Liquid Crystalline Assembly of Coil-Rod-Coil Molecules with Lateral Methyl Groups into 3-D Hexagonal and Tetragonal Assemblies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Oligomers 1a–1c
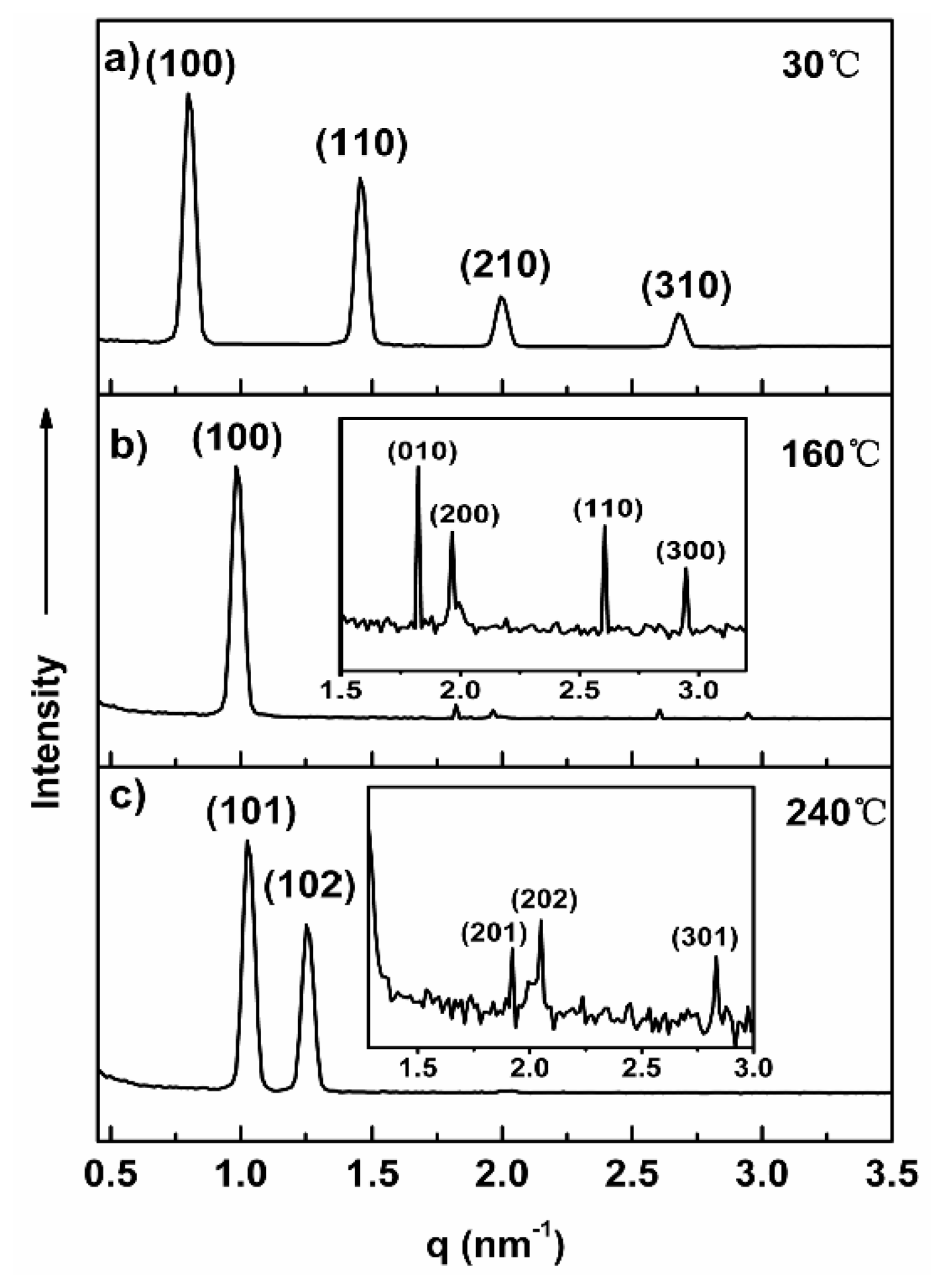
2.2. Self-Assembly of Molecules 1a–1c in the Bulk State
3. Experimental Section
3.1. Materials
3.2. Techniques
3.3. Synthesis Compounds 6a–6c
3.4. Synthesis Oligomers 1a–1c
4. Conclusions
Supplementary Information
ijms-15-05634-s001.pdfAcknowledgments
Conflicts of Interest
References and Notes
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-mediated self-assembly, disassembly and self-organization of complex systems. Chem. Rev 2009, 109, 6275–6540. [Google Scholar]
- Ligthart, G.B.; Sijbesma, R.P.; Meijer, E.W. Complementary quadruple hydrogen bonding in supramolecular copolymers. J. Am. Chem. Soc 2005, 127, 810–811. [Google Scholar]
- Hoeben, F.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P. About supramolecular assemblies of π-conjugated systems. Chem. Rev 2005, 105, 1491–1546. [Google Scholar]
- Zhao, H.X.; Guo, D.S.; Wang, L.H.; Qian, H.; Liu, Y. A novel supramolecular ternary polymer with two orthogonal host-guest interactions. Chem. Commun 2012, 48, 11319–11321. [Google Scholar]
- Roosma, J.; Mes, T.; Leclere, P.; Palmans, A.; Meijer, E.W. Supramolecular materials from benzene-1,3,5-tricarboxamide-based nanorods. J. Am. Chem. Soc 2008, 130, 1120–1121. [Google Scholar]
- Wilson, D.A.; Wilson, C.J.; Moldoveanu, C.; Resmerita, A.; Corcoran, P.; Hoang, L.M.; Rosen, B.M.; Percec, V. Neopentylglycolborylation of aryl mesylates and tosylates catalyzed by Ni-based mixed-ligand systems activated with Zn. J. Am. Chem. Soc 2010, 132, 1800–1801. [Google Scholar]
- Yue, L.; Ai, H.; Yang, Y.; Lu, W.; Wu, L. Chiral self-assembly and reversible light modulation of a polyoxometalate complex via host–guest recognition. Chem. Commun 2013, 49, 9770–9772. [Google Scholar]
- Zhang, X.; Wang, C. Supramolecular amphiphiles. Chem. Soc. Rev 2011, 40, 94–101. [Google Scholar]
- Wang, C.; Guo, Y.; Wang, Z.; Zhang, X. Superamphiphiles based on charge transfer complex: Controllable hierarchical self-assembly of nanoribbons. Langmuir 2010, 26, 14509–14511. [Google Scholar]
