The Biomolecular Basis of Adipogenic Differentiation of Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Adipose-Derived Stem Cells: Phenotypic Characterization
3. The Receptor Tyrosine Kinase Family
4. Akt Signaling and Cancer
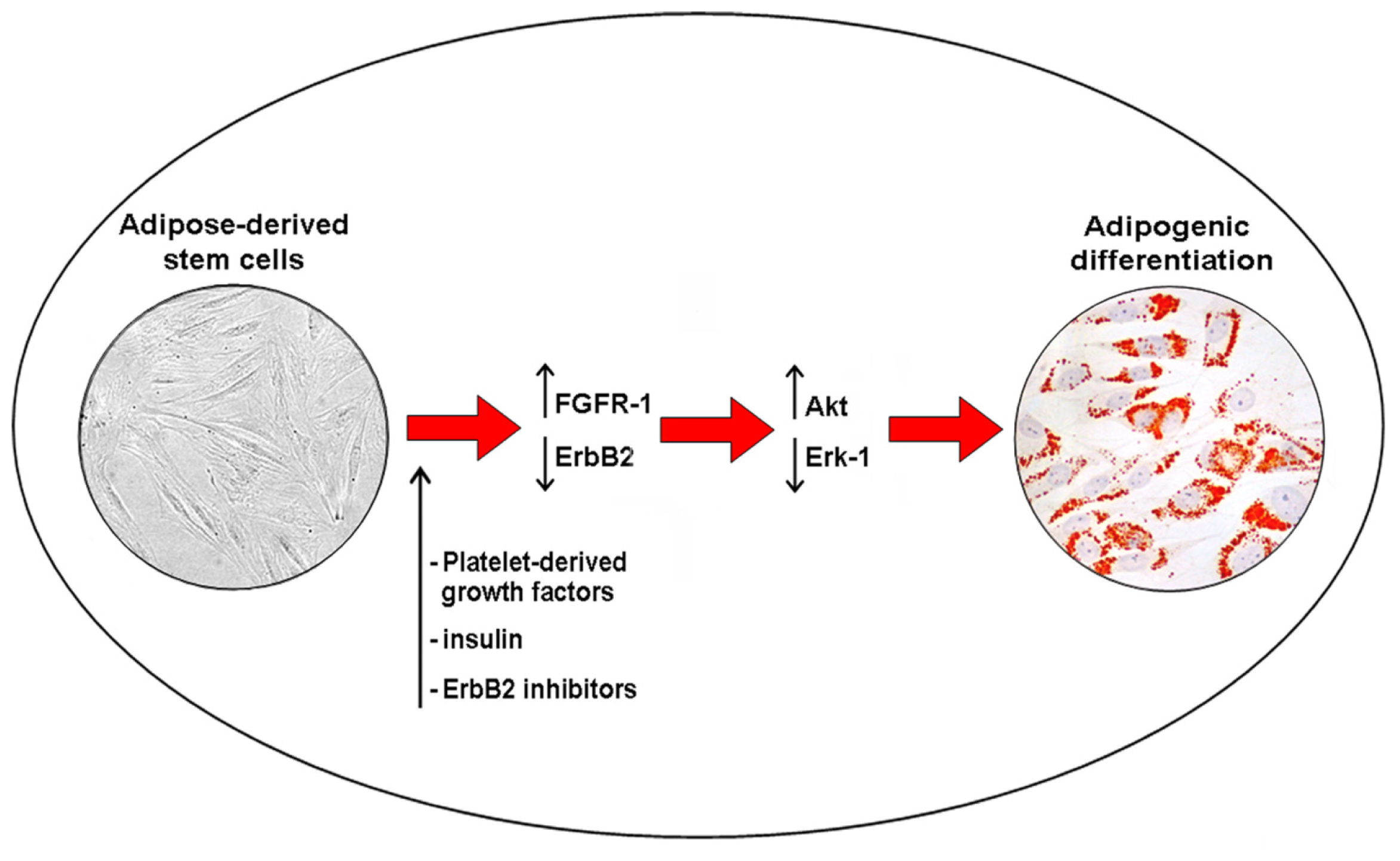
5. Akt Signaling and Adipogenic Differention of ASCs
6. Adipose-Derived Stem Cell Angiogenesis and Adipogenesis: Crosstalk and Similarities
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res 2008, 102, 77–85. [Google Scholar]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res 2007, 100, 1249–1260. [Google Scholar]
- Cervelli, V.; Scioli, M.G.; Gentile, P.; Doldo, E.; Bonanno, E.; Spagnoli, L.G.; Orlandi, A. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl. Med 2012, 1, 206–220. [Google Scholar]
- Gentile, P.; Orlandi, A.; Scioli, M.G.; di Pasquali, C.; Bocchini, I.; Cervelli, V. Concise review: Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl. Med 2012, 1, 230–236. [Google Scholar]
- Heng, B.C.; Liu, H.; Cao, T. Transplanted human embryonic stem cells as biological “catalysts” for tissue repair and regeneration. Med. Hypotheses 2005, 64, 1085–1088. [Google Scholar]
- Spitalieri, P.; Quitadamo, M.C.; Orlandi, A.; Guerra, L.; Giardina, E.; Casavola, V.; Novelli, G.; Saltini, C.; Sangiuolo, F. Rescue of murine silica-induced lung injury and fibrosis by human embryonic stem cells. Eur. Respir. J 2012, 39, 446–457. [Google Scholar]
- Xu, Y.; Malladi, P.; Wagner, D.R.; Longaker, M.T. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Curr. Opin. Mol. Ther 2005, 7, 300–305. [Google Scholar]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signaling. Nature 2001, 411, 355–365. [Google Scholar]
- Ullrich, A.; Schlessinger, J. Signal transduction by receptors with tyrosine kinase activity. Cell 1990, 61, 203–212. [Google Scholar]
- Bafico, A.; Aaronson, S.A. Signaling pathways of tyrosine kinase receptors. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Eds.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Marshall, C.J. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell 1995, 80, 179–185. [Google Scholar]
- Schlessinger, J. SH2/SH3 signaling proteins. Curr. Opin. Genet. Dev 1994, 4, 25–30. [Google Scholar]
- Zhao, M. PTEN: A promising pharmacological target to enhance epithelial wound healing. Br. J. Pharmacol 2007, 152, 1141–1144. [Google Scholar]
- Ciuffreda, L.; di Sanza, C.; Cesta Incani, U.; Eramo, A.; Desideri, M.; Biagioni, F.; Passeri, D.; Falcone, I.; Sette, G.; Bergamo, P.; et al. The mitogen-activated protein kinase (MAPK) cascade controls phosphatase and tensin homolog (PTEN) expression through multiple mechanisms. J. Mol. Med. (Berl.) 2012, 90, 667–679. [Google Scholar]
- Trisciuoglio, D.; de Luca, T.; Desideri, M.; Passeri, D.; Gabellini, C.; Scarpino, S.; Liang, C.; Orlandi, A.; del Bufalo, D. Removal of the BH4 domain from Bcl-2 protein triggers an autophagic process that impairs tumor growth. Neoplasia 2013, 15, 315–327. [Google Scholar]
