Terminal Protection of Small Molecule-Linked DNA for Small Molecule–Protein Interaction Assays
Abstract
:1. Introduction
2. Terminal Protection Assay of Small Molecule-Linked DNA
2.1. Terminal Protection of Small Molecule-Linked Single-Stranded DNA
2.2. Terminal Protection of Small Molecule-Linked Double-Stranded DNA
3. Signal Amplification in Terminal Protection Assay for Sensitive Detection of Small Molecule–Protein Interaction
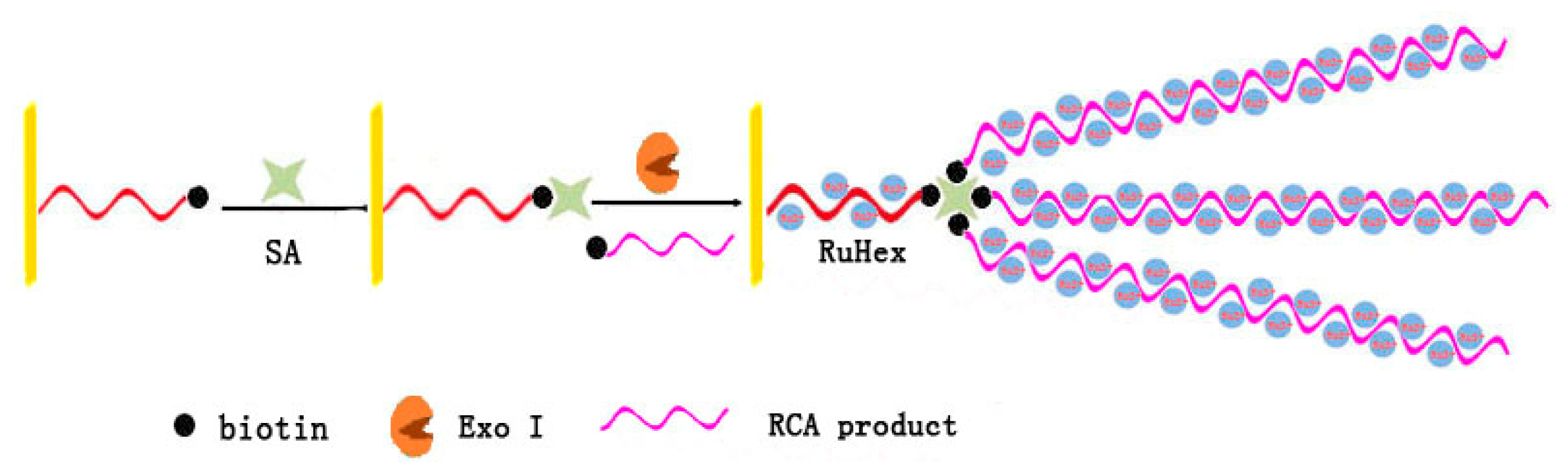
3.1. Rolling Circle Amplification
3.2. Hybridization Chain Reaction
3.3. Nuclease-Assisted Signal Amplification
3.3.1. Nickase-Assisted Signal Amplification
3.3.2. Exo III-Assisted Signal Amplification
3.4. Dual Amplification Strategy Based on Rolling Cycle and Exo III-Assisted Recycling Cleavage
4. Other Methods
4.1. Non-Nuclease-Assisted Terminal Protection Assay
4.2. DNA/Fok I Transducer
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Arkin, M.R.; Wells, J.A. Small-molecule inhibitors of protein–protein interactions: Progressing towards the dream. Nat. Rev. Drug Discov 2004, 3, 301–317. [Google Scholar]
- Zhang, H.; Li, F.; Dever, B.; Li, X.F.; Le, X.C. DNA-mediated homogeneous binding assays for nucleic acids and proteins. Chem. Rev 2012, 113, 2812–2841. [Google Scholar]
- Yakovchuk, P.; Protozanova, E.; Frank–Kamenetskii, M.D. Base-stacking and base-pairing contributions into thermal stability of the DNA double helix. Nucleic Acids Res 2006, 34, 564–574. [Google Scholar]
- Denkert, C.; Budczies, J.; Kind, T.; Weichert, W.; Tablack, P.; Sehouli, J.; Niesporek, S.; Könsgen, D.; Dietel, M.; Fiehn, O. Mass spectrometry-based metabolic profiling reveals different metabolite patterns in invasive ovarian carcinomas and ovarian borderline tumors. Cancer Res 2006, 66, 10795–10804. [Google Scholar]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol 2004, 22, 969–976. [Google Scholar]
- Michnick, S.W.; Ear, P.H.; Manderson, E.N.; Remy, I.; Stefan, E. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat. Rev. Drug Discov 2007, 6, 569–582. [Google Scholar]
- Acharya, G.; Chang, C.L.; Doorneweerd, D.D.; Vlashi, E.; Henne, W.A.; Hartmann, L.C.; Low, P.S.; Savran, C.A. Immunomagnetic diffractometry for detection of diagnostic serum markers. J. Am. Chem. Soc 2007, 129, 15824–15829. [Google Scholar]
- Preul, M.C.; Caramanos, Z.; Collins, D.L.; Villemure, J.G.; Leblanc, R.; Olivier, A.; Pokrupa, R.; Arnold, D.L. Accurate, noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nat. Med 1996, 2, 323–325. [Google Scholar]
- Guiffant, D.; Tribouillard, D.; Gug, F.; Galons, H.; Meijer, L.; Blondel, M.; Bach, S. Identification of intracellular targets of small molecular weight chemical compounds using affinity chromatography. Biotechnol. J 2007, 2, 68–75. [Google Scholar]
- Becker, F.; Murthi, K.; Smith, C.; Come, J.; Costa-Roldán, N.; Kaufmann, C.; Hanke, U.; Degenhart, C.; Baumann, S.; Wallner, W.; et al. A three-hybrid approach to scanning the proteome for targets of small molecule kinase inhibitors. Chem. Biol 2004, 11, 211–223. [Google Scholar]
- Petrov, A.; Okhonin, V.; Berezovski, M.; Krylov, S.N. Kinetic capillary electrophoresis (KCE): A conceptual platform for kinetic homogeneous affinity methods. J. Am. Chem. Soc 2005, 127, 17104–17110. [Google Scholar]
