Swelling and Shrinking Properties of Thermo-Responsive Polymeric Ionic Liquid Hydrogels with Embedded Linear pNIPAAM
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Properties of Hydrogels
2.2. Thermal Behaviour of Hydrogels
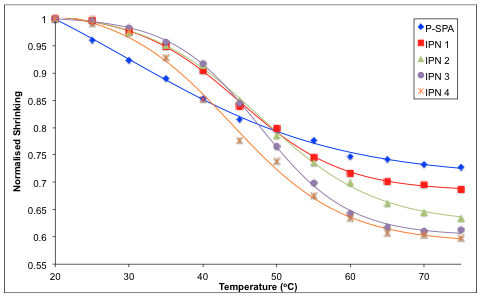
2.3. Shrinking Behaviour of Hydrogels
2.4. Reswelling Behaviour of Hydrogels
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis of pNIPAAM
3.3. Synthesis of Tributyl-Hexyl Phosphonium 3-Sulfopropylacrylate (P-SPA)
3.4. Preparation of Semi-IPN Hydrogel
3.5. Thermobehaviour of Semi-IPN Hydrogels
3.6. Measurement of Shrinking of Semi-IPN Hydrogels
3.7. Measurement of Reswelling of Semi-IPN Hydrogels
4. Conclusions
Supplementary Information
ijms-15-05337-s001.pdfAcknowledgments
Conflicts of Interest
References
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable I-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun 1992. [Google Scholar] [CrossRef]
- Wassersheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 1–380. [Google Scholar]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and cataysis. Chem. Rev 1999, 99, 2071–2084. [Google Scholar]
- Fraser, K.J.; MacFarlane, D.R. Phosphonium-based ionic liquids: An overview. Aust. J. Chem 2009, 62, 309–321. [Google Scholar]
- Ohno, H. Design of ion conductive polymers based on ionic liquids. Macromol. Symp 2007, 249–250, 551–556. [Google Scholar]
- Pont, A.-L.; Marcilla, R.; de Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. Power Sour 2009, 188, 558. [Google Scholar]
- Suzuki, K.; Yamaguchi, M.; Hotta, S.; Tanabe, N.; Yanagida, S. A new alkyl-imidazole polymer prepared as an inonic polymer electrolyte by in situ polymerization of dye sensitized solar cells. J. Photochem. Photobiol. A 2004, 164, 81–85. [Google Scholar]
- Marcilla, R.; Sanchez-Paniagua, M.; Lopez-Ruiz, B.; Lopez-Cabarcos, E.; Ochoteco, E.; Grande, H.; Mecerreyes, D. Synthesis and characterization of new polymeric ionic liquid microgels. J. Polym. Sci. A 2006, 44, 3958–3965. [Google Scholar]
- Kijima, M.; Setoh, K.; Shirakawa, H. Self-doped polyphenylenes containing electron-accepting viologen sde group. Chem. Lett 2000, 29, 936. [Google Scholar]
- Kohno, Y.; Ohno, H. Temperature-responsive ionic liquid/water interfaces: Relation between hydrophilicity of ions and dynamic phase change. Phys. Chem. Chem. Phys 2012, 14, 5063–5070. [Google Scholar]
- Fukaya, Y.; Sekikawa, K.; Murata, K.; Nakamura, N.; Ohno, H. Miscibility and phase behavior of water-dicarboxylic acid type ionic liquid mixed systems. Chem. Commun 2007, 3089–3091. [Google Scholar]
- Kohno, Y.; Ohno, H. Ionic liquid/water mixtures: From hostility to conciliation. Chem. Commun 2012, 48, 7119–7130. [Google Scholar]
- Kohno, Y.; Deguchi, Y.; Ohno, H. Ionic liquid-derived charged polymers to show highly thermoresponsive LCST-type transition with water at desired temperatures. Chem. Commun 2012, 48, 11883–11885. [Google Scholar]
