The First Insight into the Metabolite Profiling of Grapes from Three Vitis vinifera L. Cultivars of Two Controlled Appellation (DOC) Regions
Abstract
:1. Introduction
2. Results and Discussion
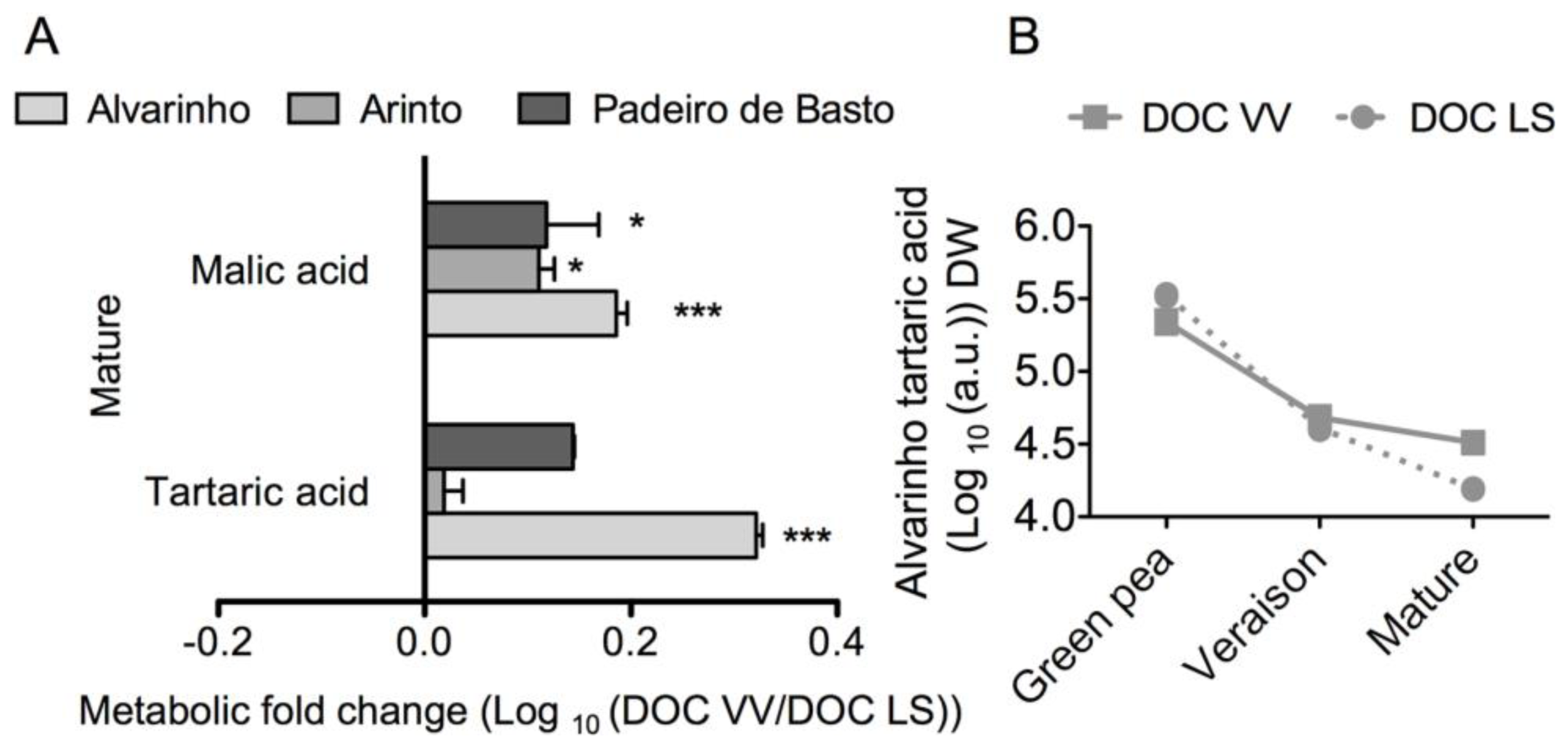
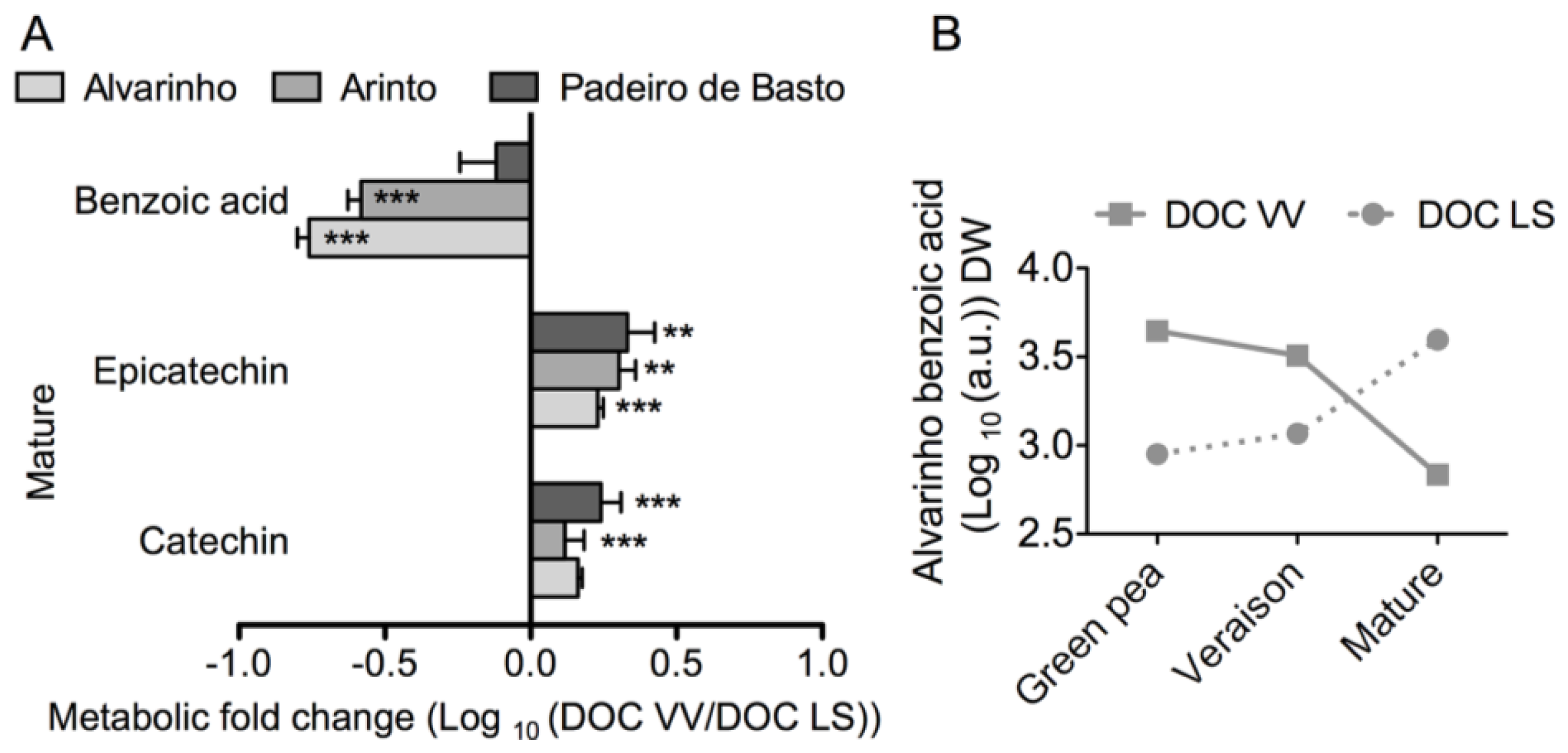
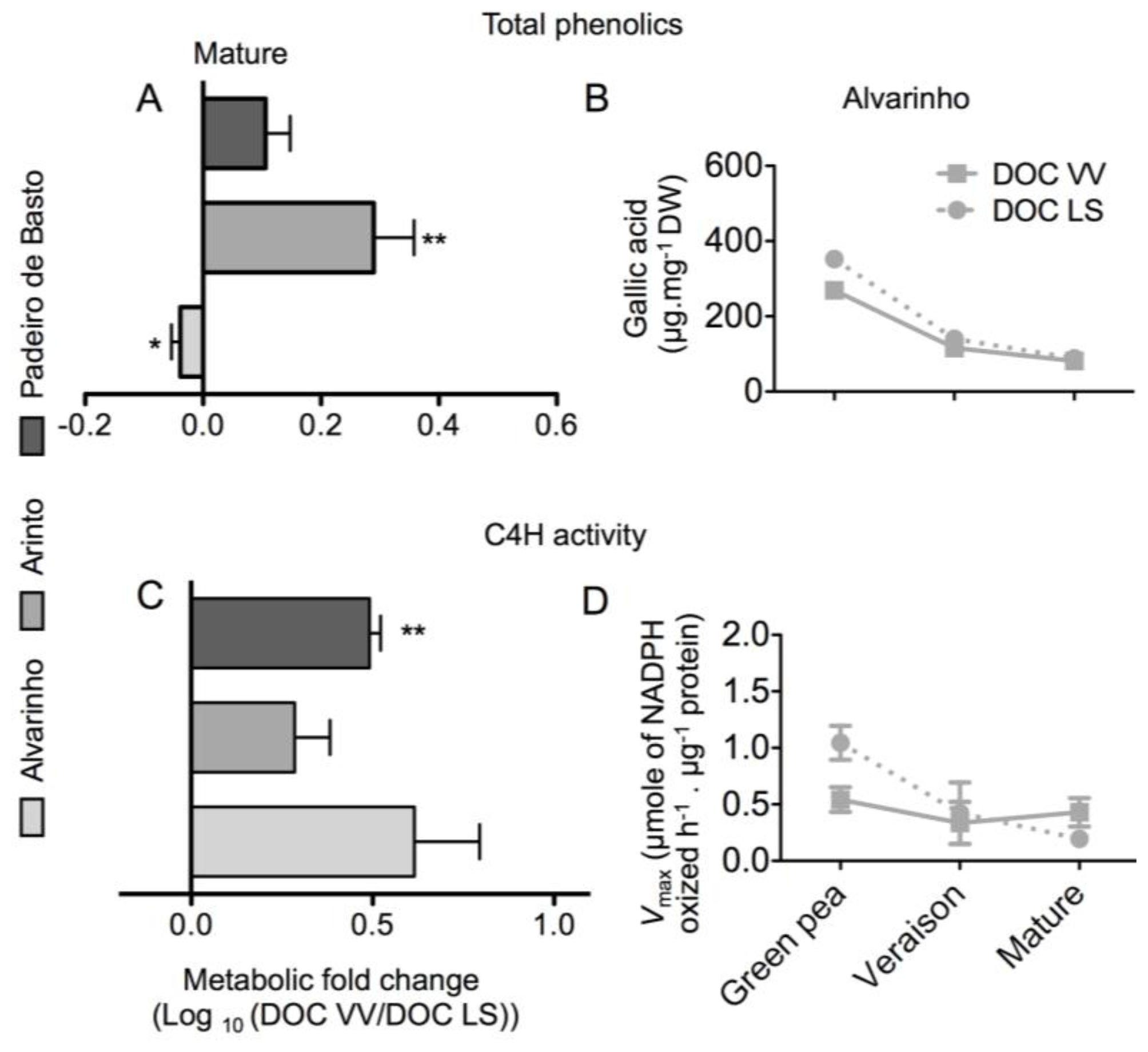
2.1. Results
2.2. Discussion
3. Experimental Section
3.1. Characterization of the Vineyard Regions and Sample Collection
3.2. Sample Preparation for Metabolome Analysis
3.3. Quantification of Free Amino Acids
3.4. Total Phenolic Content
3.5. Cinnamate 4-Hydroxylase (C4H) Activity
3.6. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-15-04237-s001.pdfAcknowledgments
Conflicts of Interest
- Author ContributionsA.T., E.-D.J. and H.G. raised the hypothesis underlying this work designed the experiments. A.T., V.M. and H.N. carried out the experiments. A.T. and V.M. performed data processing and statistical analysis and designed the figures and tables. A.T. and H.G. wrote the article. H.G. directed the study. All authors read and approved the manuscript.