- Bao, J.; Krylov, S.N. Volatile kinetic capillary electrophoresis for studies of protein–small molecule interactions. Anal. Chem 2012, 84, 6944–6947. [Google Scholar]
- Bachovchin, D.A.; Brown, S.J.; Rosen, H.; Cravatt, B.F. Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat. Biotechnol 2009, 27, 387–394. [Google Scholar]
- Thorsen, T.S.; Madsen, K.L.; Rebola, N.; Rathje, M.; Anggono, V.; Bach, A.; Moreira, I.S.; Stuhr-Hansen, N.; Dyhring, T.; Peters, D.; et al. Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proc. Natl. Acad. Sci. USA 2010, 107, 413–418. [Google Scholar]
- Redman, J.E. Surface plasmon resonance for probing quadruplex folding and interactions with proteins and small molecules. Methods 2007, 43, 302–312. [Google Scholar]
- Kanoh, N.; Kyo, M.; Inamori, K.; Ando, A.; Asami, A.; Nakao, A.; Osada, H. SPR imaging of photo-cross-linked small-molecule arrays on gold. Anal. Chem 2006, 78, 2226–2230. [Google Scholar]
- Wu, Z.; Zhen, Z.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Terminal protection of small-molecule-linked DNA for sensitive electrochemical detection of protein binding via selective carbon nanotube assembly. J. Am. Chem. Soc 2009, 131, 12325–12332. [Google Scholar]
- Zhang, B.; Liu, B.; Tang, D.; Niessner, R.; Chen, G.; Knopp, D. DNA-based hybridization chain reaction for amplified bioelectronic signal and ultrasensitive detection of proteins. Anal. Chem 2012, 84, 5392–5399. [Google Scholar]
- Ji, H.; Yan, F.; Lei, J.; Ju, H. Ultrasensitive electrochemical detection of nucleic acids by template enhanced hybridization followed with rolling circle amplification. Anal. Chem 2012, 84, 7166–7171. [Google Scholar]
- Xu, Q.; Cao, A.; Zhang, L.F.; Zhang, C.Y. Rapid and label-free monitoring of exonuclease III-assisted target recycling amplification. Anal. Chem 2012, 84, 10845–10851. [Google Scholar]
- Wang, Q.; Jiang, B.; Xu, J.; Xie, J.; Xiang, Y.; Yuan, R.; Chai, Y. Amplified terminal protection assay of small molecule/protein interactions via a highly characteristic solid-state Ag/AgCl process. Biosens. Bioelectron 2013, 43, 19–24. [Google Scholar]
- He, Y.; Xing, X.; Tang, H.; Pang, D. Graphene oxide-based fluorescent biosensor for protein detection via terminal protection of small-molecule-linked DNA. Small 2013, 12, 2097–2101. [Google Scholar]
- Zhang, H.; Li, Y.; Su, X. A small-molecule-linked DNA-graphene oxide-based fluorescence-sensing system for detection of biotin. Anal. Biochem 2013, 442, 172–177. [Google Scholar]
- Wu, Z.; Wang, H.; Guo, M.; Tang, L.J.; Yu, R.Q.; Jiang, J.H. Terminal protection of small molecule-linked DNA: A versatile biosensor platform for protein binding and gene typing assay. Anal. Chem 2011, 83, 3104–3111. [Google Scholar]
- Kong, R.M.; Zhang, X.B.; Zhang, L.L.; Huang, Y.; Lu, D.Q.; Tan, W.; Shen, G.L.; Yu, R.Q. Molecular beacon-based junction probes for efficient detection of nucleic acids via a true target-triggered enzymatic recycling amplification. Anal. Chem 2011, 83, 14–17. [Google Scholar]
- Wang, H.Q.; Liu, W.Y.; Wu, Z.; Tang, L.J.; Xu, X.M.; Yu, R.Q.; Jiang, J.H. Homogeneous label-free genotyping of single nucleotide polymorphism using ligation-mediated strand displacement amplification with DNAzyme-based chemiluminescence detection. Anal. Chem 2011, 83, 1883–1889. [Google Scholar]
