Synthesis, Properties and Applications of Biodegradable Polymers Derived from Diols and Dicarboxylic Acids: From Polyesters to Poly(ester amide)s
Abstract
:1. Introduction
2. Synthesis of Poly(alkylene dicarboxylate)s
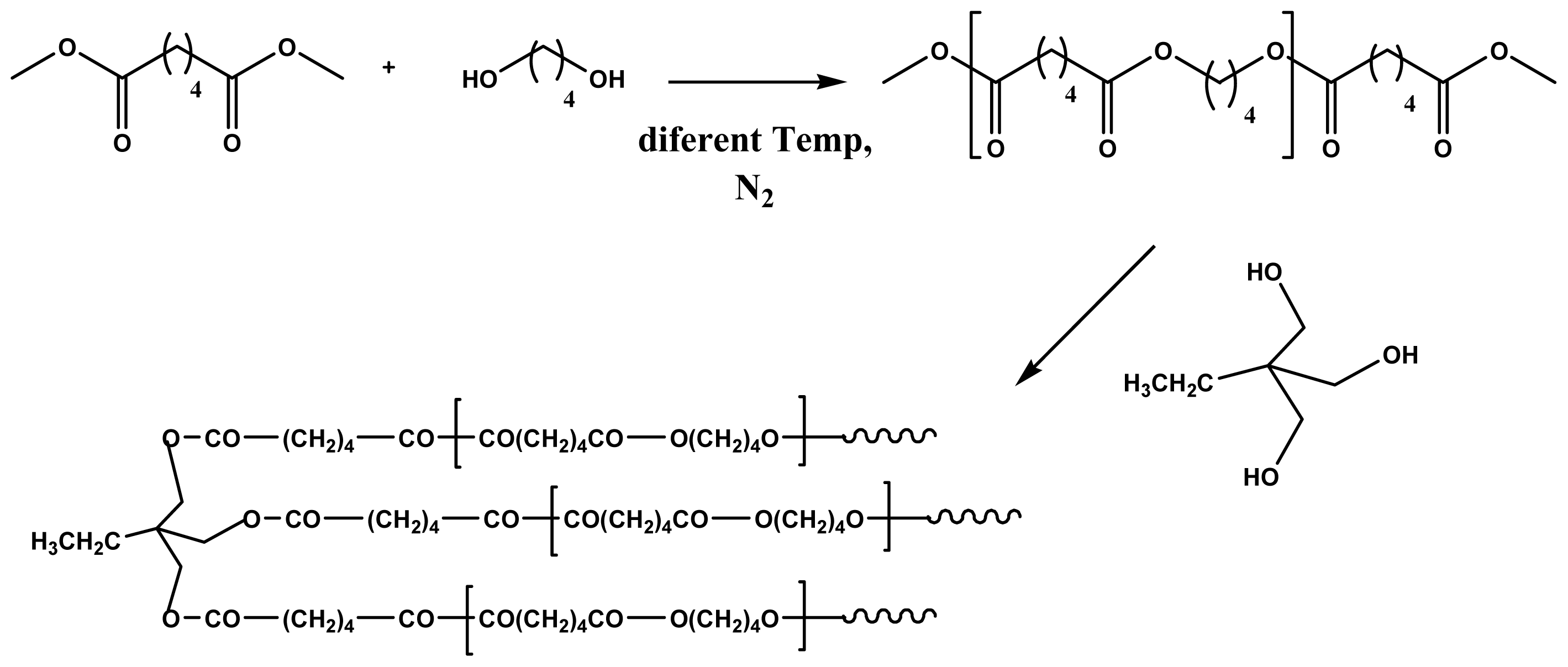
2.1. Thermal Polycondensation
2.2. Polycondensation Catalysts
2.3. Ring Opening Polymerization Methods
2.4. Poly(alkylene dicarboxylate)s from Biobased Monomers
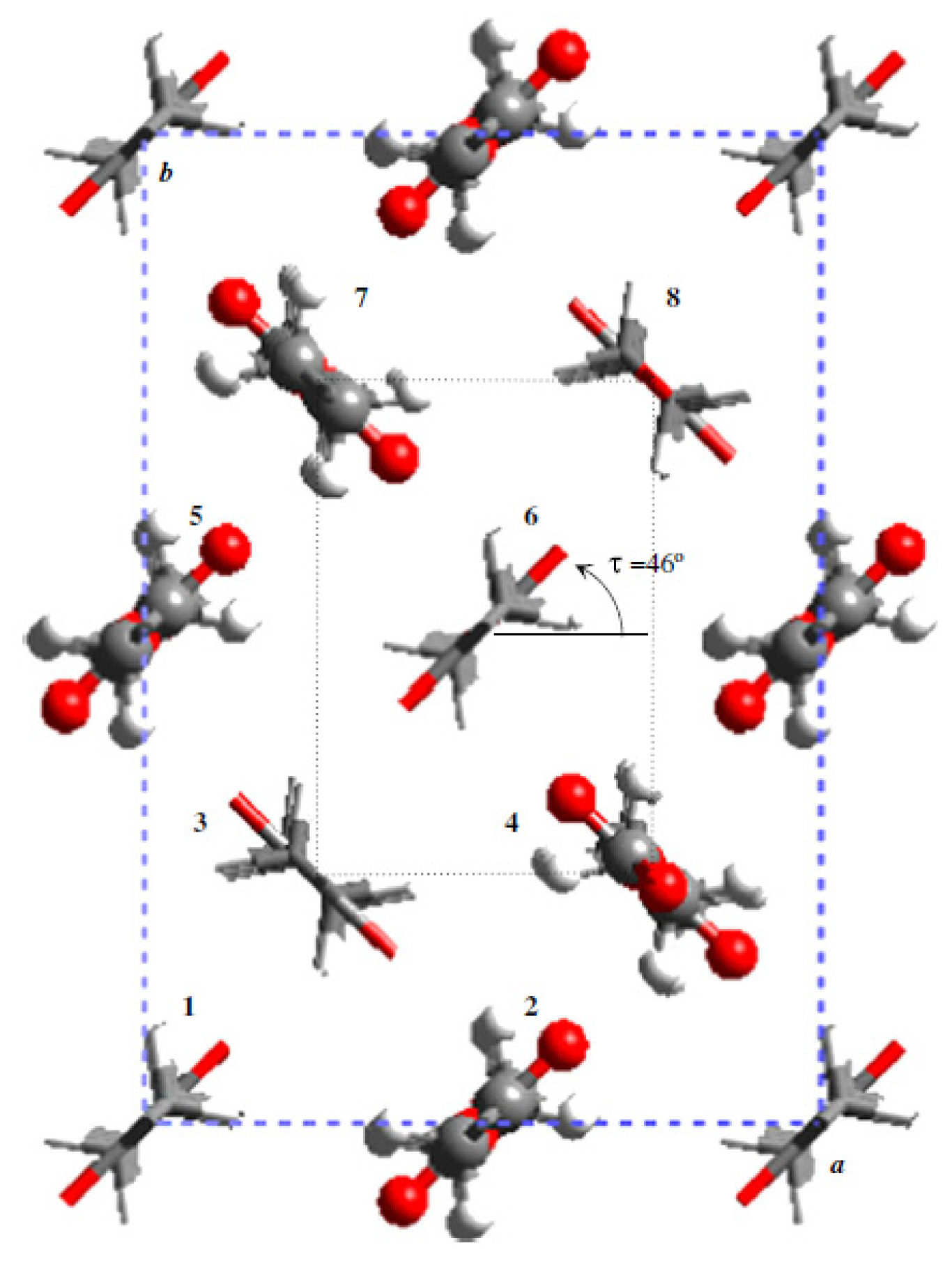
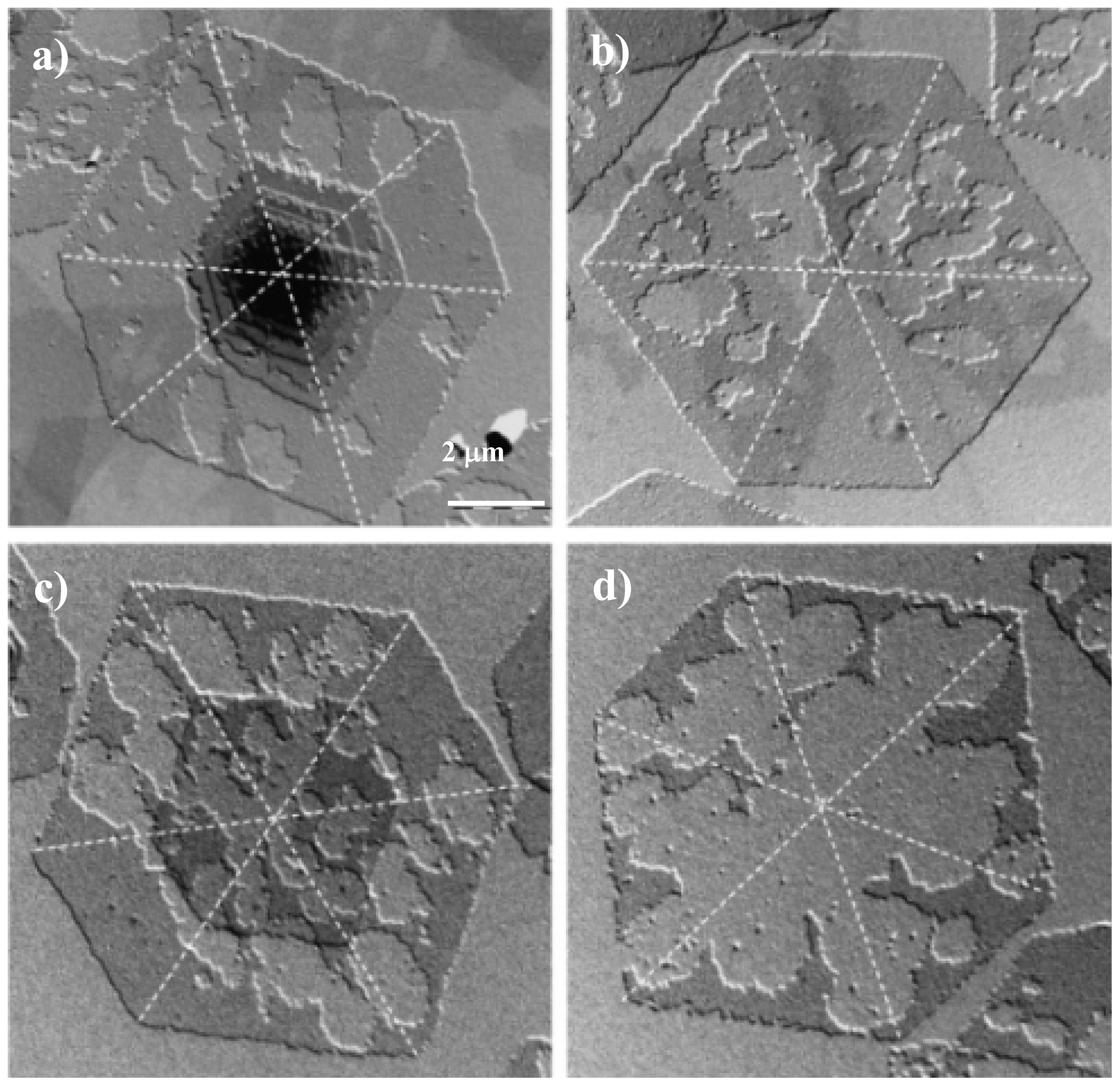
3. Crystalline Structure of Poly(alkylene dicarboxylate)s
4. Copolymers Constituted by Different Diol or/and Dicarboxylic Acid Units
5. Biodegradable Poly(alkylene dicarboxylate)s Having Rigid Aromatic or Carbohydrate Units
6. Degradation of Poly(alkylene dicarboxylate)s
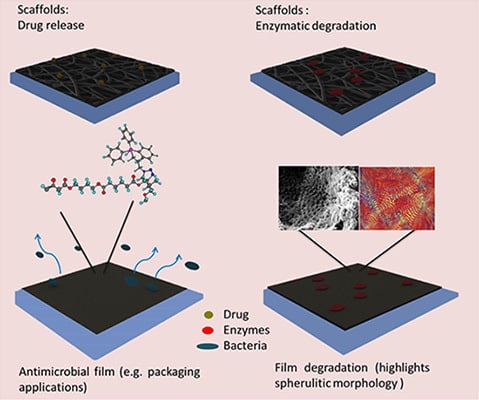
7. Applications of Poly(alkylene dicarboxylate)s
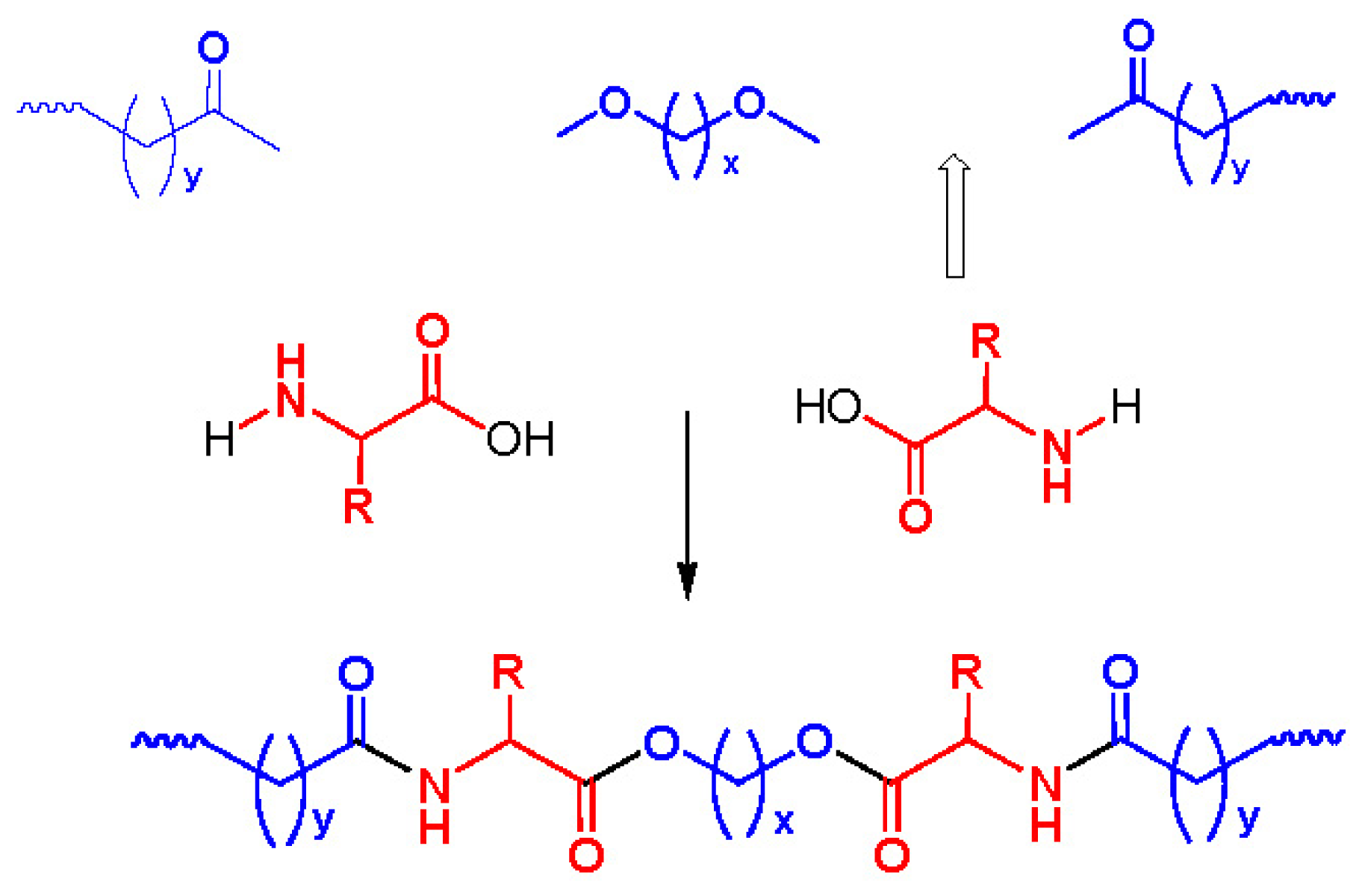
8. Poly(ester amide)s Derived from the Incorporation of Natural α-Amino Acids to Polyalkylene Dicarboxylates
8.1. Monomers for Synthesizing AABBP
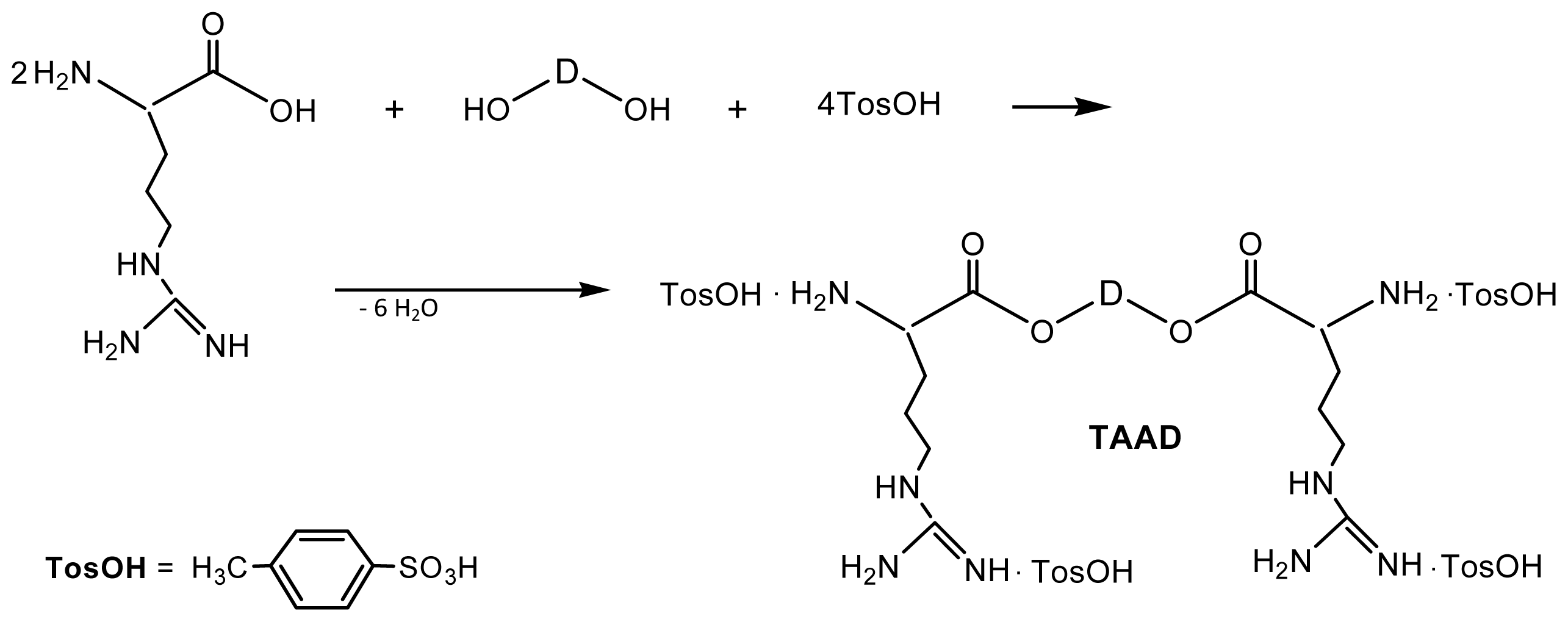
8.1.1. AA Based Monomers
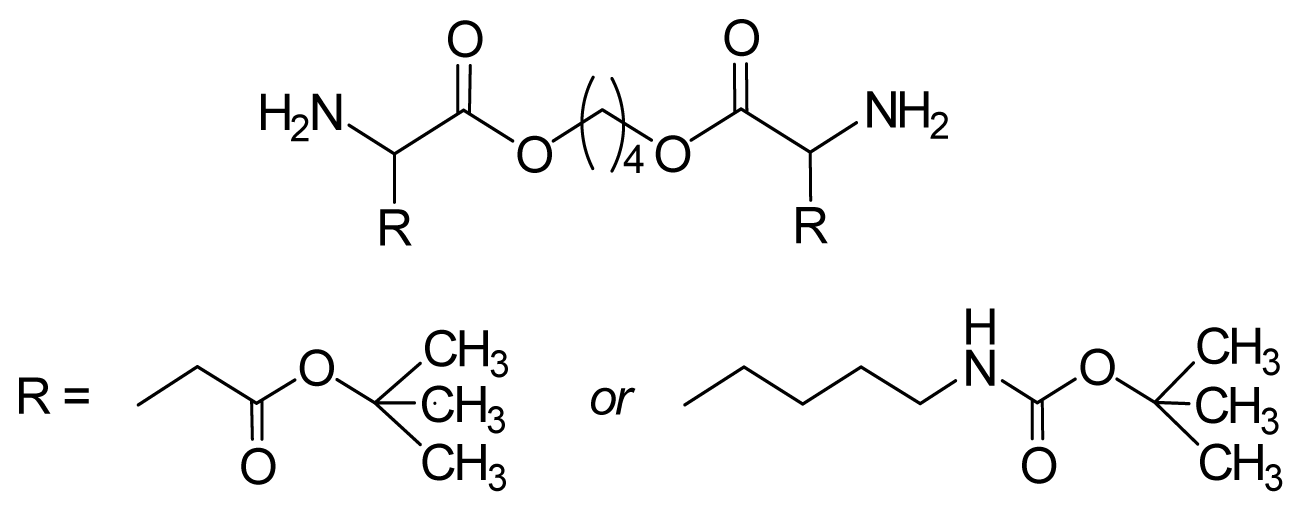
8.1.2. Monomers—Counter-Partners for Synthesizing AABBP
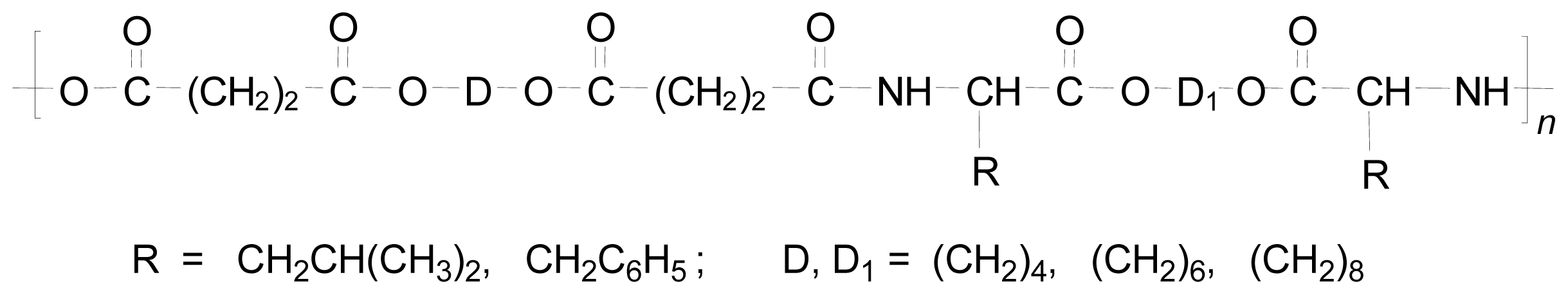
8.2. AABBP Made of AAAD Monomers
8.2.1. AABBP via Solution Active Polycondensation
Regular AABBP and Related Polymers
Functional AABBP
8.2.2. AABBP via Interfacial Polycondensation
8.3. AABBP Made of DABA-Based Monomers
8.3.1. AABBP via Thermal and Biocatalytic Polycondensation
8.3.2. AABBP via Azlactone Method
8.3.3. AABBP via Nucleophilic Substitution (NS)
8.4. AABBP Made by Combining AAAD-S and DABA Monomers
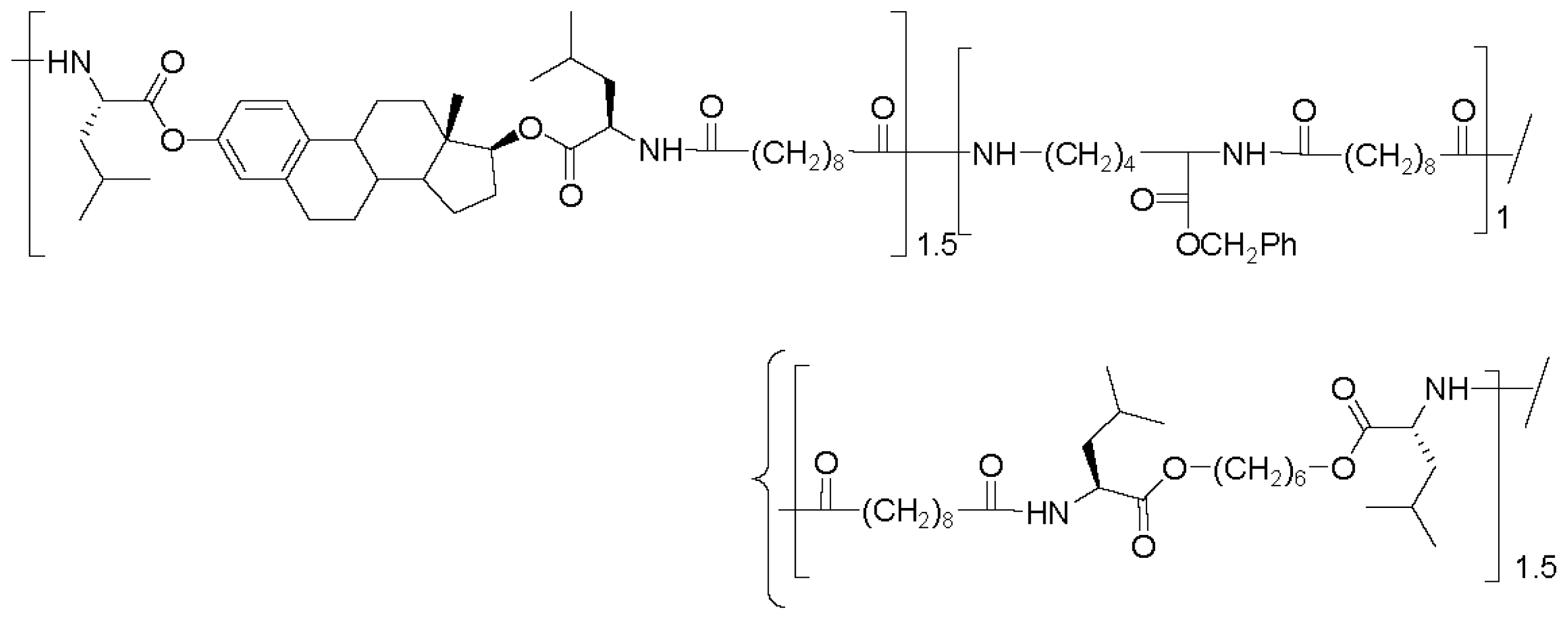
8.5. Biocompatibility and Some Applications of AABBP
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lu, T.S.; Sun, Y.M.; Wang, C.S. Novel copolyesters containing naphthalene structure. II. Copolyesters prepared from 2,6-dimethyl naphthalate, 1,4-dimethyl terephthalate, and ethylene glycol. J. Polym. Sci. A 1995, 33, 2841–2850. [Google Scholar]
- Byrom, D. Polymer synthesis by microorganisms: Technology and economics. Trends Biotechnol 1987, 5, 246–250. [Google Scholar]
- Raquez, J.M.; Nabar, Y.; Narayan, R.; Dubois, P. Novel high-performance talc/poly[(butylene adipate)-co-terephthalate)] hybrid materials. Macromol. Mater. Eng 2008, 293, 310–320. [Google Scholar]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B 2011, 49, 832–864. [Google Scholar]
- Seyednejad, H.; Ghassemi, A.H.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar]
- Vert, M. Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci 2002, 27, 87–133. [Google Scholar]
- Sharma, V.; Kundu, P.P. Condensation polymers from natural oils. Prog. Polym. Sci 2008, 33, 1199. [Google Scholar]
- Di Lorenzo, M.L.; Raimo, M.; Cascone, E.; Martuscelli, E. Poly(3-hydroxybutyrate)-based copolymers and blends: Influence of a second component on crystallization and thermal behavior. J. Macromol. Sci. B 2001, 40, 639–667. [Google Scholar]
- Andrade, A.P.; Witholt, B.; Chang, D.L.; Li, Z. Synthesis and characterization of novel thermoplastic polyester containing blocks of poly[(R)-3-hydroxyoctanoate] and poly[(R)-3-hydroxybutyrate. Macromolecules 2003, 36, 9830–9835. [Google Scholar]
- Han, Y.K.; Um, J.W.; Im, S.S.; Kim, B.C. Synthesis and characterization of high molecular weight branched PBA. J. Polym. Sci. A 2001, 39, 2143–2150. [Google Scholar]
- Zhu, C.Y.; Zhang, Z.Q.; Liu, Q.P.; Wang, Z.P.; Jin, J. Synthesis and biodegradation of aliphatic polyesters from dicarboxylic acids and diols. J. Appl. Polym. Sci 2003, 90, 982–990. [Google Scholar]
- Saulnier, B.; Coudane, J.; Garreau, H.; Vert, M. Hydroxyl-bearing poly(α-hydroxy acid)-type aliphatic degradable polyesters prepared by ring opening (co)polymerization of dilactones based on glycolic, gluconic and l-lactic acids. Polymer 2006, 47, 1921–1929. [Google Scholar]
- Kricheldorf, H.R.; Rabenstein, M.; Maskos, M.; Schmidt, M. Macrocycles. 15. The role of cyclization in kinetically controlled polycondensations. 1. Polyester syntheses. Macromolecules 2001, 34, 713–722. [Google Scholar]
- Ahn, B.D.; Kim, S.H.; Kim, Y.H.; Yang, J.S. Synthesis and characterization of the biodegradable copolymers from succinic acid and adipic acid with 1,4-butanediol. J. Appl. Polym. Sci 2001, 82, 2808–2826. [Google Scholar]
- Kricheldorf, H.R.; Behnken, G.; Schwarz, G. Telechelic polyesters of ethane diol and adipic or sebacic acid by meansof bismuth carboxylates as non-toxic catalysts. Polymer 2005, 46, 11219–11224. [Google Scholar]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev 2001, 101, 3793–3818. [Google Scholar]
- Olson, D.A.; Sheares, V.V. Preparation of unsaturated linear aliphatic polyesters using condensation polymerization. Macromolecules 2006, 39, 2808–2814. [Google Scholar]
- Liu, W.; Chen, B.; Wang, F.; Tan, T.; Deng, L. Lipase-catalyzed synthesis of aliphatic polyesters and properties characterization. Proc. Biochem 2011, 46, 1993–2000. [Google Scholar]
- Binns, F.; Roberts, M.S.; Taylor, A.; Williams, C.F. Enzymatic polymerization of an unactivated diol diacid system. J. Chem. Soc. Perkin Trans 1993, 1, 899–904. [Google Scholar]
- Linko, Y.Y.; Wang, Z.L.; Seppala, J. Lipase-catalyzed synthesis of poly(1,4-butyl sebacate) from sebacic acid or its derivatives with BDO. J. Biotechnol 1995, 40, 133–138. [Google Scholar]
- Binns, F.; Harffey, P.; Roberts, S.M.; Taylor, A. Studies of lipase-catalyzed polyesterification of an unactivated diacid/diol system. J. Polym. Sci. A 1998, 36, 2069–2079. [Google Scholar]
- Uyama, H.; Inada, K.; Kobayashi, S. Lipase-catalyzed synthesis of aliphatic polyesters by polycondensation of dicarboxylic acids and glycols in solvent-free system. Polym. J 2000, 32, 440–443. [Google Scholar]
- Kobayashi, S.; Uyama, H.; Namekawa, S. In vitro biosynthesis of polyesters with isolated enzymes in aqueous systems and organic solvents. Polym. Degrad. Stab 1998, 59, 195–201. [Google Scholar]
- Yang, J.; Zhang, S.; Liu, X.; Cao, A. A study on biodegradable aliphatic poly(tetramethylene succinate): The catalyst dependences of polyester syntheses and their thermal stabilities. Polym. Degrad. Stab 2003, 81, 1–7. [Google Scholar]
- Lim, J.S.; Hong, S.M.; Kim, D.K.; Im, S.S. Effect of isocyanate-modified fumed silica on the properties of poly(butylene succinate) nanocomposites. J. Appl. Polym. Sci 2008, 107, 3598–3608. [Google Scholar]
- Bikiaris, D.N.; Achilias, D.S. Synthesis of poly(alkylene succinate) biodegradable polyesters I. Mathematical modelling of the esterification reaction. Polymer 2006, 47, 4851–4860. [Google Scholar]
- Takasu, A.; Iio, Y.; Oishi, Y.; Narukawa, Y.; Hirabayashi, T. Environmentally benign polyester synthesis by room temperature direct polycondensation of dicarboxylic acid and diol. Macromolecules 2005, 38, 1048–1050. [Google Scholar]