References
- White, M.A.; Whalen, P.; Jones, G.V. Land and wine. Nat. Geosci. 2009, 2, 82–84. [Google Scholar]
- Chaves, M.M.; Santos, T.P.; Souza, C.R.; Ortuño, M.F.; Rodrigues, M.L.; Lopes, C.M.; Maroco, J.P.; Pereira, J.S. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol 2007, 150, 237–252. [Google Scholar]
- Costa, J.M.; Ortuño, M.F.; Chaves, M.M. Deficit irrigation as strategy to save water: Physiology and potential application to horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar]
- Coombe, B.G.; Bovio, M.; Schneider, A. Solute accumulation by grape pericarp cells V Relationship to berry size and the effects of defoliation. J. Exp. Bot. 1987, 38, 1789–1798. [Google Scholar]
- Agasse, A.; Vignault, C.; Kappel, C.; Gerós, H.; Delrot, S. Sugar Transport and Sensing in Grape. In Grapevine Molecular Physiology and Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer Nertherlands: Dordrecht, The Nertherlands, 2009; pp. 105–139. [Google Scholar]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- Kliewer, W.M. Sugars and organic acids of Vitis vinifera. Plant Physiol. 1966, 41, 923–931. [Google Scholar]
- Huang, Z.; Ough, C.S. Amino acid profiles of commercial grape juices and wines. Am. J. Enol. Vitic. 1991, 42, 261–267. [Google Scholar]
- Hernández-Orte, P.; Guitart, A.; Cacho, J. Changes in the concentration of amino acids during the ripening of Vitis vinifera Tempranillo variety from the Denomination d’Origine Somontano (Spain). Am. J. Enol. Vitic 1999, 50, 144–154. [Google Scholar]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant. Cell Environ 2007, 30, 1381–1399. [Google Scholar]
- Castellarin, S.D.; Bavaresco, L.; Falginella, L.; Gonçalves, M.I.V.Z.; di Gaspero, G.; Gerós, H.; Chaves, M.-M.; Delrot, S. Phenolics in Grape Berry and Key Antioxidants. In The Biochemistry of the Grape Berry; Bentham Science: Bussum, The Netherlands, 2012; pp. 89–110. [Google Scholar]
- Darriet, P.; Thibon, C.; Dubourdieu, D.; Gerós, H.; Chaves, M.-M.; Delrot, S. Aroma and Aroma Precursors in Grape Berry. In The Biochemistry of the Grape Berry; Bentham Science: Bussum, The Netherlands, 2012; pp. 111–136. [Google Scholar]
- Krishnan, P.; Kruger, N.J.; Ratcliffe, R.G. Metabolite fingerprinting and profiling in plants using NMR. J. Exp. Bot. 2005, 56, 255–265. [Google Scholar]
- Pereira, G.; Gaudillere, J.P.; Vanleeuwen, C.; Hilbert, G.; Lavialle, O.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. 1H NMR and chemometrics to characterize mature grape berries in four wine-growing areas in Bordeaux-France. J. Agric. Food Chem. 2005, 53, 6382–6389. [Google Scholar]
- Pereira, G.E.; Gaudillere, J.P.; Pieri, P.; Hilbert, G.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. Microclimate influence on mineral and metabolic profiles of grape berries. J. Agric. Food Chem. 2006, 54, 6765–6775. [Google Scholar]
- Son, H.-S.; Hwang, G.-S.; Ki, M.K.; Ahn, H.-J.; Park, W.-M.; van den Berg, F.; Hong, Y.-S.; Lee, C.-H. Metabolomic studies on geographical grapes and their wines using 1H NMR analysis coupled with multivariate statistics. J. Agric. Food Chem 2009, 57, 1481–1490. [Google Scholar]
- Ali, K.; Maltese, F.; Fortes, A.M.; Pais, M.S.; Choi, Y.H.; Verpoorte, R. Monitoring biochemical changes during grape berry development in Portuguese cultivars by NMR spectroscopy. Food Chem 2011, 124, 1760–1769. [Google Scholar]