- Zhao, W.; Ali, M.M.; Brook, M.A.; Li, Y. Rolling circle amplification: Applications in nanotechnology and biodetection with functional nucleic acids. Angew. Chem. Int. Ed. Engl 2008, 47, 6330–6337. [Google Scholar]
- Wang, Q.; Jiang, B.; Xie, J.; Xiang, Y.; Yuan, R.; Chai, Y. Coupling of background reduction with rolling circle amplification for highly sensitive protein detection via terminal protection of small molecule-linked DNA. Analyst 2013, 138, 5751–5756. [Google Scholar]
- Dirks, R.M.; Pierce, N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci.USA 2004, 101, 15275–15278. [Google Scholar]
- Wang, G.; He, X.; Wang, L.; Zhang, X. A folate receptor electrochemical sensor based on terminal protection and supersandwich DNAzyme amplification. Biosens. Bioelectron 2013, 42, 337–341. [Google Scholar]
- Zheleznaya, L.A.; Kachalova, G.S.; Artyukh, R.I.; Yunusova, A.K.; Perevyazova, T.A.; Matvienko, N.I. Nicking endonucleases. Biochemistry 2009, 74, 1457–1466. [Google Scholar]
- Cao, Y.; Zhu, S.; Yu, J.; Zhu, X.; Yin, Y.; Li, G. Protein detection based on small molecule-linked DNA. Anal. Chem 2012, 84, 4314–4320. [Google Scholar]
- Zhou, G.; Zhang, X.; Ji, X.; He, Z. Ultrasensitive detection of small molecule–protein interaction via terminal protection of small molecule linked DNA and Exo III-aided DNA recycling amplification. Chem. Commun 2013, 49, 8854–8856. [Google Scholar]
- Ou, L.J.; Wang, H.B.; Chu, X. Terminal protection of small-molecule-linked DNA for sensitive fluorescence detection of protein binding based on nucleic acid amplification. Analyst 2013, 138, 7218–7223. [Google Scholar]
- Fei, Y.H.; Liu, D.; Wu, Z.S.; Shen, G.L.; Yu, R.Q. DNA-encoded signal conversion for sensitive microgravimetric detection of small molecule–protein interaction. Bioconjug. Chem 2011, 22, 2369–2376. [Google Scholar]
- Zhen, Z.; Tang, L.J.; Lin, J.; Jiang, J.H.; Yu, R.Q.; Xiong, X.; Tan, W. Endonucleolytic inhibition assay of DNA/Fok I transducer as a sensitive platform for homogeneous fluorescence detection of small molecule–protein interactions. Anal. Chem 2012, 84, 5708–5715. [Google Scholar]
- Sanders, K.L.; Catto, L.E.; Bellamy, S.R.; Halford, S.E. Targeting individual subunits of the Fok I restriction endonuclease to specific DNA strands. Nucleic Acids Res 2009, 37, 2105–2115. [Google Scholar]






© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, C.; Wu, Z.; Tang, H.; Tang, L.-J.; Yu, R.-Q.; Jiang, J.-H. Terminal Protection of Small Molecule-Linked DNA for Small Molecule–Protein Interaction Assays. Int. J. Mol. Sci. 2014, 15, 5221-5232. https://doi.org/10.3390/ijms15045221
Hu C, Wu Z, Tang H, Tang L-J, Yu R-Q, Jiang J-H. Terminal Protection of Small Molecule-Linked DNA for Small Molecule–Protein Interaction Assays. International Journal of Molecular Sciences. 2014; 15(4):5221-5232. https://doi.org/10.3390/ijms15045221
Chicago/Turabian StyleHu, Cui, Zhan Wu, Hao Tang, Li-Juan Tang, Ru-Qin Yu, and Jian-Hui Jiang. 2014. "Terminal Protection of Small Molecule-Linked DNA for Small Molecule–Protein Interaction Assays" International Journal of Molecular Sciences 15, no. 4: 5221-5232. https://doi.org/10.3390/ijms15045221