- Bhosale, S.V.; Kalyankar, M.B.; Nalage, S.V.; Lalander, C.H.; Bhosale, S.V.; Langford, S.J.; Oliver, R.F. pH dependent molecular self-assembly of octaphosphonate porphyrin of nanoscale dimensions: Nanosphere and nanorod aggregates. Int. J. Mol. Sci 2011, 12, 1464–1473. [Google Scholar]
- Starnes, S.D.; Arungundram, S.; Saunders, C.H. Anion sensors based on β,β′-disubstituted porphyrin derivatives. Tetrahedron Lett 2002, 43, 7785–7788. [Google Scholar]
- Kim, H.-J.; Jeong, Y.-H.; Lee, E.; Lee, M. Channel structures from self-assembled hexameric macrocycles in laterally grafted bent rod molecules. J. Am. Chem. Soc 2009, 131, 17371–17375. [Google Scholar]
- Percec, V.; Rudick, J.G.; Peterca, M.; Heiney, P.A. Nanomechanical function from self-organizable dendronized helical polyphenylacetylenes. J. Am. Chem. Soc 2008, 130, 7503–7508. [Google Scholar]
- Motoyanagi, J.; Fukushima, T.; Ishii, N.; Aida, T. Photochemical stitching of a tubularly assembled hexabenzocoronene amphiphile by dimerization of coumarin pendants. J. Am. Chem. Soc 2006, 128, 4220–4201. [Google Scholar]
- Zhong, K.L.; Wang, Q.; Chen, T.; Jin, L.Y. Self assembly of coil-rod-coil molecules into bicontinuous cubic and oblique columnar assemblies depending on the coil chain length. Eur. Polym. J 2013, 49, 3244–3250. [Google Scholar]
- Hamley, I.W.; Castelletto, V.; Lu, Z.B.; Imrie, C.T.; Itoh, T.; Al-Hussein, M. Interplay between smectic ordering and microphase separation in a series of side-group liquid-crystal block copolymers. Macromolecules 2004, 37, 4798–4807. [Google Scholar]
- Lee, E.; Kim, J.K.; Lee, M. Reversible scrolling of two-dimensional sheets from the self-assembly of laterally grafted amphiphilic rods. Angew. Chem. Int. Ed 2008, 47, 6375–6378. [Google Scholar]
- Wu, J.; Pisula, W.; Müllen, K. Graphenes as potential material for electronics. Chem. Rev 2007, 107, 718–747. [Google Scholar]
- Rudick, J.G.; Percec, V. Helical chirality in dendronized polyarylacetylenes. New J. Chem 2007, 31, 1083–1096. [Google Scholar]
- Liu, L.B.; Moon, K.S.; Gunawidjaja, R.; Lee, E.; Tsukruk, V.; Lee, M. Molecular reorganization of paired assemblies of T-shaped rod-coil amphiphilic molecule at the air-water interface. Langmuir 2008, 24, 3930–3936. [Google Scholar]
- Lavigueur, C.; Foster, E.J.; Williams, V.E. Modular assembly of elliptical mesogens. Liq. Cryst 2007, 34, 833–840. [Google Scholar]
- Voisin, E.; Foster, J.E.; Rakotomalala, M.; Williams, V.E. Effects of symmetry on the stability of columnar liquid crystals. Chem. Mater 2009, 21, 3251–3261. [Google Scholar]