- Ishii, M.; Okazaki, M.; Shibasaki, Y.; Ueda, M.; Teranishi, T. Convenient synthesis of aliphatic polyesters by distannoxane-catalyzed polycondensation. Biomacromolecules 2001, 2, 1267–1270. [Google Scholar]
- Yashiro, T.; Kricheldorf, H.R.; Huijser, S. Syntheses of polyesters from succinic anhydride and various diols catalyzed by metal triflates. Macromol. Chem. Phys 2009, 210, 1607–1616. [Google Scholar]
- Buzin, P.; Lahcini, M.; Schwarz, G.; Kricheldorf, H.R. Aliphatic polyesters by bismuth triflate-catalyzed polycondensation of dicarboxylic acids and aliphatic diols. Macromolecules 2008, 41, 8491–8495. [Google Scholar]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.P.; Pascault, J.; Fuertes, P.; Saint-Loup, R. Synthesis and properties of poly(butylene succinate): Efficiency of different transesterification catalysts. J. Polym. Sci. A 2011, 49, 5301–5312. [Google Scholar]
- Takasu, A.; Ishii, M.; Inai, Y.; Hirabayashi, T.; Inomata, K. Threo-disyndiotactic polymerization of (e,e)-alkyl sorbates assisted by bulky organoaluminum lewis acid via “alternating turning over polymerization (atop)” mechanism. Macromolecules 2003, 36, 7055–7064. [Google Scholar]
- Takasu, A.; Oishi, Y.; Iio, Y.; Inai, Y.; Hirabayashi, T. Synthesis of aliphatic polyesters by direct polyesterification of dicarboxylic acids with diols under mild conditions catalyzed by reusable rare-earth triflate. Macromolecules 2003, 36, 1772–1774. [Google Scholar]
- Garaleh, M.; Lahcini, M.; Kricheldorf, H.R.; Weidner, S.M. Syntheses of aliphatic polyesters catalyzed by lanthanide triflates. J. Polym. Sci. A 2009, 47, 170–177. [Google Scholar]
- Sokolsky-Papkova, M.; Langerb, R.; Domb, A.J. Synthesis of aliphatic polyesters by polycondensation using inorganic acid as catalyst. Polym. Adv. Technol 2011, 22, 502–511. [Google Scholar]
- Zhi-Wei, G.; Charles, J.S. Enzymatic synthesis of macrocyclic lactones. J. Am. Chem. Soc 1998, 110, 1999–2001. [Google Scholar]
- Sugihara, S.; Toshima, K.; Matsumura, S. New strategy for enzymatic synthesis of high-molecular-weight poly(butylenesuccinate) via cyclic oligomers. Macromol. Rapid Commun 2006, 27, 203–207. [Google Scholar]
- Habeych, D.I.; Eggink, G.; Boeriu, C.G. Linear and cyclic ester Oligomers of succinic acid and 1,4-butanediol: Biocatalytic synthesis and characterization. Biocatal. Biotransform 2011, 29, 299–310. [Google Scholar]
- Hofvendahl, K.; Hahn-Hagerda, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzym. Microb. Technol 2000, 26, 87–107. [Google Scholar]
- Zeikus, J.G.; Jain, M.K.; Elankovan, P. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol 1999, 51, 545–552. [Google Scholar]
- Polen, T.; Spelberg, M.; Bott, M. Toward biotechnological production of adipic acid and precursors from biorenewables. J. Biotechnol 2013, 167, 75–84. [Google Scholar]
- Chandel, A.K.; Singh, O.V. Weedy lignocellulosic feedstock and microbial metabolic engineering: Advancing the generation of “Biofuel”. Appl. Microbiol. Biotechnol 2011, 89, 1289–1303. [Google Scholar]
- Yang, Y.; Lu, W.; Zhang, X.; Xie, W.; Cai, M.; Gross, R.A. Two-step biocatalytic route to biobased functional polyesters from ω-carboxy fatty acids and diols. Biomacromolecules 2010, 11, 259–268. [Google Scholar]
- Kaur, G.; Srivastava, A.K.; Chand, S. Advances in biotechnological production of 1,3-propanediol. Biochem. Eng. J 2012, 64, 106–118. [Google Scholar]
- Umare, S.S.; Chandure, A.S.; Pandey, R.A. Synthesis, characterization and biodegradable studies of 1,3-propanediol based polyesters. Polym. Degrad. Stab 2007, 92, 464–479. [Google Scholar]
- Quinzler, D.; Mecking, S. Linear semicrystalline polyesters from fatty acids by complete feedstock molecule utilization. Angew. Chem. Int. Ed 2010, 49, 4306–4308. [Google Scholar]
- Stempfle, F.; Quinzler, D.; Heckler, I.; Mecking, S. Long-chain linear C19 and C23 monomers and polycondensates from unsaturated fatty acid esters. Macromolecules 2011, 44, 4159–4166. [Google Scholar]
- Genas, M. Rilsan (Polyamid 11), synthese und eigenschaften. Angew. Chem. Int. Ed 1962, 74, 535–540. [Google Scholar]
- Trzaskowski, J.; Quinzler, D.; Bahrle, C.; Mecking, S. Aliphatic long-chain C20 polyesters from olefin metathesis. Macromol. Rapid Commun 2011, 32, 1352–1356. [Google Scholar]
- Huf, S.; Krügener, S.; Hirth, T.; Rupp, S.; Zibek, S. Biotechnological synthesis of long-chain dicarboxylic acids as building blocks for polymers. Eur. J. Lipid Sci. Technol 2011, 113, 548–561. [Google Scholar]
- Celli, A.; Marchese, P.; Sisti, L.; Dumand, D.; Sullalti, S.; Totaro, G. Effect of 1,4-cyclohexylene units on thermal properties of poly(1,4-cyclohexylenedimethylene adipate) and similar aliphatic polyesters. Polym. Int 2013, 62, 1210–1217. [Google Scholar]
- Berti, C.; Binassi, E.; Colonna, M.; Fiorini, M.; Kannan, G.; Karanam, S. Preparation of Bio-Based 1,4-Cyclohexane Dimethanol Used to Produce Terephthalate Polyesters. U.S. Patent 20100168373, 1 July 2010. [Google Scholar]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci 2010, 35, 578–622. [Google Scholar]
- Kricheldorf, H.R.; Wulff, D.F. Layer structures 11. Cholesteric polyesters derived from isosorbide, 2,5-bis(dodecyloxy)terephthalic acid and 4,49-dihydroxybiphenyl. Polymer 1998, 39, 6145–6151. [Google Scholar]
- Okada, M.; Tachikawa, K.; Aoi, K. Biodegradable polymers based on renewable resources. III. Copolyesters composed of 1,4:3,6-dianhydro-d-glucitol,1,1-bis(5-carboxy-2-furyl)ethane and aliphatic dicarboxylic acid units. J. Appl. Polym. Sci 1999, 74, 3342–3350. [Google Scholar]
- Okada, M.; Tsunoda, K.; Tachikawa, K.; Aoi, K. Biodegradable polymers based on renewable resources. IV. Enzymatic degradation of polyesters composed of 1,4:3.6-dianhydro-d-glucitol and aliphatic dicarboxylic acid moieties. J. Appl. Polym. Sci 2000, 77, 338–346. [Google Scholar]
- Kricheldorf, H.R.; Chatti, S.; Schwarz, G.; Kruger, R.P. Macrocycles 27: Cyclic aliphatic polyesters of isosorbide. J. Polym. Sci. A 2003, 41, 3414–3424. [Google Scholar]
- Ivan, S.; Vukic, R.N.; Cakic, S.; Simendic, O.; Ristic, V.; Budinski-Simendic, J. Synthesis and characterisation of polyester based on isosorbide and butanedioic acid. J. Polym. Environ 2012, 20, 519–527. [Google Scholar]
- Park, H.S.; Gong, M.S.; Knowles, J.C. Synthesis and biocompatibility properties of polyester containing various diacid based on isosorbide. J. Biomater. Appl 2012, 27, 99–109. [Google Scholar]
- Percec, V.; Chu, P.; Kawasumi, M. Toward “Willowlike” thermotropic dendrimers. Macromolecules 1994, 27, 4441–4453. [Google Scholar]
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architecturess synthetic strategies and major characterization aspects. Chem. Rev 2009, 109, 5924–5973. [Google Scholar]
- Wurm, F.; Frey, H. Linear–dendritic block copolymers: The state of the art and exciting perspectives. Prog. Polym. Sci 2011, 36, 1–52. [Google Scholar]
