No Stress! Relax! Mechanisms Governing Growth and Shape in Plant Cells
Abstract
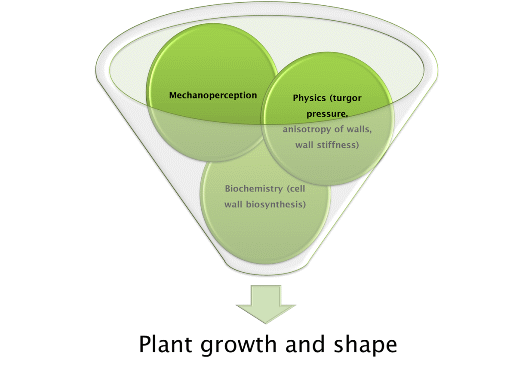
:1. Introduction
2. The Irreversible Expansion of Plant Cells
3. Factors Affecting Plant Cell Growth
4. A Special Mechanism of Global Growth: The Case of Bast Fibres
5. Differential Growth to Generate Complex Geometries
6. Tip Growth
7. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Geitmann, A.; Ortega, J.K. Mechanics and modeling of plant cell growth. Trends Plant Sci. 2009, 1, 467–478. [Google Scholar]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar]
- Humphrey, T.V.; Bonetta, D.T.; Goring, D.R. Sentinels at the wall: Cell wall receptors and sensors. New Phytol. 2007, 176, 7–21. [Google Scholar]
- Hamann, T.; Bennett, M.; Mansfield, J.; Somerville, C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. 2009, 57, 1015–1026. [Google Scholar]
- Seifert, G.J.; Blaukopf, C. Irritable walls: The plant extracellular matrix and signaling. Plant Physiol. 2010, 153, 467–478. [Google Scholar]
- Hamann, T.; Denness, L. Cell wall integrity maintenance in plants: Lessons to be learned from yeast? Plant Signal. Behav. 2011, 6, 1706–1709. [Google Scholar]
- Blaukopf, C.; Krol, M.Z.; Seifert, G.J. New insights into the control of cell growth. Methods Mol. Biol. 2011, 715, 221–244. [Google Scholar]
- Hamann, T. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 2012, 3, 77. [Google Scholar]
- Kroeger, J.H.; Zerzour, R.; Geitmann, A. Regulator or driving force? The role of turgor pressure in oscillatory plant cell growth. PLoS One 2011, 6, e18549. [Google Scholar]
- Schopfer, P. Biomechanics of plant growth. Am. J. Bot. 2006, 93, 1415–1425. [Google Scholar]
- Lockhart, J.A. An analysis of irreversible plant cell growth. J. Theor. Biol. 1965, 8, 264–275. [Google Scholar]
- Telewski, F.W. A unified hypothesis of mechanoperception in plants. Am. J. Bot. 2006, 93, 1466–1476. [Google Scholar]
- Schopfer, P. Is the loss of stability theory a realistic concept for stress relaxation-mediated cell wall expansion during plant growth? Plant Physiol. 2008, 147, 935–936; author reply 936–938. [Google Scholar]
- Zonia, L.; Munnik, T. Life under pressure: Hydrostatic pressure in cell growth and function. Trends Plant Sci. 2007, 12, 90–97. [Google Scholar]
- Wei, C.; Lintilhac, P.M. Loss of stability—A new model for stress relaxation in plant cell walls. J. Theor. Biol. 2003, 224, 305–312. [Google Scholar]
- Wei, C.; Lintilhac, L.S.; Lintilhac, P.M. Loss of stability pH and the anisotropic extensibility of Chara cell walls. Planta 2006, 223, 1058–1067. [Google Scholar]
- Wei, C.; Lintilhac, P.M. Loss of stability: A new look at the physics of cell wall behavior during plant cell growth. Plant Physiol. 2007, 145, 763–772. [Google Scholar]
- Geitmann, A. Mechanical modelling and structural analysis of the primary plant cell wall. Curr. Opin. Plant Biol. 2010, 13, 693–699. [Google Scholar]
- Hamant, O.; Heisler, M.G.; Jönsson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M.; et al. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1655. [Google Scholar]
- Snegireva, A.V.; Ageeva, M.V.; Amenitskii, S.I.; Chernova, T.E.; Ebskamp, M.; Gorshkova, T.A. Intrusive growth of sclerenchyma fibers. Russ. J. Plant Physiol. 2010, 57, 342–355. [Google Scholar]