- Hong, D.-J.; Jeong, H.; Lee, J.-K.; Zin, W.-C.; Nguyen, T.D.; Glotzer, S.C.; Lee, M. Solid-state scrolls from hierarchical self-assembly of T-shaped rod-coil molecules. Angew. Chem. Int. Ed 2009, 48, 1664–1668. [Google Scholar]
- Lee, M.; Cho, B.K.; Zin, W.C. Supramolecular structures from rod-coil block copolymers. Chem. Rev 2001, 101, 3869–3892. [Google Scholar]
- Zhu, J.; Zhong, K.; Liang, Y.; Wang, Z.; Chen, T.; Jin, L.Y. Synthesis and self-assembly of oligomers containing cruciform 9,10-bis(arylethynyl)anthracene unit: Formation of supramolecular nanostructures based on rod-length-dependent organization. Tetrahedron 2014. [Google Scholar] [CrossRef]
- Vriezema, D.M.; Aragones, M.C.; Elemans, J.A.A.W.; Comelissen, J.; Rowan, A.E.; Nolte, R.J.M. Self-assembled nanoreactors. Chem. Rev 2005, 105, 1445–1490. [Google Scholar]
- Kim, J.-K.; Hong, M.-K.; Ahn, J.-H.; Lee, M. Liquid crystalline assembly from rigid wedge-flexible coil diblock molecules. Angew. Chem. Int. Ed 2005, 44, 328–332. [Google Scholar]
- Chen, L.; Zhong, K.L.; Jin, L.Y. Supramolecular honeycomb and columnar assemblies formed by self-assembly of coil-rod-coil molecules with a conjugated rod segment. Macromol. Res 2010, 18, 800–805. [Google Scholar]
- Lee, E.; Huang, Z.; Ryu, J.-H.; Lee, M. Rigid-flexible block molecules based on a laterally extended aromatic segment: Hierarchical assembly into single fibers, flat ribbons, and twisted ribbons. Chem. Eur. J 2008, 14, 6957–6966. [Google Scholar]
- Wang, Z.S.; Cui, J.J.; Liang, Y.R.; Chen, T.; Lee, M.; Yin, B.Z.; Jin, L.Y. Supramolecular nanostructures from self-assembly of T-shaped rod building block oligomers. J. Polym. Sci. Part A Polym. Chem 2013, 51, 5021–5028. [Google Scholar]
- Tian, L.R.; Zhong, K.-L.; Liu, Y.J.; Huang, Z.G.; Jin, L.Y.; Hirst, L.S. Synthesis and self-assembly of coil-rod-coil molecules with lateral methyland ethyl groups in the center of the rod segment. Soft Matter 2010, 6, 5993–5998. [Google Scholar]
- The Avogadro constant (symbols: NA) is defined as the number of constituent particles (usually atoms or molecules) in one mole of a given substance. So, the average number (n) of molecules in a single rod bundle can be calculated according to following equation: n = m × NA/MW = v × ρ × NA/MW = (abc × sinγ) × ρ × NA/MW.