- Samperi, F.; Battiato, S.; Puglisi, C.; Scamporrino, A.; Ambrogi, V.; Ascione, L.; Carfagna, C. Combined techniques for the characterization of linear-hyperbranched hybrid poly(butylene adipate) copolymers. J. Polym. Sci. Part A 2011, 49, 3615–3630. [Google Scholar]
- Fujimaki, T. Processability and properties of aliphatic polyesters, “BIONOLLE”,” synthesized by polycondensation reaction. Polym. Degrad. Stab 1998, 59, 209–214. [Google Scholar]
- Ichikawa, Y.; Mizukoshi, T. Bionolle (polybutylenesuccinate). Adv. Polym. Sci 2012, 245, 285–314. [Google Scholar]
- Zhao, J.B.; Wu, X.F.; Yang, W.T. Synthesis of aliphatic polyesters by a chain-extending reaction with octamethylcyclotetrasilazane and hexaphenylcyclotrisilazane as chain extenders. J. Appl. Polym. Sci 2004, 92, 3333–3337. [Google Scholar]
- Huang, C.Q.; Luo, S.Y.; Xu, S.Y.; Zhao, J.B.; Liang, S.L.; Yang, W.T. Catalyzed chain extension of poly(butylene adipate)and poly(butylene succinate) with 2,2′-(1,4-phenylene)-bis(2-oxazoline). J. Appl. Polym. Sci 2010, 115, 1555–1565. [Google Scholar]
- Mckee, M.G.; Unal, S.; Wilkes, G.L.; Long, T.E. Branched polyesters: Recent advances in synthesis and performance. Prog. Polym. Sci 2005, 30, 507–539. [Google Scholar]
- Chae, H.G.; Kim, B.C.; Im, S.S.; Han, Y.K. Effect of molecular weight and branch structure on the crystallization and rheological properties of poly(butylene adipate). Polym. Eng. Sci 2001, 41, 1133–1139. [Google Scholar]
- Kim, E.K.; Bae, J.S.; Im, S.S.; Kim, B.C.; Han, Y.K. Preparation and properties of branched polybutylenesuccinate. J. Appl. Polym. Sci 2001, 80, 1388–1394. [Google Scholar]
- Wang, G.; Guo, B.; Li, R. Synthesis, characterization, and properties of long-chain branched poly(butylene succinate). J. Appl. Polym. Sci 2012, 124, 1271–1280. [Google Scholar]
- Jin, H.J.; Park, J.K.; Park, K.H.; Kim, M.N.; Yoon, J.S. Properties of aliphatic polyesters with n-paraffinic side branches. J. Appl. Polym. Sci 2000, 77, 547–555. [Google Scholar]
- Kim, M.N.; Kim, K.H.; Jin, H.J.; Park, J.K.; Yoon, J.S. Biodegradability of ethyl and n-octyl branched poly(ethylene adipate) and poly(butylene succinate). Eur. Polym. J 2001, 37, 1843–1847. [Google Scholar]
- Li, F.X.; Xu, X.J.; Li, Q.B.; Li, Y.; Zhang, H.Y.; Yu, J.Y. Thermal degradation and their kinetics of biodegradable poly(butylene succinate-co-butylene terephthalate)s under nitrogen and air atmospheres. Polym. Degrad. Stab 2006, 91, 1685–1693. [Google Scholar]
- Li, F.; Xu, X.J.; Hao, Q.G.; Li, Q.B.; Yu, J.Y.; Cao, A.M. Effects of comonomer sequential structure on thermal and crystallization behaviors of biodegradable poly(butylene succinate-co-butylene terephthalate)s. J. Polym. Sci. B 2006, 44, 1635–1644. [Google Scholar]
- Ueda, A.S.; Chatani, Y.; Tadokoro, H. Structural studies of polyesters. IV. Molecular and crystal structures of poly(ethylene succinate) and poly(ethylene oxalate). Polym. J 1971, 2, 387–397. [Google Scholar]
- Turner-Jones, A.; Bunn, C.W. The crystal structures of polyethylene adipate and polyethylene suberate. Acta Crystallogr 1962, 15, 105–113. [Google Scholar]
- Chatani, Y.; Hasegawa, R.; Tadokoro, H. Structure of polyesters. Polym. Prepr. Jpn 1971, 20, 420. [Google Scholar]
- Minke, R.; Blacwell, J. Polymorphic structures of poly(tetramethylene adipate). J. Macromol. Sci. Phys 1979, B16, 407–417. [Google Scholar]
- Minke, R.; Blacwell, J. Single crystals of poly(tetramethylene adipate). J. Macromol. Sci. Phys 1980, B18, 233–255. [Google Scholar]
- Aylwin, P.A.; Boyd, R.H. Aliphatic polyesters as models for relaxation processes in crystalline polymers: 1. Characterization. Polymer 1984, 25, 323–329. [Google Scholar]
- Alemán, C.; Puiggalí, J. Effect of the folding of methylene units in the conformational preferences of small diesters. J. Org. Chem 1997, 62, 3076–3080. [Google Scholar]
- Kanamoto, T.; Tanaka, K. Growth and morphology of single crystals of linear aliphatic polyesters. J. Polym. Sci. A 1971, 9, 2043–2060. [Google Scholar]
- Brandrup, J.; Immergut, E.H. Polymer Handbook; Wiley-Interscience: New York, NY, USA, 1989. [Google Scholar]
- Liau, W.B.; Boyd, R.H. Structure and packing in crystalline aliphatic polyesters. Macromolecules 1990, 23, 1531–1539. [Google Scholar]
- Ichikawa, Y.; Kondo, H.; Igarashi, Y.; Noguchi, K.; Okuyama, K.; Washiyama, J. Crystal structures of a and b forms of poly(tetramethylene succinate). Polymer 2000, 41, 4719–4727. [Google Scholar]
- Armelin, E.; Casas, M.T.; Puiggalí, J. Structure of poly(hexamethylene sebacate). Polymer 2001, 42, 5695–5699. [Google Scholar]
- Armelin, E.; Almontassir, A.; Franco, L.; Puiggalí, J. Crystalline structure of poly(dodecamethylene sebacate). Repercussions on lamellar folding surfaces. Macromolecules 2002, 35, 3630–3635. [Google Scholar]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci 2009, 34, 605–640. [Google Scholar]
- Almontassir, A.; Gestí, S.; Franco, L.; Puiggalí, J. Molecular packing of polyesters derived from 1,4-butanediol and even aliphatic dicarboxylic acids. Macromolecules 2004, 37, 5300–5309. [Google Scholar]
- Gestí, S.; Almontassir, A.; Casas, M.T.; Puiggalí, J. Molecular packing and crystalline morphologies of biodegradable poly(alkylene dicarboxylate)s derived from 1,6-hexanediol. Polymer 2004, 45, 8845–8861. [Google Scholar]
- Gestí, S.; Almontassir, A.; Casas, M.T.; Puiggalí, J. Crystalline structure of poly(hexamethylene adipate). Study on the morphology and the enzymatic degradation of single crystals. Biomacromolecules 2006, 7, 799–808. [Google Scholar]
- Gestí, S.; Casas, M.T.; Puiggalí, J. Crystalline structure of poly(hexamethylene succinate) and single crystal degradation studies. Polymer 2007, 48, 5088–5097. [Google Scholar]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable poly(ethylene succinate) (PES). 1. Crystal growth kinetics and morphology. Biomacromolecules 2000, 1, 704–712. [Google Scholar]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable poly(ethylene succinate) (PES). 2. Crystal morphology of melt-crystallized ultrathin film and its change after enzymatic degradation. Biomacromolecules 2000, 1, 713–720. [Google Scholar]
- Gan, Z.; Abe, H.; Doi, Y. Crystallization, melting, and enzymatic degradation of biodegradable poly(butylene succinate-co-14 mol % ethylene succinate) copolyester. Biomacromolecules 2001, 2, 313–321. [Google Scholar]
- Iwata, T.; Doi, Y. Crystal structure and biodegradation of aliphatic polyester crystals. Macromol. Chem. Phys 1999, 200, 2429–2442. [Google Scholar]
- Mochizuki, M.; Mukai, K.; Yamada, K.; Ichise, N.; Murase, S.; Iwaya, Y. Structural effects upon enzymatic hydrolysis of poly(butylene succinate-co-ethylene succinate)s. Macromolecules 1997, 30, 7403–7407. [Google Scholar]
- Li, X.; Hong, Z.; Sun, J.; Geng, Y.; Huang, Y.; An, H. Identifying the phase behavior of biodegradable poly(hexamethylene succinate-co-hexamethylene adipate) copolymers with FTIR. J. Phys. Chem 2009, 113, 2695–2704. [Google Scholar]
