Human Adipose-Derived Mesenchymal Stem Cells Are Resistant to HBV Infection during Differentiation into Hepatocytes in Vitro
Abstract
:1. Introduction
2. Results and Discussion
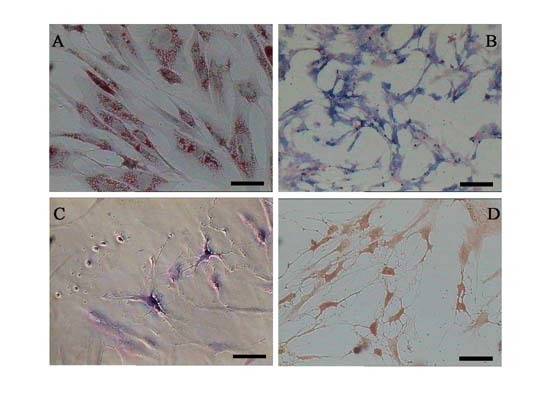
2.1. Characterization of Adipose-Derived Mesenchymal Stem Cells (AD-MSCs)
2.2. AD-MSCs Differentiated into Functional Hepatocytes
2.3. Hepatitis B Virus (HBV) Failed to Infect AD-MSCs and Hepatic Differentiation AD-MSCs
3. Experimental Section
3.1. Adipose Tissue and Bone Marrow from Human Subjects
3.2. MSCs Isolation and Culture
3.3. Cell Line
3.4. Infection Serum Source
3.5. MTT Assay for Cells Growth Curve
3.6. Analysis of MSCs DNA Content
3.7. Flow Cytometric Analysis Cell Surface Phenotype
3.8. Differentiation of MSCs into Adipocyt, Osteoblasts and Neurons
3.9. Hepatic Differentiation Protocol
3.10. AD-MSCs and Hepatocyte-Like Cells Derived from AD-MSCs HBV Infection
3.11. Immunocytochemical Analysis
3.12. Western Blotting Analysis
3.13. Enzyme-Linked Immunosorbent Assay (ELISA) for Albumin Secretion
3.14. Periodic Acid-Schiff (PAS) Staining for Glycogen Deposits
3.15. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Liaw, Y.F.; Chu, C.M. Hepatitis B virus infection. Lancet 2009, 337, 582–592. [Google Scholar]
- Navarro-Alvarez, N.; Soto-Gutierre, A. Hopatocyte transplantation: A step forward. Organ Transplant 2007, 12, 652–658. [Google Scholar]
- Dhawan, A.; Puppi, J. Human hepatocyte transplantation: Current experience and future challenges. Nat. Rev. Gastroenterol. Hepatol 2010, 7, 288–298. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar]
- In’t Anker, P.S.; Scherjon, S.A. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar]
- Bieback, K.; Kern, S. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar]
- Shih, D.T.; Lee, D.C. Isolation and characterization of neurogenic masenchymal stem cells in human scalp tissue. Stem Cells 2005, 23, 1012–1020. [Google Scholar]
- Mezey, E.; Chandross, K.J. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000, 290, 1779–1782. [Google Scholar]
- Krause, D.S.; Theise, N.D. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001, 105, 369–377. [Google Scholar]
- Woodbury, D.; Schwarz, E.J. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res 2000, 61, 364–370. [Google Scholar]
- Schwartz, R.E.; Reyes, M. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J. Clin. Investig 2002, 109, 1291–1302. [Google Scholar] [Green Version]
- Kuo, T.K.; Hung, S.P. Stem cell therapy for liver disease: Parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology 2008, 134, 2111–2121. [Google Scholar]
- Mohamadnejad, M.; Alimoghaddam, K. Phase I trial of autologous one marrow mesenchymal stem cells transplantation in patients with decompensated liver cirrhosis. Arch. Iran. Med 2007, 10, 459–466. [Google Scholar]
- Zhong, Y.S.; Lin, N. Deficient proliferation of bone marrow-derived mesenchymal stem cells in patients with chronic hepatitis B viral infections and cirrhosis of the liver. Dig. Dis. Sci 2010, 55, 438–445. [Google Scholar]
- Zuk, P.A.; Zhu, M. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 2001, 7, 211–228. [Google Scholar]
- Zuk, P.A.; Zhu, M. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar]
- Kern, S.; Eichler, H. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar]
- Izadpanah, R.; Trygg, C. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J. Cell. Biochem 2006, 99, 1285–1297. [Google Scholar]