- Toffali, K.; Zamboni, A.; Anesi, A.; Stocchero, M.; Pezzotti, M.; Levi, M.; Guzzo, F. Novel aspects of grape berry ripening and post-harvest withering revealed by untargeted LC-ESI-MS metabolomics analysis. Metabolomics 2011, 7, 424–436. [Google Scholar]
- Liang, Z.; Owens, C.L.; Zhong, G.Y.; Cheng, L. Polyphenolic profiles detected in the ripe berries of Vitis vinifera germplasm. Food Chem. 2011, 129, 940–950. [Google Scholar]
- Hrazdina, G.; Parsons, G.F.; Mattick, L.R. Physiological and biochemical events during development and maturation of grape berries. Am. J. Enol. Vitic 1984, 35, 220–227. [Google Scholar]
- Chen, J.Y.; Wen, P.F.; Kong, W.F.; Pan, Q.H.; Wan, S.B.; Huang, W.D. Changes and subcellular localizations of the enzymes involved in phenylpropanoid metabolism during grape berry development. J. Plant Physiol. 2006, 163, 115–127. [Google Scholar]
- Coombe, B.G. Research on development and ripening of the grape berry. Am. J. Enol. Vitic. 1992, 43, 101–110. [Google Scholar]
- Jackson, D.; Lombard, P. Environmental and management practices affecting grape composition and wine quality—A review. Am. J. Enol. Vitic 1993, 44, 409–430. [Google Scholar]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic 2005, 56, 170–181. [Google Scholar]
- Clingeleffer, P.R. Plant management research: Status and what it can offer to address challenges and limitations. Aust. J. Grape Wine Res 2010, 16, 25–32. [Google Scholar]
- Shiraishi, M. Comparison in changes in sugars organic acids and amino acids during berry ripening of sucrose- and hexose-accumulating grape cultivars. J. Jpn. Soc. Hortic. Sci 2000, 69, 141–148. [Google Scholar]
- Liu, H.F.; Wu, B.H.; Fan, P.G.; Xu, H.Y.; Li, S.H. Inheritance of sugars and acids in beries of grape (Vitis vinifera L). Euphytica 2006, 153, 99–107. [Google Scholar]
- Shiraishi, M.; Fujishima, H.; Chijiwa, H. Evaluation of table grape genetic resources for sugar organic acid and amino acid composition of berries. Euphytica 2010, 174, 1–13. [Google Scholar]
- Keller, M.; Mills, L.J.; Wample, R.L.; Spayd, S.E. Bunch thinning effects on three deficit-irrigated Vitis vinifera cultivars. Am. J. Enol. Vitic 2005, 56, 91–103. [Google Scholar]
- Li, Z.; Palmer, W.M.; Martin, A.P.; Wang, R.; Rainsford, F.; Jin, Y.; John, W.; Patrick, J.W.; Yang, Y.; Ruan, Y.L. High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into and heat tolerance of young fruit. J. Exp. Bot. 2012, 63, 1155–1166. [Google Scholar]
- Gaudillère, J.P.; van-Leeuwen, C.; Ollat, N. Carbon isotope composition of sugars in grapevine an integrated indicator of vineyard water status. J. Exp. Bot 2002, 53, 757–763. [Google Scholar]
- Castellarin, S.D.; Matthews, M.A.; di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 2009, 10, 212. [Google Scholar]
- Ruffner, H.P. Metabolism of tartaric and malic acids in Vitis: A review—Part B. Vitis 1982, 21, 346–358. [Google Scholar]
- Ruffner, H.P. Metabolism of tartaric and malic acids in Vitis: A review—Part A. Vitis 1982, 21, 247–259. [Google Scholar]