- Fujita, K.; MacFarlane, D.; Forsyth, M.; Yoshizawa-Fujita, M.; Murata, K.; Nakamura, N.; Ohno, H. Solubility and stability of cytochrome c in hydrated ionic liquids: Effect of oxo acid residues and kosmotropicity. Biomacromolecules 2007, 8, 2080–2086. [Google Scholar]
- Kohno, Y.; Ohno, H. Key factors to prepare polyelectrolytes showing temperature-sensitive lower critical solution temperature-type phase transitions in water. Aust. J. Chem 2011, 65, 91–94. [Google Scholar]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci 1992, 17, 163–249. [Google Scholar]
- Men, Y.; Schlaad, H.; Yuan, J. Cationic poly(ionic liquid) with tunable lower critical solution temperature-type phase transition. ACS Macro Lett 2013, 2, 456–459. [Google Scholar]
- Ziolkowski, B.; Diamond, D. Thermoresponsive poly(ionic liquid) hydrogels. Chem. Commun 2013, 49, 10308–10310. [Google Scholar]
- Zhang, X.-Z.; Xu, X.-D.; Cheng, S.-X.; Zhuo, R.-X. Strategies to improve the response rate of thermosensitive PNIPAAm hydrogels. Soft Matter 2008, 4, 385–391. [Google Scholar]
- Zhang, X.-Z.; Chu, C.-C. Synthesis and properties of the semi-interpenetrating polymer network-like, thermosensitive poly(N-isopropylacrylamide) hydrogel. J. Appl. Polym. Sci 2003, 89, 1935–1942. [Google Scholar]
- Zhang, X.-Z.; Yang, Y.-Y.; Chung, T.-S.; Ma, K.-X. Preparation and characterization of fast response macroporous poly(N-isopropylacrylamide) hydrogels. Langmuir 2001, 17, 6094–6099. [Google Scholar]
- Xu, X.-D.; Wang, B.; Wang, Z.-C.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Fabrication of fast responsive, thermosensitive poly(N-isopropylacrylamide) hydrogels by using diethyl ether as precipitation agent. J. Biomed. Mater. Res. A 2008, 86, 1023–1032. [Google Scholar]
- Wu, X.S.; Hoffman, A.S.; Yager, P. Synthesis and characterization of thermally reversible macroporous poly(N-isopropylacrylamide) hydrogels. J. Polym. Sci. A 1992, 30, 2121–2129. [Google Scholar]
- Guilherme, M.R.; Campese, G.M.; Radovanovic, E.; Rubira, A.F.; Tambourgi, E.B.; Muniz, E.C. Thermo-responsive sandwiched-like membranes of IPN-PNIPAAm/PAAm hydrogels. J. Membr. Sci 2006, 275, 187–194. [Google Scholar]
- Guilherme, M.R.; da Silva, R.; Rubira, A.F.; Geuskens, G.; Muniz, E.C. Thermo-sensitive hydrogels membranes from PAAm networks and entangled PNIPAAm: Effect of temperature, cross-linking and PNIPAAm contents on the water uptake and permeability. React. Funct. Polym 2004, 61, 233–243. [Google Scholar]
- Vidal, F.; Plesse, C.; Randriamahazaka, H.; Teyssie, D.; Chevrot, C. Long-life air working semi-IPN/ionic liquid: New precursor of artificial muscles. In Mol. Cryst. Liq. Cryst; 2006; Volume 448, pp. 287–291. [Google Scholar]