- Zhong, K.L.; Huang, Z.; Man, Z.; Jin, L.Y.; Yin, B.; Lee, M. Synthesis and self-assembly of rod-coil molecules with n-shaped rod building block. J. Polym. Sci. Part A Polym. Chem 2010, 48, 1415–1422. [Google Scholar]
- Zhong, K.; Man, Z.; Huang, Z.; Chen, T.; Yin, B.; Jin, L.Y. Supramolecular columnar nanostructures from self-organization of coil-rod-coil molecules incorporating an anthracene unit. Polym. Int 2011, 60, 845–850. [Google Scholar]





| Molecule | Phase transitions (°C) and corresponding enthalpy changes (in brackets) (kJ/mol) | |
|---|---|---|
| Heating | Cooling | |
| 1a | HPL 106.4 (2.0) Colrec 243.6 (0.9) Colob 292.4 (0.4) i | i 274.5 (0.8) Colob 219.7 (1.0) Colrec 104.8 (1.8) HPL |
| 1b | Colrec 86.4 (1.9) Colob 224.6 (0.9) HCP 274.3 (0.4) i | i 249.6 (0.8) HCP 195.9 (1.1) Colob 80.7 (1.7) Colrec |
| 1c | Colhex 72.9(1.6) Mtet 225.9 (1.4) i | i 205.3 (1.5) Mtet 36.4 (0.8) Colhex |
| Mesophase (lattice constants) | Reflections/nm | Miller indices (h k l) | |
|---|---|---|---|
| qobsd | qcalcd | ||
| HPL at 30 °C; a = 6.48 nm; c = 10.38 nm | 1.125 | 1.125 | 100 |
| 1.210 | 1.211 | 002 | |
| 2.037 | 2.036 | 2–11 | |
| 2.321 | 2.321 | 201 | |
| Colrec at 160 °C; a = 6.49 nm; b = 3.43 nm | 0.968 | 0.968 | 100 |
| 2.064 | 2.065 | 110 | |
| 2.663 | 2.662 | 210 | |
| 2.904 | 2.905 | 300 | |
| Colob at 230 °C; a = 8.61 nm; b = 4.66 nm; γ = 135 | 1.025 | 1.025 | 100 |
| 1.908 | 1.908 | 010 | |
| 2.733 | 2.734 | 110 | |
| 3.089 | 3.089 | 300 | |
| Mesophase (lattice constants) | Reflections/nm | Miller indices (h k l) | |
|---|---|---|---|
| qobsd | qcalcd | ||
| Colrec at 30 °C a = 7.9 nm b = 5.2 nm | 0.797 | 0.797 | 100 |
| 1.453 | 1.453 | 110 | |
| 1.994 | 1.994 | 210 | |
| 2.677 | 2.676 | 310 | |
| Colob at 160 °C a = 9.04 nm b = 4.89 nm γ = 135 | 0.983 | 0.983 | 100 |
| 1.822 | 1.823 | 010 | |
| 1.963 | 1.965 | 200 | |
| 2.605 | 2.605 | 110 | |
| 2.945 | 2.947 | 300 | |
| HCP at 230 °C a = 7.75 nm b = 15.2 nm | 1.025 | 1.025 | 101 |
| 1.253 | 1.253 | 102 | |
| 1. 922 | 1.922 | 201 | |
| 2.050 | 2.050 | 202 | |
| 2.833 | 2.833 | 301 | |
| Mesophase (lattice constants) | Reflections/nm | Miller indices (h k l) | |
|---|---|---|---|
| qobsd | qcalcd | ||
| Colh at 30 °C a = 7.4 nm | 0.983 | 0.983 | 100 |
| 1.694 | 1.694 | 110 | |
| 1.965 | 1.965 | 200 | |
| 2.590 | 2.591 | 210 | |
| 2.946 | 2.947 | 300 | |
| Mtet at 160 °C a = 9.8 nm b = 9.02nm | 0.911 | 0.911 | 110 |
| 1.396 | 1.396 | 002 | |
| 1.594 | 1. 594 | 211 | |
| 1.809 | 1.808 | 220 | |
| 2.308 | 2.307 | 230 | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Z.; Lan, Y.; Zhong, K.; Liang, Y.; Chen, T.; Jin, L.Y. Liquid Crystalline Assembly of Coil-Rod-Coil Molecules with Lateral Methyl Groups into 3-D Hexagonal and Tetragonal Assemblies. Int. J. Mol. Sci. 2014, 15, 5634-5648. https://doi.org/10.3390/ijms15045634
Wang Z, Lan Y, Zhong K, Liang Y, Chen T, Jin LY. Liquid Crystalline Assembly of Coil-Rod-Coil Molecules with Lateral Methyl Groups into 3-D Hexagonal and Tetragonal Assemblies. International Journal of Molecular Sciences. 2014; 15(4):5634-5648. https://doi.org/10.3390/ijms15045634
Chicago/Turabian StyleWang, Zhuoshi, Yu Lan, Keli Zhong, Yongri Liang, Tie Chen, and Long Yi Jin. 2014. "Liquid Crystalline Assembly of Coil-Rod-Coil Molecules with Lateral Methyl Groups into 3-D Hexagonal and Tetragonal Assemblies" International Journal of Molecular Sciences 15, no. 4: 5634-5648. https://doi.org/10.3390/ijms15045634