- Gorshkova, T.A.; Sal’nikov, V.V.; Chemikosova, S.B.; Ageeva, M.V.; Pavlencheva, N.V. The snap point: A transition point in Linum usitatissimum bast fiber development. Ind. Crop. Prod. 2003, 18, 213–221. [Google Scholar]
- Cai, G.; Faleri, C.; del Casino, C.; Emons, A.M.; Cresti, M. Distribution of callose synthase cellulose synthase and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules. Plant Physiol. 2011, 155, 1169–1190. [Google Scholar]
- Cai, G. How do microtubules affect deposition of cell wall polysaccharides in the pollen tube? Plant Signal. Behav. 2011, 6, 732–735. [Google Scholar]
- Zhang, C.; Halsey, L.; Szymanski, D.B. The development and geometry of shape change in Arabidopsis thaliana cotyledon pavement cells. BMC Plant Biol. 2011, 11, 27. [Google Scholar]
- Lunn, D.; Gaddipati, S.R.; Tucker, G.A.; Lycett, G.W. Null mutants of individual RABA genes impact the proportion of different cell wall components in stem tissue of Arabidopsis thaliana. PLoS One 2013, 8, e75724. [Google Scholar]
- Li, S.; Chen, M.; Yu, D.; Ren, S.; Sun, S.; Liu, L.; Ketelaar, T.; Emons, A.M.; Liu, C.M. EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell 2013, 25, 1774–1786. [Google Scholar]
- Hao, H.; Chen, T.; Fan, L.; Li, R.; Wang, X. 2 6-dichlorobenzonitrile causes multiple effects on pollen tube growth beyond altering cellulose synthesis in Pinus bungeana Zucc. PLoS One 2013, 8, e76660. [Google Scholar]
- Peng, L.; Zhang, L.; Cheng, X.; Fan, L.S.; Hao, H.Q. Disruption of cellulose synthesis by 26-dichlorobenzonitrile affects the structure of the cytoskeleton and cell wall construction in Arabidopsis. Plant Biol. (Stuttg.) 2013, 15, 405–414. [Google Scholar]
- Mathur, J.; Hülskamp, M. Microtubules and microfilaments in cell morphogenesis in higher plants. Curr. Biol. 2002, 12, R669–R676. [Google Scholar]
- Wasteneys, G.O.; Galway, M.E. Remodeling the cytoskeleton for growth and form: An overview with some new views. Annu. Rev. Plant Biol. 2003, 54, 691–722. [Google Scholar]
- Paredez, A.R.; Somerville, C.R.; Ehrhardt, D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006, 312, 1491–1495. [Google Scholar]
- Bringmann, M.; Li, E.; Sampathkumar, A.; Kocabek, T.; Hauser, M.T.; Persson, S. POM-POM2/cellulose synthase interacting1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. Plant Cell 2012, 24, 163–177. [Google Scholar]
- Smith, L.G. Cytoskeletal control of plant cell shape: Getting the fine points. Curr. Opin. Plant Biol. 2003, 6, 63–73. [Google Scholar]
- Thitamadee, S.; Tuchihara, K.; Hashimoto, T. Microtubule basis for left-handed helical growth in Arabidopsis. Nature 2002, 417, 193–196. [Google Scholar]
- Rajangam, A.S.; Kumar, M.; Aspeborg, H.; Guerriero, G.; Arvestad, L.; Pansri, P.; Brown, C.J.; Hober, S.; Blomqvist, K.; Divne, C.; et al. MAP20 a microtubule-associated protein in the secondary cell walls of hybrid aspen is a target of the cellulose synthesis inhibitor 26-dichlorobenzonitrile. Plant Physiol. 2008, 148, 1283–1294. [Google Scholar]
- Mathur, J. Local interactions shape plant cells. Curr. Opin. Cell Biol. 2006, 18, 40–46. [Google Scholar]