- Li, X.; Sun, J.; Huang, Y.; Geng, Y.; Wang, X.; Ma, Z.; Shao, C.G.; An, H.N.; Yan, T.Z.; Li, L.B. Inducing new crystal structures through random copolymerization of biodegradable aliphatic polyester. Macromolecules 2008, 41, 3162–3168. [Google Scholar]
- Liang, Z.; Pan, P.; Zhu, B.; Dong, T.; Hua, L.; Inoue, Y. Crystalline phase of isomorphic poly(hexamethylene sebacate-co-hexamethylene adipate) copolyester: Effects of comonomer composition and crystallization temperature. Macromolecules 2010, 43, 2925–2932. [Google Scholar]
- Liang, Z.; Pan, P.; Zhu, B.; Inoue, Y. Isomorphic crystallization of aliphatic copolyesters derived from 1,6-hexanediol: Effect of the chemical structure of comonomer units on the extent of cocrystallization. Polymer 2011, 52, 2667–2676. [Google Scholar]
- Díaz, A.; Franco, L.; Puiggalí, J. Study on the crystallization of poly(butylene azelate-co-butylene succinate) copolymers. Thermochim. Acta 2014, 575, 45–54. [Google Scholar]
- Díaz, A.; Franco, L.; Estrany, F.; del Valle, L.J.; Puiggalí, J. Poly(butylene azelate-co-butylene succinate) copolymers: Crystalline morphologies and degradation. Polym. Degrad. Stab 2014, 99, 80–91. [Google Scholar]
- Witt, U.; Müller, R.J.; Deckwer, W.D. Studies on sequence distribution on aliphatic/aromatic copolyesters by high-resolution 13C nuclear magnetic resonance spectroscopy for evaluation of biodegradability. Macromol. Chem. Phys 1996, 197, 1525–1535. [Google Scholar]
- Kleeberg, I.; Hetz, C.; Kroppenstedt, R.M.; Müller, R.J.; Deckwer, W.D. Biodegradation of aliphatic/aromatic copolyesters by thermophilic actinomycetes. Appl. Environ. Microbiol 1998, 64, 1731–1735. [Google Scholar]
- Witt, U.; Einig, T.; Yamamoto, M.; Kleeberg, I.; Deckwer, W.-D.; Müller, R.J. Biodegradation of aliphatic–aromatic copolyesters: Evaluation of the final biodegradability and ecotoxicological impact of degradation intermediates. Chemosphere 2001, 44, 289–299. [Google Scholar]
- Kleeberg, I.; Welzel, K.; van den Heuvel, J.; Müller, R.J.; Deckwer, W.D. Characterization of a new extracellular hydrolase from thermobifida fusca degrading aliphatic-aromatic copolyesters. Biomacromolecules 2005, 6, 262–270. [Google Scholar]
- Herrera, R.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. characterization and degradation behavior of poly(butylene adipate-co-terephthalate)s. J. Polym. Sci. A 2002, 40, 4141–4157. [Google Scholar]
- Marten, E.; Müller, R.J.; Deckwer, W.D. Studies on the enzymatic hydrolysis of polyesters. II. aliphatic-aromatic copolyesters. Polym. Degrad. Stab 2005, 88, 371–381. [Google Scholar]
- Jaisankar, V.; Nanthini, R.; Ravi, A.; Karunanidhi, M. A study on biodegradation of aliphatic-aromatic random copolyesters. J. Polym. Mater 2009, 26, 157–166. [Google Scholar]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.; Ngouajio, M.; Fernandez, R.T. Biodegradation and hydrolysis rate of aliphatic aromatic polyester. Polym. Degrad. Stab 2010, 95, 2641–2647. [Google Scholar]
- Javadi, A.; Kramschuster, A.J.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.-S. Processing and characterization of microcellular PHBV/PBAT blends. Polym. Eng. Sci 2010, 50, 1440–1448. [Google Scholar]
- Zarrinbakhsh, N.; Mohanty, A.K.; Misra, M. Improving the interfacial adhesion in a new renewable resourcebased biocomposites from biofuel coproduct and biodegradable plastic. J. Mater. Sci 2013, 48, 6025–6038. [Google Scholar]
- Nobrega, M.M.; Olivato, J.B.; Müller, C.M.O.; Yamashita, F. addition of saturated fatty acids to biodegradable films: Effect on the crystallinity and viscoelastic characteristics. J. Polym. Environ 2013, 21, 166–171. [Google Scholar]
- Madera-Santana, T.J.; Misra, M.; Drzal, L.T.; Robledo, D.; Freile-Pelegrin, Y. Preparation and characterization of biodegradable agar/poly(butylene adipate-co-terephatalate) composites. Polym. Eng. Sci 2009, 49, 1117–1126. [Google Scholar]
- Someya, Y.; Sugahara, Y.; Shibata, M. Nanocomposites based on poly(butylene adipate-co-terephthalate) and montmorillonite. J. Appl. Polym. Sci 2005, 95, 386–392. [Google Scholar]
- Chivrac, F.; Kadlecová, Z.; Pollet, E.; Avérous, L. Aromatic copolyester-based nano-biocomposites: Elaboration, structural characterization and properties. J. Polym. Environ 2006, 14, 393–401. [Google Scholar]
- Celli, A.; Barbiroli, G.; Berti, C.; di Credico, F.; Lorenzetti, C.; Marchese, P.; Marianucci, E. Thermal properties of poly(alkylene dicarboxylate)s derived from 1,12-dodecanedioic acid and even aliphatic diols. J. Polym. Sci. B 2007, 45, 1053–1067. [Google Scholar]
- Berti, C.; Celli, A.; Marchese, P.; Marianucci, E.; Barbiroli, G.; di Credico, F. The effect of aliphatic chain length on thermal properties of poly(alkylene dicarboxylate)s. Polymers 2013, 7, 658–675. [Google Scholar]
- Berti, C.; Celli, A.; Marchese, P.; Barbiroli, G.; di Credico, F.; Verney, V.; Commereuc, S. Novel copolyesters based on poly(alkylene dicarboxylate)s: 1. Thermalbehavior and biodegradation of aliphatic-aromatic random copolymers. Eur. Polym. J 2008, 44, 3650–3661. [Google Scholar]
- Zheng, L.; Li, C.; Zhang, D.; Guan, G.; Xiao, Y.; Wang, D. Novel biodegradable multiblock copolymers comprising poly(butylene succinate) and poly(1,2-propylene terephthalate) with hexamethylene diisocyanate as a chain extender. Polym. Int 2011, 60, 666–675. [Google Scholar]
- Kint, D.P.R.; Wigström, E.; de Ilarduya, A.; Alla, A.; Muñoz-Guerra, S. Poly(ethylene terephthalate) copolyesters derived from (2S,3S)-2,3-dimethoxy-1,4-butanediol. J. Polym. Sci 2001, 39, 3250–3262. [Google Scholar]
- Zamora, F.; Hakkou, K.; Alla, A.; Rivas, M.; Roffé, I.; Mancera, M.; Muñoz-Guerra, S. Aromatic polyesters fromnaturally occurring monosaccharides: Poly(ethylene terephthalate) and poly(ethylene isophthalate) analogs derived from d-mannitol and galactitol. J. Polym. Sci 2005, 43, 4570–4574. [Google Scholar]
- Zamora, F.; Hakkou, K.; Alla, A.; Espartero, J.L.; Muñoz-Guerra, S.; Galbis, J.A. Aromatic homo- and co-polyesters from naturally occurring monosaccharides: PET and PEI analogs derived from l-arabinitol and xylitol. J. Polym. Sci 2005, 43, 6394–6410. [Google Scholar]
- Zamora, F.; Hakkou, K.; Alla, A.; Marín-Bernabé, A.; de Paz, M.V.; Mtz de Ilarduya, A.; Muñoz-Guerra, S. Polyesters analogous to PET and PBT based on O-benzyl ethers of xylitol and l-arabinitol. J. Polym. Sci 2008, 46, 5167–5179. [Google Scholar]
- Zamora, F.; Hakkou, K.; Alla, A.; Rivas, M.; Mtz de Ilarduya, A.; Muñoz-Guerra, S.; Galbis, J.A. Butylene copolyesters based on aldaric and terephthalic acids. Synthesis and characterization. J. Polym. Sci 2009, 47, 1168–1177. [Google Scholar]
- Jansen, M.A.G.; Wu, L.H.; Goossens, J.G.P.; de Wit, G.; Bailly, C.; Koning, C.E.; Portale, G. The incorporation of rigid diol monomers into poly(butylene terephthalate) via solid-state copolymerization and melt copolymerization. J. Polym. Sci. A 2008, 46, 1203–1217. [Google Scholar]