- Banas, A.; Teratani, T. Adipose tissue-derived mesenchymal stem cells as a sources of human hepatocytes. Hepatology 2007, 46, 219–228. [Google Scholar]
- Talens-Visconto, R.; Bonora, A. Human mesenchymal stem cells from adipose tissue: Differertiation into hepatic lineage. Toxicol. in Vitro 2007, 21, 324–329. [Google Scholar]
- Aurich, H.; Sgodda, M. Hepatocyte differentiation of mesenchymal stem cell from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 2009, 58, 570–581. [Google Scholar]
- Meirelles, L.S.; Nardi, N.B. Methodology, biology and clinical applications of mesenchymal stem cells. Front. Biosci 2009, 14, 4281–4298. [Google Scholar]
- Aurich, I.; Mueller, L.P. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut 2007, 56, 405–415. [Google Scholar]
- Batholomew, A.; Sturgeon, C. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar]
- De Ugarte, D.A.; Morizono, K. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar]
- Seo, M.J.; Such, S.Y. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 328, 258–264. [Google Scholar]
- Fan, B.J.; Piao, Y.F. Bone marrow mesenchymal stem cell from chronic hepatitis B patients differentiation into hepatocyte-like cells. Afr. J. Microbiol. Res 2012, 6, 3866–3873. [Google Scholar]
- Dominici, M.; Le Blanc, K. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar]
- Okura, H.; Komoda, H. Properties of hepatocyte-like cell clusters from human adipose tissue-derived mesenchymal stem cells. Tissue Eng 2010, 16, 761–770. [Google Scholar]
- Lee, J.H.; Lee, K.H. Possibility of undifferentiated human thigh adipose stem cells differentiating into functional hepatocyte. Arch. Plast. Surg 2012, 39, 593–599. [Google Scholar]
- Schmelzer, E.; Wauthier, E.; Reid, L.M. The phenotype of pluripotent human hepatic progenitors. Stem Cells 2006, 24, 1852–1858. [Google Scholar]
- Xie, C.; Zheng, Y.B. Human bone marrow mesenchymal stem cells are resistant to HBV infection during differentiation into hepatocytes in vivo and in vitro. Cell Biol. Int. 2009, 33, 493–500. [Google Scholar]
- Ma, R.; Xing, Q. Hepatitis B virus infection and replication in human bone marrow mesenchymal stem cell. Virol. J 2011, 8, 486–494. [Google Scholar]
- Alonso, M.; Claros, S. The effect of type I collagen on osteochondrogenic differentiation in adipose-derived stromal cellsin vivo. Cytotherapy 2008, 10, 597–610. [Google Scholar]












© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.; Wang, F.; Zhao, H.; Zhang, X.; Chen, H.; Zhang, K. Human Adipose-Derived Mesenchymal Stem Cells Are Resistant to HBV Infection during Differentiation into Hepatocytes in Vitro. Int. J. Mol. Sci. 2014, 15, 6096-6110. https://doi.org/10.3390/ijms15046096
Wang Y, Wang F, Zhao H, Zhang X, Chen H, Zhang K. Human Adipose-Derived Mesenchymal Stem Cells Are Resistant to HBV Infection during Differentiation into Hepatocytes in Vitro. International Journal of Molecular Sciences. 2014; 15(4):6096-6110. https://doi.org/10.3390/ijms15046096
Chicago/Turabian StyleWang, Ying, Feng Wang, Hongchang Zhao, Xiaohe Zhang, Haiying Chen, and Kaiyu Zhang. 2014. "Human Adipose-Derived Mesenchymal Stem Cells Are Resistant to HBV Infection during Differentiation into Hepatocytes in Vitro" International Journal of Molecular Sciences 15, no. 4: 6096-6110. https://doi.org/10.3390/ijms15046096