- Mayer, U.; Herzog, U.; Berger, F.; Inzé, D.; Jürgens, G. Mutations in the pilz group genes disrupt the microtubule cytoskeleton and uncouple cell cycle progression from cell division in Arabidopsis embryo and endosperm. Eur. J. Cell Biol. 1999, 78, 100–108. [Google Scholar]
- Steinborn, K.; Maulbetsch, C.; Priester, B.; Trautmann, S.; Pacher, T.; Geiges, B.; Küttner, F.; Lepiniec, L.; Stierhof, Y.D.; Schwarz, H.; et al. The Arabidopsis PILZ group genes encode tubulin-folding cofactor orthologs required for cell division but not cell growth. Genes Dev. 2002, 16, 959–971. [Google Scholar]
- Perrin, R.M.; Wang, Y.; Yuen, C.Y.; Will, J.; Masson, P.H. WVD2 is a novel microtubule-associated protein in Arabidopsis thaliana. Plant J. 2007, 49, 961–971. [Google Scholar]
- Schwab, B.; Mathur, J.; Saedler, R.; Schwarz, H.; Frey, B.; Scheidegger, C.; Hülskamp, M. Regulation of cell expansion by the DISTORTED genes in Arabidopsis thaliana: Actin controls the spatial organization of microtubules. Mol. Genet. Genomics 2003, 269, 350–360. [Google Scholar]
- Van der Honing, H.S.; Kieft, H.; Emons, A.M.; Ketelaar, T. Arabidopsis VILLIN2 and VILLIN3 are required for the generation of thick actin filament bundles and for directional organ growth. Plant Physiol. 2012, 158, 1426–1438. [Google Scholar]
- Stoppin-Mellet, V.; Gaillard, J.; Vantard, M. Functional evidence for in vitro microtubule severing by the plant katanin homologue. Biochem J. 2002, 365, 337–342. [Google Scholar]
- Uyttewaal, M.; Burian, A.; Alim, K.; Landrein, B.; Borowska-Wykręt, D.; Dedieu, A.; Peaucelle, A.; Ludynia, M.; Traas, J.; Boudaoud, A.; et al. Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell 2012, 149, 439–451. [Google Scholar]
- Hall, H.; Ellis, B. Transcriptional programming during cell wall maturation in the expanding Arabidopsis stem. BMC Plant Biol. 2013, 13, 14. [Google Scholar]
- Ageeva, M.V.; Petrovská, B.; Kieft, H.; Sal’nikov, V.V.; Snegireva, A.V.; van Dam, J.E.; van Veenendaal, W.L.; Emons, A.M.; Gorshkova, T.A.; van Lammeren, A.A. Intrusive growth of flax phloem fibers is of intercalary type. Planta 2005, 222, 565–574. [Google Scholar]
- Sanati Nezhad, A.; Geitmann, A. The cellular mechanics of an invasive lifestyle. J. Exp. Bot. 2013, 64, 4709–4728. [Google Scholar]
- Siedlecka, A.; Wiklund, S.; Péronne, M.A.; Micheli, F.; Lesniewska, J.; Sethson, I.; Edlund, U.; Richard, L.; Sundberg, B.; Mellerowicz, E.J. Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol. 2008, 146, 554–565. [Google Scholar]
- Lev-Yadun, S. Plant fibers: Initiation growth model plants and open questions. Russ. J. Plant Physiol. 2010, 57, 305–315. [Google Scholar]
- Guerriero, G.; Sergeant, K.; Hausman, J.-F. Integrated -Omics: A powerful approach to understanding the heterogeneous lignification of fibre crops. Int. J. Mol. Sci. 2013, 14, 10958–10978. [Google Scholar]
- Roach, M.J.; Deyholos, M.K. Microarray analysis of developing flax hypocotyls identifies novel transcripts correlated with specific stages of phloem fibre differentiation. Ann. Bot. 2008, 102, 317–330. [Google Scholar]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar]