- Sablong, R.; Duchateau, R.; Koning, C.E.; de Wit, G.; van Es, D.; Koelewijn, R.; van Haveren, J. Incorporation of isosorbide into poly(butylene terephthalate) via solid-state polymerization. Biomacromolecules 2008, 9, 3090–3097. [Google Scholar]
- Lavilla, C.; Gubbels, E.; Martínez de Ilarduya, A.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Solid-state modification of pbt with cyclic acetalized galactitol and d-mannitol: Influence of composition and chemical microstructure on thermal properties. Macromolecules 2013, 46, 4335–4345. [Google Scholar]
- Gubbels, E.; Lavilla, C.; Martínez de Ilarduya, A.; Noordover, B.A.J.; Koning, C.E.; Muñoz-Guerra, S. Partially renewable copolyesters prepared from acetalized d-glucitol by solid-state modification of poly(butylene terephthalate). J. Polym. Sci. A 2014, 52, 164–177. [Google Scholar]
- Noordover, B.A.J.; van Staalduinen, V.G.; Duchateau, R.; Koning, C.E.; van Benthem, R.A.T.M.; Mak, M.; Heise, A.; Frissen, A.E.; van Haveren, J. Co- and terpolyesters based on isosorbide and succinic acid for coating applications: Synthesis and characterization. Biomacromolecules 2006, 7, 3406–3416. [Google Scholar]
- Wu, J.; Eduard, P.; Thiyagarajan, S.; van Haveren, J.; van Es, D.S.; Koning, C.E.; Lutz, M.; Fonseca Guerra, C. Isohexide derivatives from renewable resources as chiral building blocks. ChemSusChem 2011, 4, 599–603. [Google Scholar]
- Wu, J.; Eduard, P.; Thiyagarajan, S.; Jasinska-Walc, L.; Rozanski, A.; Fonseca Guerra, C.; Noordover, B.A.J.; van Haveren, J.; van Es, D.S.; Koning, C.E. Semicrystalline polyesters based on a novel renewable building block. Macromolecules 2012, 45, 5069–5080. [Google Scholar]
- Lavilla, C.; Alla, A.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. High Tg. bio-based aliphatic polyesters from bicyclic d-mannitol. Biomacromolecules 2013, 14, 781–793. [Google Scholar]
- Shih, Y.F.; Chieh, Y.C. Thermal degradation behavior and kinetic analysis of biodegradable polymers using various comparative models, 1. Macromol. Theory Simul 2007, 16, 101–110. [Google Scholar]
- Rizzarelli, P.; Carroccio, S. Thermo-oxidative processes in biodegradable poly(butylene succinate). Polym. Degrad. Stab 2009, 94, 1825–1838. [Google Scholar]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal degradation mechanism of poly(ethylene succinate) and poly(butylene succinate): Comparative study. Thermochim. Acta 2005, 435, 142–150. [Google Scholar]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Effect of molecular weight on thermal degradation mechanism of the biodegradable polyester poly(ethylene succinate). Thermochim. Acta 2006, 440, 166–175. [Google Scholar]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal degradation kinetics of the biodegradable aliphatic polyester, poly(propylene succinate). Polym. Degrad. Stab 2006, 91, 60–68. [Google Scholar]
- Soccio, M.; Lotti, N.; Finelli, L.; Gazzano, M.; Munari, A. Aliphatic poly(propylene dicarboxylate)s: Effect of chain length on thermal properties and crystallization kinetics. Polymer 2007, 48, 3125–3136. [Google Scholar]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal degradation kinetics and decomposition mechanism of new aliphatic biodegradable polyesters poly(propylene glutarate) and poly(propylene suberate). Thermochim. Acta 2010, 505, 59–68. [Google Scholar]
- Chrissafis, K.; Paraskevopoulos, K.M.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal decomposition of poly(propylene sebacate) and poly(propylene azelate) biodegradable polyesters: Evaluation of mechanisms using TGA, FTIR and GC/MS. J. Anal. Appl. Pyrol 2011, 92, 123–130. [Google Scholar]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Giliopoulos, D.J.; Stergiou, C.A. Correlation between chemical and solid-state structures and enzymatic hydrolysis in novel biodegradable polyesters. The Case of Poly(propylene alkanedicarboxylate)s. Macromol. Biosci 2008, 8, 728–740. [Google Scholar]
- Tokiwa, Y.; Suzuki, T. Hydrolysis of copolyesters containing aromatic and aliphatic ester blocks by lipase. J. Appl. Polym. Sci 1981, 26, 441–448. [Google Scholar]
- Nagata, M.; Kiyotsukuri, T.; Ibuki, H.; Tsutsumi, N.; Sakai, W. Synthesis and enzymatic degradation of regular network aliphatic polyesters. React. Funct. Polym 1996, 30, 165–171. [Google Scholar]
- Nagata, M.; Ibuki, H.; Sakai, W.; Tsutsumi, N. Synthesis, characterization, and enzymatic degradation of novel regular network aliphatic polyesters based on pentaerythritol. Macromolecules 1997, 30, 6525–6530. [Google Scholar]
- Nagata, M.; Machida, T.; Sakai, W.; Tsutsumi, N. Synthesis, characterization, and enzymatic degradation studies on novel network aliphatic polyesters. Macromolecules 1998, 31, 6450–6454. [Google Scholar]
- Murase, T.; Iwata, T.; Doi, Y. Direct observation of enzymatic degradation behavior of poly[(R)-3-hydroxybutyrate] lamellar single crystals by atomic force microscopy. Macromolecules 2001, 34, 5848–5853. [Google Scholar]
- Kikkawa, Y.; Hirota, T.; Numata, K.; Tsuge, T.; Abe, H.; Iwata, T.; Doi, Y. In-situ atomic force microscopy observation of enzymatic degradation in poly(hydroxyalkanoic acid) thin films: Normal and constrained conditions. Macromol. Biosci 2004, 4, 276–285. [Google Scholar]
- Iwata, T.; Doi, Y. Morphology and enzymatic degradation of poly(e-caprolactone) single crystals: does a polymer single crystal consist of micro-crystals? Polym. Int 2002, 51, 852–858. [Google Scholar]
- Iwata, T.; Doi, Y. Morphology and enzymatic degradation of poly(l-lactic acid) single crystals. Macromolecules 1998, 31, 2461–2467. [Google Scholar]
- Iwata, T.; Doi, Y. morphology and enzymatic degradation of solution-grown single crystals of poly(ethylene succinate). Macromolecules 2001, 34, 7343–7348. [Google Scholar]
- Iwata, T.; Kobatashi, S.; Tabata, K.; Yonezawa, N.; Doi, D. Crystal structure, thermal behavior and enzymatic degradation of poly(tetramethylene adipate) solution-grown chain-folded Lamellar crystals. Macromol. Biosci 2004, 4, 296–307. [Google Scholar]
- Casas, M.T.; Puiggalí, J. Enzymatic degradation of poly(octamethylene suberate) lamellar crystals. Polym. Degrad. Stab 2009, 94, 1941–1947. [Google Scholar]
- Ding, M.; Zhang, M.; Yang, J.; Qiu, J.H. Study on the enzymatic degradation of aliphatic polyester–pbs and its copolymers. J. Appl. Polym. Sci 2012, 124, 2902–2907. [Google Scholar]
- Chen, C.H.; Lu, H.Y.; Chen, M.; Peng, J.S.; Tsai, C.J.; Yang, C.S. Synthesis and characterization of poly(ethylene succinate) and its copolyesters containing minor amounts of butylene succinate. J. Appl. Polym. Sci 2009, 111, 1433–1439. [Google Scholar]
- Cao, A.M.; Okamura, T.; Nakayamab, K.; Inoued, Y.; Masuda, T. Studies on synthesis and physical properties of biodegradable aliphatic poly(butylene succinate-co-ethylene succinate)s and poly(butylene succinate-co-diethylene glycol succinate)s. Polym. Degrad. Stab 2002, 78, 107–177. [Google Scholar]