- Spayd, S.; Tarara, J. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv Merlot berries. Am. J. Enol. Vitic 2002, 3, 171–182. [Google Scholar]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv Agiorgitiko (Vitis vinifera L) Effects on wine phenolic and aroma components. J. Agric. Food Chem 2006, 54, 5077–5086. [Google Scholar]
- Salazar-Parra, C.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Morales, F. Effects of climate change scenarios on Tempranillo grapevine (Vitis vinifera L) ripening: Response to a combination of elevated CO2 and temperature and moderate drought. Plant Soil 2010, 337, 179–191. [Google Scholar]
- Kliewer, W.M.; Lider, L.A. Influence of leafroll virus on composition of Burger fruits. Am. J. Enol. Vitic. 1976, 27, 118–124. [Google Scholar]
- Stines, A.P.; Grubb, J.; Gockowiak, H.; Henschke, P.A.; Hoj, P.; van Heeswijck, R. Proline and arginine accumulation in developing berries of Vitis Vinifera L in Australian vineyards: Influence of vine cultivar berry maturity and tissue type. Aust. J. Grape Wine Res 2000, 6, 150–158. [Google Scholar]
- Bell, S.; Henschke, P.A. Implication of nitrogen nutrition for grapes fermentation and wine. Aust. J. Grape Wine Res 2005, 11, 242–295. [Google Scholar]
- Kliewer, W.M. Free amino acids and other nitrogenous fractions in wine grapes. J. Food Sci 1970, 35, 17–21. [Google Scholar]
- Huang, Z.; Ough, C.S. Effect of vineyard locations varieties and rootstocks on the juice amino acid composition of several cultivars. Am. J. Enol. Vitic. 1989, 40, 135–139. [Google Scholar]
- Asensio, M.L.; Valdés, E.; Cabello, F. Characterisation of some Spanish white grapevine cultivars by morphology and amino acid analysis. Sci. Hortic 2002, 93, 289–299. [Google Scholar]
- Coruzzi, G.; Last, R. Amino Acids. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 358–410. [Google Scholar]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism regulation and signalling. J. Exp. Bot 2007, 58, 2339–2358. [Google Scholar]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between varietal amino acid profile of grapes and wine aromatic composition Experiments with model solutions and chemometric study. J. Agric. Food Chem 2002, 50, 2891–2899. [Google Scholar]
- Peña-Neira, A.; Hernández, T.; García-Vallejo, C.; Estrella, I.; Suarez, J.A. A survey of phenolic compounds in Spanish wines from different geographical origins. Eur. Food Res. Technol. 2000, 210, 445–448. [Google Scholar]
- Pozo-Bayón, M.A.; Hernández, M.T.; Martín-Alvarez, P.J.; Polo, M.C. Study of low molecular weight phenolic compounds during the aging of sparkling wines manufactured with red and white grape varieties. J. Agric. Food Chem 2003, 51, 2089–2095. [Google Scholar]
- Monagas, M.; Suárez, R.; Gómez-Cordovés, C.; Bartolomé, B. Simultaneous determination of nonanthocyanin phenolic compounds in red wines by HPLC-DAD/ESI-MS. Am. J. Enol. Vitic 2005, 56, 139–147. [Google Scholar]
- Haselgrove, L.; Botting, D.; Heeswijck, R.; Hog, P.B.; Dry, P.R.; Ford, C.; Iland, P. Canopy microclimate and berry composition: The effect of bunch exposure on the phenolic composition of Vitis vinifera L cv Shiraz grape berries. Aust. J. Grape Wine Res 2000, 6, 141–149. [Google Scholar]
- Food and Agriculture Organization (FAO), World Reference Base for Soil Resources 2006. World Soil Resources Reports, No. 103; FAO: Rome, Italy, 2006.