- Mathur, J.; Mathur, N.; Kernebeck, B.; Srinivas, B.P.; Hülskamp, M. Mutations in actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. Plant Cell 2003, 15, 1632–1645. [Google Scholar]
- Yang, Z.; Lavagi, I. Spatial control of plasma membrane domains: ROP GTPase-based symmetry breaking. Curr. Opin. Plant Biol. 2012, 15, 601–607. [Google Scholar]
- Fu, Y.; Gu, Y.; Zheng, Z.; Wasteneys, G.; Yang, Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 2005, 120, 687–700. [Google Scholar]
- Fu, Y.; Xu, T.; Zhu, L.; Wen, M.; Yang, Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr. Biol. 2009, 19, 1827–1832. [Google Scholar]
- Ambrose, C.; Debono, A.; Wasteneys, G. Cell Geometry Guides the Dynamic Targeting of Apoplastic GPI-Linked Lipid Transfer Protein to Cell Wall Elements and Cell Borders in Arabidopsis thaliana. PLoS One 2013, 8, e81215. [Google Scholar]
- Krichevsky, A.; Kozlovsky, S.V.; Tian, G.W.; Chen, M.H.; Zaltsman, A.; Citovsky, V. How pollen tubes grow. Dev. Biol. 2007, 303, 405–420. [Google Scholar]
- Dresselhaus, T.; Franklin-Tong, N. Male-female cross-talk during pollen germination tube growth and guidance and double fertilization. Mol. Plant 2013, 6, 1018–1036. [Google Scholar]
- Gu, F.; Nielsen, E. Targeting and regulation of cell wall synthesis during tip growth in plants. J. Integr. Plant Biol. 2013, 55, 835–846. [Google Scholar]
- Hepler, P.K.; Rounds, C.M.; Winship, L.J. Control of cell wall extensibility during pollen tube growth. Mol. Plant 2013, 6, 998–1017. [Google Scholar]
- McKenna, S.T.; Kunkel, J.G.; Bosch, M.; Rounds, C.M.; Vidali, L.; Winship, L.J.; Hepler, P.K. Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 2009, 21, 3026–3040. [Google Scholar]
- Parton, R.M.; Fischer-Parton, S.; Watahiki, M.K.; Trewavas, A.J. Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J. Cell Sci. 2001, 114, 2685–2695. [Google Scholar]
- Cai, G.; Cresti, M. Organelle motility in the pollen tube: A tale of 20 years. J. Exp. Bot. 2009, 60, 495–508. [Google Scholar]
- Vidali, L.; Rounds, C.M.; Hepler, P.K.; Bezanilla, M. Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS One 2009, 4, e5744. [Google Scholar]
- Röckel, N.; Wolf, S.; Kost, B.; Rausch, T.; Greiner, S. Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis and reflects the distribution of esterified and de-esterified pectins. Plant J. 2008, 53, 133–143. [Google Scholar]
- Del Duca, S.; Faleri, C.; Iorio, R.A.; Cresti, M.; Serafini-Fracassini, D.; Cai, G. Distribution of transglutaminase in pear pollen tubes in relation to cytoskeleton and membrane dynamics. Plant Physiol. 2013, 161, 1706–1721. [Google Scholar]
- Bove, J.; Vaillancourt, B.; Kroeger, J.; Hepler, P.K.; Wiseman, P.W.; Geitmann, A. Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol. 2008, 147, 1646–1658. [Google Scholar]
- Bosch, M.; Cheung, A.Y.; Hepler, P.K. Pectin methylesterase a regulator of pollen tube growth. Plant Physiol. 2005, 138, 1334–1346. [Google Scholar]
- Wang, W.; Wang, L.; Chen, C.; Xiong, G.Y.; Tan, X.Y.; Yang, K.Z.; Wang, Z.C.; Zhou, Y.H.; Ye, D.; Chen, L.Q. Arabidopsis CSLD1 and CSLD4 are required for cellulose deposition and normal growth of pollen tubes. J. Exp. Bot. 2011, 62, 5161–5177. [Google Scholar]
- Parre, E.; Geitmann, A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 2005, 220, 582–592. [Google Scholar]