- Wendel, H.G.; de Stanchina, E.; Fridman, J.S.; Malina, A.; Ray, S.; Kogan, S.; Cordon-Cardo, C.; Pelletier, J.; Lowe, S.W. Survival signaling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 2004, 428, 332–337. [Google Scholar]
- Le Page, C.; Koumakpayi, I.H.; Alam-Fahmy, M.; Mes-Masson, A.M.; Saad, F. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br. J. Cancer 2006, 94, 1906–1912. [Google Scholar]
- Torsvik, A.; Bjerkvig, R. Mesenchymal stem cell signaling in cancer progression. Cancer Treat. Rev 2013, 39, 180–188. [Google Scholar]
- Flågeng, M.H.; Knappskog, S.; Haynes, B.P.; Lønning, P.E.; Mellgren, G. Inverse regulation of EGFR/HER1 and HER2–4 in normal and malignant human breast tissue. PLoS One 2013, 8, e74618. [Google Scholar]
- Nguyen, P.T.; Tsunematsu, T.; Yanagisawa, S.; Kudo, Y.; Miyauchi, M.; Kamata, N.; Takata, T. The FGFR1 inhibitor PD173074 induces mesenchymal-epithelial transition through the transcription factor AP-1. Br. J. Cancer 2013, 109, 2248–2258. [Google Scholar]
- Quarto, N.; Longaker, M.T. Differential expression of specific FGF ligands and receptor isoforms during osteogenic differentiation of mouse Adipose-derived Stem Cells (mASCs) recapitulates the in vivo osteogenic pattern. Gene 2008, 424, 130–140. [Google Scholar]
- Liu, B.; Ordonez-Ercan, D.; Fan, Z.; Huang, X.; Edgerton, S.M.; Yang, X.; Thor, A.D. Estrogenic promotion of ErbB2 tyrosine kinase activity in mammary tumor cells requires activation of ErbB3 signaling. Mol. Cancer Res 2009, 7, 1882–1892. [Google Scholar]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [Green Version]
- Zhang, H.H.; Huang, J.; Düvel, K.; Boback, B.; Wu, S.; Squillace, R.M.; Wu, C.L.; Manning, B.D. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One 2009, 4, e6189. [Google Scholar]
- Fasshauer, M.; Klein, J.; Kriauciunas, K.M.; Ueki, K.; Benito, M.; Kahn, C.R. Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol. Cell. Biol 2001, 21, 319–329. [Google Scholar]
- Normanno, N.; di Maio, M.; de Maio, E.; de Luca, A.; de Matteis, A.; Giordano, A.; Perrone, F. NCI-Naple Breast Cancer Group. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr.-Relat. Cancer 2005, 12, 721–747. [Google Scholar]
- Grose, R.; Fantl, V.; Werner, S.; Chioni, A.M.; Jarosz, M.; Rudling, R.; Cross, B.; Hart, I.R.; Dickson, C. The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. EMBO J 2007, 26, 1268–1278. [Google Scholar]
- Turner, N.; Pearson, A.; Sharpe, R.; Lambros, M.; Geyer, F.; Lopez-Garcia, M.A.; Natrajan, R.; Marchio, C.; Iorns, E.; Mackay, A.; et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 2010, 70, 2085–2094. [Google Scholar]
- Cao, Y. Angiogenesis modulates adipogenesis and obesity. J. Clin. Investig 2007, 117, 2362–2368. [Google Scholar]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res 2004, 94, 678–685. [Google Scholar]
- Selgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther 2010, 5, 103–110. [Google Scholar]