- Xu, Y.X.; Xu, J.; Guo, B.H.; Xie, X.M. Crystallization kinetics and morphology of biodegradable poly(butylene succinate-co-propylene succinate)s. J. Polym. Sci. B 2007, 45, 420–428. [Google Scholar]
- Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis Cocrystallization enzymatic degradation of novel poly(butylene-co-propylene succinate) copolymers. Biomacromolecules 2007, 8, 2437–2449. [Google Scholar]
- Lu, S.F.; Chen, M.; Shih, Y.C.; Chen, C.H. Nonisothermal crystallization kinetics of biodegradable poly(butylene succinate-co-propylene succinate)s. J. Polym. Sci. B 2010, 48, 1299–1308. [Google Scholar]
- Nikolic, M.S.; Djonlagic, J. Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene adipate)s. Polym. Degrad. Stab 2001, 74, 263–270. [Google Scholar]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable aliphatic polyesters. Part I. Properties and biodegradation of poly(butylene succinate-co-butylene adipate). Polym. Degrad. Stab 2006, 91, 367–376. [Google Scholar]
- Zhu, Q.-Y.; He, Y.-S.; Zeng, J.-B.; Huang, Q.; Wang, Y.-Z. Synthesis and characterization of a novel multiblock copolyester containing poly(ethylene succinate) and poly(butylene succinate). Mater. Chem. Phys 2011, 130, 943–949. [Google Scholar]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. Control of biodegradability of polylactide via nanocomposite technology. Macromol. Mater. Eng 2003, 288, 203–208. [Google Scholar]
- Singh, N.K.; Purkayastha, B.D.; Roy, J.K.; Banik, R.M.; Yashpal, M.; Singh, G. Nanoparticle-induced controlled biodegradation and its mechanism in poly(ɛ-caprolactone). ACS Appl. Mater. Interfaces 2010, 2, 69–81. [Google Scholar]
- Han, S.I.; Lim, J.S.; Kim, D.K.; Kim, M.N.; Im, S.S. In situ polymerized poly(butylene succinate)/silica nanocomposites: Physical properties and biodegradation. Polym. Degrad. Stab 2008, 93, 889–895. [Google Scholar]
- Chouzouri, G.; Xanthos, M. In vitro bioactivity and degradation of polycaprolactone composites containing silicate fillers. Acta Biomater 2007, 3, 745–756. [Google Scholar]
- Lee, S.R.; Park, H.M.; Lim, H.; Kang, T.; Li, X.; Cho, W.J.; Ha, C.-S. Microstructure, tensile properties, and biodegradability of aliphatic polyester/clay nanocomposites. Polymer 2002, 43, 2495–2500. [Google Scholar]
- Maiti, P.; Batt, C.A.; Giannelis, E.P. New biodegradable polyhydroxybutyrate/layered silicate nanocomposites. Biomacromolecules 2007, 8, 3393–3400. [Google Scholar]
- Bikiaris, D.N.; Nianias, N.P.; Karagiannidou, E.G.; Docoslis, A. Effect of different nanoparticles on the properties and enzymatic hydrolysis mechanism of aliphatic polyesters. Polym. Degrad. Stab 2012, 97, 2077–2089. [Google Scholar]
- Chrissafis, K.; Roumeli, E.; Paraskevopoulos, K.M.; Nianias, N.; Bikiaris, D.N. Effect of different nanoparticles on thermal decomposition of poly(propylene sebacate)/nanocomposites: Evaluation of mechanisms using TGA and TG–FTIR–GC/MS. J. Anal. Appl. Pyrol 2012, 96, 92–99. [Google Scholar]
- Vassiliou, A.A.; Bikiaris, D.; El Mabrouk, K.; Kontopoulou, M. Effect of evolved interactions in poly(butylene succinate)/fumed silica biodegradable in situ prepared nanocomposites on molecular weight, material properties, and biodegradability. J. Appl. Polym. Sci 2011, 119, 2010–2024. [Google Scholar]
- Billiet, L.; Fournier, D.; du Prez, F. Combining “click” chemistry and step-growth polymerization for the generation of highly functionalized polyesters. J. Polym. Sci. A 2008, 46, 6552–6564. [Google Scholar]
- Anthierens, T.; Billiet, L.; Devlieghere, F.; du Prez, F. Poly(butylene adipate) functionalized with quaternary phosphonium groups as potential antimicrobial packaging material. Innov. Food Sci. Emerg 2012, 15, 81–85. [Google Scholar]
- Aloy, P.; Russell, R.B. Structure-based systems biology: A zoom lens for the cell. FEBS Lett 2005, 579, 1854–1858. [Google Scholar]
- LeDuc, P.R.; Bellin, R.M. Nanoscale intracellular organization and functional architecture mediating cellular behavior. Ann. Biomed. Eng 2006, 34, 102–113. [Google Scholar]
- Fukuda, J.; Khademhosseini, A.; Yeh, J.; Eng, G.; Cheng, J.J.; Farokhzad, O.C.; Langer, R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials 2006, 27, 1479–1486. [Google Scholar]
- Coutinho, D.F.; Gomes, M.E.; Neves, N.M.; Reis, R.L. Development of micropatterned surfaces of poly(butylene succinate) by micromolding for guided tissue engineering. Acta Biomater 2012, 8, 1490–1497. [Google Scholar] [Green Version]
- Yin, D.; Wang, X.H.; Yan, Y.; Zhang, R. Preliminary studies on peripheral nerve regeneration using a new polyurethane conduit. J. Bioact. Compat. Polym 2007, 22, 143–159. [Google Scholar]
- Pego, A.P.; Siebum, B.; van Luyn, M.J.; Gallego y van Seijen, X.J.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Preparation of degradable porous structures based on 1,3-trimethylene carbonate and d,l-lactide (co) polymers for heart tissue engineering. Tissue Eng 2003, 9, 981–994. [Google Scholar]
- Guarino, V.; Alvarez-Perez, M.; Cirillo, V.; Ambrosio, L. HMSC interaction with PCL and PCL/gelatin platforms: A comparative study on films and electrospun membranes. J. Bioact. Compat. Polym 2011, 26, 144–160. [Google Scholar]
- Bruggeman, J.P.; de Bruin, B.-J.; Bettinger, C.J.; Langer, R. Biodegradable poly(polyol sebacate) polymers. Biomaterials 2008, 29, 4726–4735. [Google Scholar]
- Soccio, M.; Lotti, N.; Finelli, L.; Munari, A. Influence of transesterification reactions on the miscibility andthermal properties of poly(butylene/diethylene succinate) copolymers. Eur. Polym. J 2008, 44, 1722–1732. [Google Scholar]
- Soccio, M.; Lotti, N.; Finelli, L.; Munari, A. Miscibility of biodegradable poly(propylene succinate)/poly(propylene adipate) blends: Effect of the transesterification reactions. Eur. Polym. J 2009, 45, 3236–3248. [Google Scholar]
- Gualandi, C.; Soccio, M.; Govoni, M.; Valente, S.; Lotti, N.; Munari, A.; Giordano, E.; Pasquinelli, G.; Focarete, M.L. Poly(butylene/diethyleneglycol succinate) multiblock copolyester as a candidate biomaterial for soft tissue engineering: Solid-state properties, degradability, and biocompatibility. J. Bioact. Compat. Polym 2012, 27, 244–264. [Google Scholar]
- Wang, Y.; Kim, Y.M.; Langer, R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. A 2003, 66, 192–197. [Google Scholar]
- Bettinger, C.J.; Weinberg, E.J.; Kulig, K.M.; Vacanti, J.P.; Wang, Y.; Borenstein, J.T.; Langer, R. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv. Mater 2006, 18, 165–169. [Google Scholar]
- Qiu, H.; Yang, J.; Kodali, P.; Koh, J.; Ameer, G.A. A citric acid-based hydroxyapatite composite for orthopedic implants. Biomaterials 2006, 27, 5845–5854. [Google Scholar]
- Webb, A.R.; Kumar, V.A.; Ameer, G.A. Biodegradable poly(diol citrate) nanocomposite elastomers for soft tissue engineering. J. Mater. Chem 2007, 17, 900–906. [Google Scholar]