- Zhang, G.Y.; Feng, J.; Wu, J.; Wang, X.W. BoPMEI1 a pollen-specific pectin methylesterase inhibitor has an essential role in pollen tube growth. Planta 2010, 231, 1323–1334. [Google Scholar]
- Holdaway-Clarke, T.L.; Weddle, N.M.; Kim, S.; Robi, A.; Parris, C.; Kunkel, J.G.; Hepler, P.K. Effect of extracellular calcium pH and borate on growth oscillations in Lilium formosanum pollen tubes. J. Exp. Bot. 2003, 54, 65–72. [Google Scholar]
- Zonia, L.; Munnik, T. Understanding pollen tube growth: The hydrodynamic model versus the cell wall model. Trends Plant Sci. 2011, 16, 347–352. [Google Scholar]
- Winship, L.J.; Obermeyer, G.; Geitmann, A.; Hepler, P.K. Under pressure cell walls set the pace. Trends Plant Sci. 2010, 15, 363–369. [Google Scholar]
- Vogler, H.; Draeger, C.; Weber, A.; Felekis, D.; Eichenberger, C.; Routier-Kierzkowska, A.L.; Boisson-Dernier, A.L.; Ringli, C.; Nelson, B.J.; Smith, R.S.; et al. The pollen tube: A soft shell with a hard core. Plant J. 2012, 73, 617–267. [Google Scholar]
- Kroeger, J.; Geitmann, A. The pollen tube paradigm revisited. Curr. Opin. Plant Biol. 2012, 15, 618–624. [Google Scholar]
- Kroeger, J.H.; Geitmann, A.; Grant, M. Model for calcium dependent oscillatory growth in pollen tubes. J. Theor. Biol. 2008, 253, 363–374. [Google Scholar]
- Hwang, J.U.; Gu, Y.; Lee, Y.J.; Yang, Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell 2005, 16, 5385–5399. [Google Scholar]
- Lee, Y.J.; Szumlanski, A.; Nielsen, E.; Yang, Z. Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 2008, 181, 1155–1168. [Google Scholar]
- Yan, A.; Xu, G.; Yang, Z.B. Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proc. Natl. Acad. Sci. USA 2009, 106, 22002–22007. [Google Scholar]
- Li, Y.Q.; Zhang, H.Q.; Pierson, E.S.; Huang, F.Y.; Linskens, H.F.; Hepler, P.K.; Cresti, M. Enforced growth-rate fluctuation causes pectin ring formation in the cell wall of Lilium longiflorum pollen tubes. Planta 1996, 200, 41–49. [Google Scholar]
- Rojas, E.R.; Hotton, S.; Dumais, J. Chemically mediated mechanical expansion of the pollen tube cell wall. Biophys. J. 2011, 101, 1844–1853. [Google Scholar]
- Ferguson, C.; Teeri, T.T.; Siika-aho, M.; Read, S.M.; Bacic, A. Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 1998, 206, 452–460. [Google Scholar]
- Parre, E.; Geitmann, A. More than a leak sealant The mechanical properties of callose in pollen tubes. Plant Physiol. 2005, 137, 274–286. [Google Scholar]
- Abercrombie, J.M.; O’Meara, B.C.; Moffatt, A.R.; Williams, J.H. Developmental evolution of flowering plant pollen tube cell walls: Callose synthase (CalS) gene expression patterns. Evodevo 2011, 2, 14. [Google Scholar]
- Williams, J.H. Pollen tube growth rates and the diversification of flowering plant reproductive cycles. Int. J. Plant Sci. 2012, 173, 649–661. [Google Scholar]
- Turner, A.; Bacic, A.; Harris, P.J.; Read, S.M. Membrane fractionation and enrichment of callose synthase from pollen tubes of Nicotiana alata Link et Otto. Planta 1998, 205, 380–388. [Google Scholar]
- Li, H.J.; Bacic, A.; Read, S.M. Role of a callose synthase zymogen in regulating wall deposition in pollen tubes of Nicotiana alata Link et Otto. Planta 1999, 208, 528–538. [Google Scholar]