- Zhang, J.; Peppas, N.A. Molecular interactions in poly(methacrylic acid)/poly(N-isopropyl acrylamide) interpenetrating polymer networks. J. Appl. Polym. Sci 2001, 82, 1077–1082. [Google Scholar]
- Maeda, Y.; Higuchi, T.; Ikeda, I. FTIR spectroscopic and calorimetric studies of the phase transitions of N-isopropylacrylamide copolymers in water. Langmuir 2001, 17, 7535–7539. [Google Scholar]
- Muniz, E.C.; Geuskens, G. Polyacrylamide hydrogels and semi-interpenetrating networks (IPNs) with poly(N-isopropylacrylamide): Mechanical properties by measure of compressive elastic modulus. J. Mater. Sci 2001, 12, 879–881. [Google Scholar]
- Walsh, S.; Diamond, D. SOLVER: Nonlinear curve-fitting using microsoft excel solver. Talanta 1995, 42, 561–572. [Google Scholar]
- Earle, M.; Esperanca, J.; Gilea, M.; Lopes, J.; Rebelo, L.; Magee, J.; Seddon, K.; Widegren, J. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar]





| Materials | Sample ID | ||||
|---|---|---|---|---|---|
| P-SPA | IPN 1 | IPN 2 | IPN 3 | IPN 4 | |
| P-SPA (μmol) | 800 | 800 | 800 | 800 | 800 |
| pNIPAAM (μmol) | 0 | 240 | 480 | 800 | 960 |
| PPO 800 a (μmol) | 40 | 40 | 40 | 40 | 40 |
| HMPP b (μmol) | 16 | 16 | 16 | 16 | 16 |
| P-SPA:pNIPAAM (mol ratio) | 1:0 | 1:0.3 | 1:0.6 | 1:1 | 1:1.2 |
| Sample | Initial Swollen Diameter a (mm) | Contracted Gel Diameter b (mm) | % Shrinking c | Slope d |
|---|---|---|---|---|
| P-SPA | 5.33 (0.07) | 3.88 (0.07) | 27.2 | −0.007 |
| IPN 1 | 5.61 (0.01) | 3.85 (0.06) | 31.2 | −0.011 |
| IPN 2 | 6.11 (0.17) | 3.88 (0.11) | 36.4 | −0.012 |
| IPN 3 | 6.22 (0.07) | 3.81 (0.14) | 38.7 | −0.016 |
| IPN 4 | 5.73 (0.09) | 3.43 (0.04) | 40.2 | −0.014 |
| Sample | Contracted Gel Diameter a (mm) | Reswollen Gel Diameter b (mm) | % Reswelling c | Slope d (min−1) |
|---|---|---|---|---|
| P-SPA | 3.88 (0.07) | 5.34 (0.08) | 37.6 | 0.014 |
| IPN 1 | 3.85 (0.06) | 5.62 (0.01) | 45.8 | 0.016 |
| IPN 2 | 3.88 (0.11) | 6.16 (0.16) | 58.8 | 0.023 |
| IPN 3 | 3.81 (0.14) | 6.27 (0.06) | 64.4 | 0.044 |
| IPN 4 | 3.43 (0.04) | 5.75 (0.09) | 67.6 | 0.062 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gallagher, S.; Florea, L.; Fraser, K.J.; Diamond, D. Swelling and Shrinking Properties of Thermo-Responsive Polymeric Ionic Liquid Hydrogels with Embedded Linear pNIPAAM. Int. J. Mol. Sci. 2014, 15, 5337-5349. https://doi.org/10.3390/ijms15045337
Gallagher S, Florea L, Fraser KJ, Diamond D. Swelling and Shrinking Properties of Thermo-Responsive Polymeric Ionic Liquid Hydrogels with Embedded Linear pNIPAAM. International Journal of Molecular Sciences. 2014; 15(4):5337-5349. https://doi.org/10.3390/ijms15045337
Chicago/Turabian StyleGallagher, Simon, Larisa Florea, Kevin J. Fraser, and Dermot Diamond. 2014. "Swelling and Shrinking Properties of Thermo-Responsive Polymeric Ionic Liquid Hydrogels with Embedded Linear pNIPAAM" International Journal of Molecular Sciences 15, no. 4: 5337-5349. https://doi.org/10.3390/ijms15045337