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.R.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-complian studies. Plant J. 2008, 53, 691–704. [Google Scholar]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.R. Setup and annotation ofmetabolomic experiments by integrating biological and mass spectrometric metadata. Data Integrat. Life Sci. Proc 2005, 3615, 224–239. [Google Scholar]
- Waterhouse, A. Determination of total phenolics. Curr. Protoc. Food Anal. Chem 2002, 1–8. [Google Scholar]
- Stoop, J.M.H.; Pharr, D.M. Effect of different carbon sources on relative growth rate internal carbohydrates and mannitol 1-oxidoreductase activity in celery suspension cultures. Plant Physiol 1993, 103, 1001–1008. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Santo, S.D.; Tornielli, G.B.; Zenoni, S.; Fasoli, M.; Farina, L.; Anesi, A.; Guzzo, F.; Delledonne, M.; Pezzotti, M. The plasticity of the grapevine berry transcriptome. Genome Biol. 2013, 14, R54. [Google Scholar]





| Amino acids μg/g DW | Alvarinho | Arinto | Padeiro de Basto | |||
|---|---|---|---|---|---|---|
| DOC VV | DOC LS | DOC VV | DOC LS | DOC VV | DOC LS | |
| Arg | 1,237 | 1,034 | 990 | 579 | 1,047 | 324 |
| His | 72 | 70 | 59 | 43 | 139 | 70 |
| Lys | 21 | 20 | 19 | 16 | 20 | 16 |
| Asp | 187 | 74 | 288 | 180 | 163 | 47 |
| Glu | 502 | 95 | 188 | 89 | 452 | 80 |
| Gly | 18 | 12 | 19 | 10 | 10 | 12 |
| Ser | 254 | 161 | 191 | 98 | 97 | 64 |
| Tyr | 102 | 36 | 65 | 49 | 58 | 33 |
| Cys | ||||||
| Gln | 172 | 128 | 319 | 116 | 258 | 105 |
| Thr | 328 | 274 | 321 | 177 | 80 | 43 |
| Asn | 30 | 91 | 43 | 20 | 76 | 42 |
| Phe | 136 | 116 | 68 | 74 | 53 | 31 |
| Leu | 93 | 74 | 49 | 32 | 67 | 26 |
| Trp | 122 | 90 | 147 | 128 | 132 | 56 |
| Pro | 504 | 365 | 394 | 403 | 300 | 230 |
| Ile | 64 | 49 | 45 | 29 | 21 | 15 |
| Met | 15 | 15 | 15 | 15 | 15 | 15 |
| Val | 124 | 93 | 62 | 44 | 36 | 31 |
| Ala | 434 | 259 | 487 | 238 | 326 | 166 |
| Total | 4,415 | 3,056 | 3,769 | 2,340 | 3,350 | 1,406 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Teixeira, A.; Martins, V.; Noronha, H.; Eiras-Dias, J.; Gerós, H. The First Insight into the Metabolite Profiling of Grapes from Three Vitis vinifera L. Cultivars of Two Controlled Appellation (DOC) Regions. Int. J. Mol. Sci. 2014, 15, 4237-4254. https://doi.org/10.3390/ijms15034237
Teixeira A, Martins V, Noronha H, Eiras-Dias J, Gerós H. The First Insight into the Metabolite Profiling of Grapes from Three Vitis vinifera L. Cultivars of Two Controlled Appellation (DOC) Regions. International Journal of Molecular Sciences. 2014; 15(3):4237-4254. https://doi.org/10.3390/ijms15034237
Chicago/Turabian StyleTeixeira, António, Viviana Martins, Henrique Noronha, José Eiras-Dias, and Hernâni Gerós. 2014. "The First Insight into the Metabolite Profiling of Grapes from Three Vitis vinifera L. Cultivars of Two Controlled Appellation (DOC) Regions" International Journal of Molecular Sciences 15, no. 3: 4237-4254. https://doi.org/10.3390/ijms15034237