- Derksen, J.; Janssen, G.J.; Wolters-Arts, M.; Lichtscheidl, I.; Adlassnig, W.; Ovecka, M.; Doris, F.; Steer, M. Wall architecture with high porosity is established at the tip and maintained in growing pollen tubes of Nicotiana tabacum. Plant J. 2011, 68, 495–506. [Google Scholar]
- Aouar, L.; Chebli, Y.; Geitmann, A. Morphogenesis of complex plant cell shapes: The mechanical role of crystalline cellulose in growing pollen tubes. Sex. Plant Reprod. 2010, 23, 15–27. [Google Scholar]
- Goubet, F.; Misrahi, A.; Park, S.K.; Zhang, Z.N.; Twell, D.; Dupree, P. AtCSLA7 a cellulose synthase-like putative glycosyltransferase is important for pollen tube growth and embryogenesis in Arabidopsis. Plant Physiol. 2003, 131, 547–557. [Google Scholar]
- Anderson, J.R.; Barnes, W.S.; Bedinger, P. 26-dichlorobenzonitrile a cellulose biosynthesis inhibitor affects morphology and structural integrity of petunia and lily pollen tubes. J. Plant Physiol. 2002, 159, 61–67. [Google Scholar]
- Crowell, E.F.; Gonneau, M.; Stierhof, Y.D.; Höfte, H.; Vernhettes, S. Regulated trafficking of cellulose synthases. Curr. Opin. Plant Biol. 2010, 13, 700–705. [Google Scholar]
- Crowell, E.F.; Bischoff, V.; Desprez, T.; Rolland, A.; Stierhof, Y.D.; Schumacher, K.; Gonneau, M.; Höfte, H.; Vernhettes, S. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 2009, 21, 1141–1154. [Google Scholar]
- Sampathkumar, A.; Gutierrez, R.; McFarlane, H.; Bringmann, M.; Lindeboom, J.; Emons, A.M.; Samuels, L.; Ketelaar, T.; Ehrhardt, D.; Persson, S. Patterning and life-time of plasma membrane localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiol. 2013, 162, 675–688. [Google Scholar]
- Gossot, O.; Geitmann, A. Pollen tube growth: Coping with mechanical obstacles involves the cytoskeleton. Planta 2007, 226, 405–416. [Google Scholar]
- Laitiainen, E.; Nieminen, K.M.; Vihinen, H.; Raudaskoski, M. Movement of generative cell and vegetative nucleus in tobacco pollen tubes is dependent on microtubule cytoskeleton but independent of the synthesis of callose plugs. Sex. Plant Reprod. 2002, 15, 195–204. [Google Scholar]
- Geitmann, A.; Li, Y.-Q.; Cresti, M. The role of cytoskeleton and dictyosome activity in the pulsatory growth of Nicotiana tabacum and Petunia hybrida pollen tubes. Bot. Acta 1996, 109, 102–109. [Google Scholar]
- Moscatelli, A.; Idilli, A.I. Pollen tube growth: A delicate equilibrium between secretory and endocytic pathways. J. Integr. Plant Biol. 2009, 51, 727–739. [Google Scholar]






© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guerriero, G.; Hausman, J.-F.; Cai, G. No Stress! Relax! Mechanisms Governing Growth and Shape in Plant Cells. Int. J. Mol. Sci. 2014, 15, 5094-5114. https://doi.org/10.3390/ijms15035094
Guerriero G, Hausman J-F, Cai G. No Stress! Relax! Mechanisms Governing Growth and Shape in Plant Cells. International Journal of Molecular Sciences. 2014; 15(3):5094-5114. https://doi.org/10.3390/ijms15035094
Chicago/Turabian StyleGuerriero, Gea, Jean-Francois Hausman, and Giampiero Cai. 2014. "No Stress! Relax! Mechanisms Governing Growth and Shape in Plant Cells" International Journal of Molecular Sciences 15, no. 3: 5094-5114. https://doi.org/10.3390/ijms15035094