- Cesca, M.; Bizzarro, F.; Zucchetti, M.; Gavazzi, R. Tumor delivery of chemotherapy combined with inhibitors of angiogenesis and vascular targeting agents. Front. Oncol 2013, 3, 259. [Google Scholar]
- Tarallo, V.; Vesci, L.; Capasso, O.; Esposito, M.T.; Riccioni, T.; Pastore, L.; Orlandi, A.; Pisano, C.; de Falco, S. A placental growth factor variant unable to recognize vascular endothelial growth factor (VEGF) receptor-1 inhibits VEGF-dependent tumor angiogenesis via heterodimerization. Cancer Res 2010, 70, 1804–1813. [Google Scholar]
- Cassinelli, G.; Zuco, V.; Petrangolini, G.; de Cesare, M.; Tortoreto, M.; Lanzi, C.; Cominetti, D.; Zaffaroni, N.; Orlandi, A.; Passeri, D.; et al. The curative efficacy of namitecan (ST1968) in preclinical models of pediatric sarcoma is associated with antiangiogenic effects. Biochem. Pharmacol 2012, 84, 163–171. [Google Scholar]
- Orlandi, A.; Bennett, M. Progenitor cell-derived smooth muscle cells in vascular disease. Biochem. Pharmacol 2010, 79, 1706–1713. [Google Scholar]
- Ferlosio, A.; Arcuri, G.; Doldo, E.; Scioli, M.G.; de Falco, S.; Spagnoli, L.G.; Orlandi, A. Age-related increase of stem marker expression influences vascular smooth muscle cell properties. Atherosclerosis 2012, 224, 51–57. [Google Scholar]
- Campagnolo, L.; Costanza, G.; Francesconi, A.; Arcuri, G.; Moscatelli, I.; Orlandi, A. Sortilin expression is essential for pro-nerve growth factor-induced apoptosis of rat vascular smooth muscle cells. PLoS One 2014, 9, e84969. [Google Scholar]
- Bekhite, M.M.; Finkensieper, A.; Rebhan, J.; Huse, S.; Schultze-Mosgau, S.; Figulla, H.R.; Sauer, H.; Wartenberg, M. Hypoxia, leptin, and vascular endothelial growth factor stimulate vascular endothelial cell differentiation of human adipose tissue-derived stem cells. Stem Cells Dev 2014, 23, 333–351. [Google Scholar]
- Song, S.H.; Lee, M.O.; Lee, J.S.; Jeong, H.C.; Kim, H.G.; Kim, W.S.; Hur, M.; Cha, H.J. Genetic modification of human adipose-derived stem cells for promoting wound healing. J. Dermatol. Sci 2012, 66, 98–107. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Scioli, M.G.; Bielli, A.; Gentile, P.; Mazzaglia, D.; Cervelli, V.; Orlandi, A. The Biomolecular Basis of Adipogenic Differentiation of Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2014, 15, 6517-6526. https://doi.org/10.3390/ijms15046517
Scioli MG, Bielli A, Gentile P, Mazzaglia D, Cervelli V, Orlandi A. The Biomolecular Basis of Adipogenic Differentiation of Adipose-Derived Stem Cells. International Journal of Molecular Sciences. 2014; 15(4):6517-6526. https://doi.org/10.3390/ijms15046517
Chicago/Turabian StyleScioli, Maria Giovanna, Alessandra Bielli, Pietro Gentile, Donatella Mazzaglia, Valerio Cervelli, and Augusto Orlandi. 2014. "The Biomolecular Basis of Adipogenic Differentiation of Adipose-Derived Stem Cells" International Journal of Molecular Sciences 15, no. 4: 6517-6526. https://doi.org/10.3390/ijms15046517





