.C.; Leu, T.H. Activation of toll-like receptors induces macrophage migration via the iNOS/Src/FAK pathway. Biomedicine 2011, 1, 11–15. [Google Scholar]
- Shi, Z.D.; Wang, H.; Tarbell, J.M. Heparan sulfate proteoglycans mediate interstitial flow mechanotransduction regulating MMP-13 expression and cell motility via FAK-ERK in 3D collagen. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Loeser, R.F.; Forsyth, C.B.; Samarel, A.M.; Im, H.J. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J. Biol. Chem. 2003, 278, 24577–24585. [Google Scholar]
- Kim, M.S.; Park, M.J.; Kim, S.J.; Lee, C.H.; Yoo, H.; Shin, S.H.; Song, E.S.; Lee, S.H. Emodin suppresses hyaluronic acid-induced MMP-9 secretion and invasion of glioma cells. Int. J. Oncol. 2005, 27, 839–846. [Google Scholar]
- Park, J.B.; Kwak, H.J.; Lee, S.H. Role of hyaluronan in glioma invasion. Cell Adh. Migr. 2008, 2, 202–207. [Google Scholar]
- Ding, L.; Sun, X.; You, Y.; Liu, N.; Fu, Z. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in human gliomas is associated with unfavorable overall survival. Transl. Res. 2010, 156, 45–52. [Google Scholar]
- Chen, J.H.; Huang, S.M.; Chen, C.C.; Tsai, C.F.; Yeh, W.L.; Chou, S.J.; Hsieh, W.T.; Lu, D.Y. Ghrelin induces cell migration through GHS-R CaMKII AMPK and NF-kappaB signaling pathway in glioma cells. J. Cell. Biochem. 2011, 112, 2931–2941. [Google Scholar]
- Lu, D.Y.; Chen, J.H.; Tan, T.W.; Huang, C.Y.; Yeh, W.L.; Hsu, H.C. Resistin protects against 6-hydroxydopamine-induced cell death in dopaminergic-like MES235 cells. J. Cell. Physiol. 2013, 228, 563–571. [Google Scholar]
- Lin, H.Y.; Yeh, W.L.; Huang, B.R.; Lin, C.; Lai, C.H.; Lin, H.; Lu, D.Y. Desipramine protects neuronal cell death and induces heme oxygenase-1 expression in Mes235 dopaminergic neurons. PLoS One 2012, 7, e50138. [Google Scholar]
- Chen, J.H.; Huang, S.M.; Tan, T.W.; Lin, H.Y.; Chen, P.Y.; Yeh, W.L.; Chou, S.C.; Tsai, C.F.; Wei, I.H.; Lu, D.Y. Berberine induces heme oxygenase-1 up-regulation through phosphatidylinositol 3-kinase/AKT and NF-E2-related factor-2 signaling pathway in astrocytes. Int. Immunopharmacol. 2012, 12, 94–100. [Google Scholar]
- Huang, S.M.; Chen, T.S.; Chiu, C.M.; Chang, L.K.; Liao, K.F.; Tan, H.M.; Yeh, W.L.; Chang, G.R.; Wang, M.Y.; Lu, D.Y. GDNF increases cell motility in human colon cancer through VEGF–VEGFR1 interaction. Endocr. Relat. Cancer 2014, 21, 73–84. [Google Scholar]
- Bizzarri, M.; Pasqualato, A.; Cucina, A.; Pasta, V. Physical forces and non linear dynamics mould fractal cell shape: Quantitative morphological parameters and cell phenotype. Histol. Histopathol. 2013, 28, 155–174. [Google Scholar]




© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, C.-F.; Yeh, W.-L.; Chen, J.-H.; Lin, C.; Huang, S.-S.; Lu, D.-Y. Osthole Suppresses the Migratory Ability of Human Glioblastoma Multiforme Cells via Inhibition of Focal Adhesion Kinase-Mediated Matrix Metalloproteinase-13 Expression. Int. J. Mol. Sci. 2014, 15, 3889-3903. https://doi.org/10.3390/ijms15033889
Tsai C-F, Yeh W-L, Chen J-H, Lin C, Huang S-S, Lu D-Y. Osthole Suppresses the Migratory Ability of Human Glioblastoma Multiforme Cells via Inhibition of Focal Adhesion Kinase-Mediated Matrix Metalloproteinase-13 Expression. International Journal of Molecular Sciences. 2014; 15(3):3889-3903. https://doi.org/10.3390/ijms15033889
Chicago/Turabian StyleTsai, Cheng-Fang, Wei-Lan Yeh, Jia-Hong Chen, Chingju Lin, Shiang-Suo Huang, and Dah-Yuu Lu. 2014. "Osthole Suppresses the Migratory Ability of Human Glioblastoma Multiforme Cells via Inhibition of Focal Adhesion Kinase-Mediated Matrix Metalloproteinase-13 Expression" International Journal of Molecular Sciences 15, no. 3: 3889-3903. https://doi.org/10.3390/ijms15033889