- Bat, E.; Plantinga, J.A.; Harmsen, M.C.; van Luyn, M.J.A.; Zhang, Z.; Grijpma, D.W. In vivo behavior of trimethylene carbonate and epsilon-caprolactone-based copolymer networks: Degradation and tissue response. J. Biomed. Mater. Res. A 2010, 95A, 940–949. [Google Scholar]
- Yang, J.; Webb, A.R.; Ameer, G.A. Novel citric acid-based biodegradable elastomers for tissue engineering. Adv. Mater 2004, 16, 511–516. [Google Scholar]
- Nijst, C.L.E.; Bruggeman, J.P.; Karp, J.M.; Ferreira, L.; Zumbuehl, A.; Bettinger, C.J. Synthesis and characterization of photocurable elastomers from poly(glycerol-co-sebacate). Biomacromolecules 2007, 8, 3067–3073. [Google Scholar]
- Stammen, J.A.; Williams, S.; Ku, D.N.; Guldberg, R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar]
- Djordjevic, I.; Choudhury, N.R.; Dutta, N.K.; Kumar, S. Poly[octanediol-co-(citric acid)-co-(sebacic acid)] elastomers: Novel bio-elastomersfor tissue engineering. Polym. Int 2011, 60, 333–343. [Google Scholar]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci 2012, 37, 1051–1078. [Google Scholar]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar]
- Frenot, A.; Chronakis, I.S. Polymer nanofibres assembled by electrospinning. Curr. Opin. Colloid Interface Sci 2003, 8, 64–75. [Google Scholar]
- Jeong, E.H.; Im, S.S.; Youk, J.H. Electrospinning and structural characterization of ultrafine poly(butylene succinate) fibers. Polymer 2005, 46, 9538–9543. [Google Scholar]
- Sutthiphong, S.; Pavasant, P.; Supaphol, P. Electrospun 1,6-diisocyanatohexane-extended poly(1,4-butylene succinate) fiber mats and their potential for use as bone scaffolds. Polymer 2009, 50, 1548–1558. [Google Scholar]
- Zhang, D.; Chang, J.; Zeng, Y. Fabrication of fibrous poly(butylene succinate)/wollastonite/apatite composite scaffolds by electrospinning and biomimetic process. J. Mater. Sci 2008, 19, 443–449. [Google Scholar]
- Tian, L.; Wang, P.; Zhao, Z.; Ji, J. Antimicrobial activity of electrospun poly(butylenes succinate) fiber mats containing pvp-capped silver nanoparticles. Appl. Biochem. Biotech 2013, 171, 1890–1899. [Google Scholar]
- Xu, L.; Ren, Z. Drug-loaded pbs micro spheres with chinese drug by electrospinning. J. Polym. Eng 2008, 28, 27–31. [Google Scholar]
- Yi, F.; LaVan, D.A. Poly(glycerol sebacate) Nanofiber scaffolds by core/shell electrospinning. Macromol. Biosci 2008, 8, 803–806. [Google Scholar]
- Xu, B.; Li, Y.; Fang, X.; Thouas, G.A.; Cook, W.D.; Newgreen, D.F.; Chen, Q. Mechanically tissue-like elastomeric polymers and their potential as a vehicle to deliver functional cardiomyocytes. J. Mech. Behav. Biomed 2013, 28, 354–365. [Google Scholar]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Poly(glycerol sebacate)/gelatin core/shell fibrous structure for regeneration of myocardial infarction. Tissue Eng. A 2011, 17, 1363–1373. [Google Scholar]
- Kharaziha, M.; Nikkhah, M.; Shin, S.-R.; Annabi, N.; Masoumi, N.; Gaharwar, A.K.; Camci-Unal, G.; Khademhosseini, A. PGS: Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials 2013, 34, 6355–6366. [Google Scholar]
- Almeida, L.R.; Martins, A.R.; Fernandes, E.M.; Oliveira, M.B.; Correlo, V.M.; Pashkuleva, I.; Marques, A.P.; Ribeiro, A.S.; Durães, N.F.; Silva, C.J.; et al. New biotextiles for tissue engineering: Development, characterization and in vitro cellular viability. Acta Biomater 2013, 9, 8167–8181. [Google Scholar]
- Tang, J.; Zhang, Z.; Song, Z.; Chen, L.; Hou, X.; Yao, K. Synthesis and characterization of elastic aliphatic polyesters from sebacic acid, glycol and glycerol. Eur. Polym. J 2006, 42, 3360–3366. [Google Scholar]
- Simitzis, J.; Soulis, S.; Triantou, D.; Zoumpoulakis, L.; Zotali, P. Synthesis and characterization of hydrolytically degradable copolyester biomaterials based on glycolic acid, sebacic acid and ethylene glicol. J. Mater. Sci 2011, 22, 2673–2684. [Google Scholar]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol 2005, 69, 1–8. [Google Scholar]
- Vocus/PRWEB. Available online: http://www.prweb.com/releases/2011/2/prweb8151116.htm accessed on 21 February 2011.
- Jacoby, M. Custom-made biomaterials. Chem. Eng. News 2001, 79, 30–35. [Google Scholar]
- Nathan, A.; Kohn, J. Chapter 3: Amino acid derived polymers. In Biomedical Polymers: Design-to-Degrade Systems; Shalaby, S.W., Ed.; Hanser Publishers: New York, NY, USA, 1994; p. 117. [Google Scholar]
- Sun, H.; Meng, F.; Dias, A.A.; Hendriks, M.; Feijen, J.; Zhong, Z. α-Amino acid containing degradable polymers as functional biomaterials: Rational design, synthetic pathway, and biomedical applications. Biomacromolecules 2011, 12, 1937–1955. [Google Scholar]
- Rodríguez-Galán, A.; Franco, L.; Puiggalí, J. Degradable poly(ester amide)s for biomedical applications. Polymers 2011, 3, 65–99. [Google Scholar]
- Rodríguez-Galán, A.; Franco, L.; Puiggalí, J. Chapter 4: Biodegradable poly(ester amide)s: Synthesis and applications. In Biodegradable Polymers: Processing, Degradation; Felton, G.P., Ed.; Nova Science Publisher: New York, NY, USA, 2011; p. 207. [Google Scholar]
- Bourke, S.L.; Kohn, J. Polymers derived from the amino acid l-tyrosine: Polycarbonates, polyarylates and copolymers with poly(ethylene glycol). Adv. Drug Deliv. Rev 2003, 55, 447–466. [Google Scholar]
- Katsarava, R.; Gomurashvili, Z. Chapter 5: Biodegradable polymers composed of naturally occurring α-amino acids. In Handbook of Biodegradable Polymers—Isolation, Synthesis, Characterization and Applications; Lendlein, A., Sisson, A., Eds.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2011; p. 107. [Google Scholar]
- Katsarava, R.; Kharadze, D.; Japaridze, N.; Omiadze, T.; Avalishvili, L. Heterochain polymers based on natural amino acids. Synthesis of polyamides from Nα,Nɛ-bis-(trimethylsilyl)-lysine alkyl esters. Makromol. Chem 1985, 186, 939–954. [Google Scholar]
- Gomurashvili, Z.; Kricheldorf, H.R.; Katsarava, R. Amino acid based bioanalogous polymers. Synthesis and study of new regular poly(ester amides)s composed of hydrophobic α-amino acids and dianhydrohexitoles. J. Macromol. Sci 2000, 37, 215–227. [Google Scholar]
- Okada, M.; Yamada, M.; Yokoe, M.; Aoi, K. Biodegradable polymers based on renewable resources. V. Synthesis and biodegradation behavior of poly(ester amide)s composed of 1,4:3,6-dianhydro-d-glucitol, α-amino acid, and aliphatic dicarboxylic acid units. J. Appl. Polym. Sci 2001, 81, 2721–2734. [Google Scholar]
- Guo, K.; Chu, C.C. Synthesis, characterization and biodegradation of novel poly(ether ester amide)s based on l-phenylalanine and oligoethylene glycol. Biomacromolecules 2007, 8, 2851–2861. [Google Scholar]
- Memanishvili, T.; Kupatadze, N.; Tugushi, D.; Torchilin, V.P.; Katsarava, R. Biodegradable arginine-based polymers with PEG-like backbones as potential non-viral gene delivery system. Proceedings of 1st Russian-Hellenic Symposium with International Participation Biomaterials and Bionanomaterials: Recent Advances and Safety-Toxicology Issues, Iraklion, Greece, 3–9 May 2010.
- Wu, J.; Yamanouchi, D.; Liu, B.; Chu, C.C. Biodegradable arginine-based poly(ether ester amide)s as a non-viral DNA delivery vector and their structure-function study. J. Mater. Chem 2012, 22, 18983–18991. [Google Scholar]
- Memanishvili, T.; Zavradashvili, N.; Kupatadze, N.; Tugushi, D.; Gverdtsiteli, M.; Torchilin, V.P.; Wandrey, C.; Baldi, L.; Manoli, S.S.; Katsarava, R. Arginine-based biodegradable ether-ester polymers of low cytotoxicity as potential gene carriers. Biomacromolecules 2014. submitted for publication. [Google Scholar]
- De Wit, M.A.; Wang, Z.; Atkins, K.M.; Mequanint, K.; Gillies, E.R. Syntheses, characterization, and functionalization of poly(ester amide)s with pendant amine functional groups. J. Polym. Sci. A 2008, 46, 6376–6392. [Google Scholar]
- Atkins, K.M.; Lopez, D.; Knight, D.K.; Mequanint, K.; Gillies, E.R. A versatile approach for the syntheses of poly(ester amide)s with pendant functional groups. J. Polym. Sci. A 2009, 47, 3757–3772. [Google Scholar]
- Cleaver, C.S.; Pratt, B.C. Synthesis of 2,2′-bis-[5(4H)-oxazolones]. J. Am. Chem. Soc 1955, 77, 1544–1546. [Google Scholar]
- Katsarava, R.; Kharadze, D.; Kirmelashvili, L. Heterochain polymers based on natural α-amino acids. Bis-oxazolinone method of the synthesis of polyamides containing enzymatically cleavable bonds in the main chains. Acta Polym 1985, 36, 29–38. [Google Scholar]
- Kharadze, D.; Kirmelashvili, L.; Medzmariashvili, N.; Beridze, V.; Tsitlanadze, G.; Tughushi, D.; Chu, C.C.; Katsarava, R. Synthesis and α-chymotrypsinolysis of regular poly(ester amides)s based on phenylalanine, diols and terephthalic acid. Polym. Sci. A 1999, 41, 883–890. [Google Scholar]
- Kharadze, D.; Omiadze, T.; Tsitlanadze, G.; Goguadze, T.S.; Arabuli, N.; Gomurashvili, Z.; Katsarava, R. New biodegradable polymers derived from N,N′-diacyl-bis-phenylalanine. Polym. Sci. A 1994, 36, 1214–1218. [Google Scholar]
- Asín, L.; Armelin, E.; Montane, J.; Rodríguez-Galán, A.; Puiggalí, J. Sequential poly(ester amide)s based on glycine, diols, and dicarboxylic acids: Thermal polyesterification vs. interfacial polyamidation. J. Polym. Sci. A 2001, 39, 4283–4293. [Google Scholar]
- Katsarava, R.; Kharadze, D.; Avalishvili, L. Synthesis of high-molecular-weight polysuccinamides by polycondesation of active succinates with diamines. Makromol. Chem 1986, 187, 2053–2062. [Google Scholar]
- Katsarava, R. Progress and problems in activated polycondensation. Russ. Chem. Rev 1991, 60, 722–737. [Google Scholar]
- Katsarava, R. Active polycondensation: from peptide chemistry to amino acid based biodegradable polymers. Macromol. Symp 2003, 199, 419–429. [Google Scholar]
- Morgan, P.W. Condensation Polymers: By Interfacial and Solution Methods; Interscience Publishers: New York, NY, USA, 1965; p. 561. [Google Scholar]
- Katsarava, R.; Kharadze, D. The study of stability of active phenyl esters of carboxylic acids in aprotic polar solvents. Zhurn. Obshch. Khim 1991, 61, 2413–2418. [Google Scholar]
- Katsarava, R.; Kharadze, D.; Avalishvili, L. Synthesis of polyamides using activated bis-N-oxysuccinimide esters of dicarboxylic acids. Vysokomolek. Soed 1984, A26, 1489–1497. [Google Scholar]
- Kricheldorf, H.R.; Shwarz, G. Cyclic polymers by kinetically controlled step—Growth polymerization. Macromol. Rapid Commun 2003, 24, 359–381. [Google Scholar]
- Kricheldorf, H.R. The role of self-dilution in step—Growth polymerizations. Macromol. Rapid Commun 2008, 29, 1695–1704. [Google Scholar]
- Saotome, Y.; Miyazawa, T.; Endo, T. Novel enzymatically degradable polymers comprising α-amino acid, 1,2-ethanediol and adipic acid. Chem. Lett 1991, 1, 21–24. [Google Scholar]
- Saotome, Y.; Tashiro, M.; Miyazawa, T.; Endo, T. Enzymatic degrading solubilization of a polymer comprising glycine, phenylalanine, 1,2-ethanediol, and adipic acid. Chem. Lett 1991, 1, 153–154. [Google Scholar]
- Arabuli, N.; Tsitlanadze, G.; Edilashvili, L.; Kharadze, D.; Goguadze, T.; Beridze, V.; Gomurashvili, Z.; Katsarava, R. Heterochain polymers based on natural amino acids. Synthesis and enzymatic hydrolysis of regular poly(ester amide)s based on bis(l-phenylalanine)-α,ω-alkylene diesters and adipic acid. Macromol. Chem. Phys 1994, 195, 2279–2289. [Google Scholar]
- Katsarava, R.; Arabuli, N.; Beridze, V.; Kharadze, D.; Chu, C.C.; Won, C.Y. Amino acid-based bioanalogous polymers. Synthesis, and study of regular poly(ester amides) based on bis(α–amino acid)-α,ω-alkylene diesters, and aliphatic dicarboxylic acids. J. Polym. Sci. A 1999, 37, 391–407. [Google Scholar]
- Katsarava, R.D.; Kharadze, D.P.; Bendiashvili, T.M.; Urman, Ya.G.; Slonim, I.Ya.; Alekseeva, S.G.; Cefelin, P.; Janout, V. Synthesis of polyamides by active polycondensation. The structure and kenetical aspects of active esters aminolysis reactions. Acta Polym 1988, 39, 523–533. [Google Scholar]
- Katsarava, R.; Tugushi, D. Agricultural University of Georgia, Tbilisi, GA, USA. Unpublished work. 2014.
- Katsarava, R.; Mazanashvili, N.; Mchedlishvili, N.; Gomurashvili, Z. Alkylene-Dicarboxylate-Containing Biodegradable Poly(ester-amides) and Methods of Use. U.S. Patent 20070287987, 13 December 2007. [Google Scholar]
- Katsarava, R.; Ochkhikidze, N.; Tugushi, D.; Gomurashvili, Z. Aabb-Poly(depsipeptide). Biodegradable Polymers and Methods of Use. U.S. Patent 20100040664, 18 February 2010. [Google Scholar]
- Ochkhikidze, N.; Razmadze, E.; Tugushi, D.; Kupatadze, N.; Gomurashvili, Z.; Katsarava, R. AABB—Poly(depsipeptide)s—A new class of amino acid based biodegradable polymers. Proceedings of International Symposium “Polycondensation-2008”,”, Tokyo, Japan, 8–11 September 2008.
- Jokhadze, G.; Machaidze, M.; Panosyan, H.; Chu, C.C.; Katsarava, R. Synthesis and characterrization of functional elastomeric poly(ester amide) copolymers. J. Biomater. Sci. Polym. Ed 2007, 18, 411–438. [Google Scholar]
- DeFife, K.M.; Grako, K.; Cruz-Aranda, G.; Price, S.; Chantung, R.; Macpherson, K.; Khoshabeh, R.; Gopalan, S.; Turnell, W.G. Poly(ester amide) co-polymers promote blood and tissuecompatibility. J. Biomater. Sci 2009, 20, 1495–1511. [Google Scholar]
- Lee, S.H.; Szinai, I.; Carpenter, K.; Katsarava, R.; Jokhadze, G.; Chu, C.C.; Huang, Y.; Verbeken, E.; Bramwell, O.; de Scheerder, I.; et al. In vivo biocompatibility evaluation of stents coated with a new biodegradable elastomeric and functional polymer. Coron. Artery Dis 2002, 13, 237–241. [Google Scholar]
- Gomurashvili, Z.; Zhang, H.; Da, J.; Jenkins, T.D.; Hughes, J.; Wu, M.; Lambert, L.; Grako, K.A.; DeFife, K.M.; Macpherson, K.; et al. Chapter 1: From drug-eluting stents to biopharmaceuticals: Poly(Ester Amide): A versatile new bioabsorbable biopolymer. In ACS Symposium Series 977: Polymers for Biomedical Applications; Mahapatro, A., Kulshrestha, A.S., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 10–26. [Google Scholar]
- Yamanouchi, D.; Wu, J.; Lazar, A.N.; Kent, K.C.; Chu, C.C.; Liu, B. Biodegradable arginine-based poly(ester-amide)s as non-viral gene delivery reagents. Biomaterials 2008, 29, 3269–3277. [Google Scholar]
- Knight, D.K.; Mequanint, K.; Gillies, E.R. Strategies in functional poly(ester amide) syntheses to study human coronary artery smooth muscle cell interactions. Biomacromolecules 2011, 12, 2475–2487. [Google Scholar]
- Chu, C.C.; Katsarava, R.; Guo, K. Unsaturated Poly(ester-amide) Biomaterials. U.S. Patent 7863406, 4 January 2011. [Google Scholar]
- Guo, K.; Chu, C.C.; Chkhaidze, E.; Katsarava, R. Synthesis and characterization of novel biodegradable unsaturated poly(ester amide)s. J. Polym. Sci. A 2005, 43, 1463–1477. [Google Scholar]
- Chkhaidze, E.; Tugushi, D.; Kharadze, D.; Gomurashvili, Z.; Chu, C.C.; Katsarava, R. New unsaturated biodegradable poly(ester amide)s composed of fumaric acid, l-leucine and α,ω-alkylene diols. J. Polym. Sci. A 2011, 48, 544–555. [Google Scholar]
- Zavradashvili, N.; Jokhadze, G.; Gverdtsiteli, M.; Otinashvili, G.; Kupatadze, N.; Gomurashvili, Z.; Tugushi, D.; Katsarava, R. Amino acid based epoxy-poly(ester amide)s—A new class of functional biodegradable polymers: Synthesis and chemical transformations. J. Macromol. Sci. A 2013, 50, 449–465. [Google Scholar]
- Neparidze, N.; Machaidze, M.; Zavradashvili, N.; Mazanashvili, N.; Tabidze, V.; Tugushi, D.; Katsarava, R. Biodegradable copoly(ester amide)s with hydrophobic lateral substituents. Polim. I Meditsina 2006, 2, 27–33. (In Russian) [Google Scholar]
- Legashvili, I.; Nepharidze, N.; Katsarava, R.; Sannigrahi, B.; Khan, I.M. Non-covalent nano-adducts of co-poly(ester amide) and poly(ethylene glycol): Preparation, characterization and model drug-release studies. J. Biomater. Sci 2007, 18, 673–685. [Google Scholar]
- Gomurashshvili, Z.; Turnell, W.G.; Vassilev, V.; Chowdar, N.S. Biodegradable Water Soluble Polymers. U.S. Patent 20070282011, 6 December 2007. [Google Scholar]
- Paredes, N.; Rodríguez-Galán, A.; Puiggalí, J. Synthesis and characterization of a family of biodegradable poly(ester amides) derived from glycine. J. Polym. Sci. A 1998, 36, 1271–1282. [Google Scholar]
- Paredes, N.; Casas, M.T.; Puiggalí, J.; Lotz, B. Structural data on the packing of poly(ester amide)s derived from glycine, hexanediol, and odd-numbered dicarboxylic acids. J. Polym. Sci. A 1999, 37, 2521–2533. [Google Scholar]
- Paredes, N.; Rodríguez-Galán, A.; Puiggalí, J.; Peraire, C. Studies on the biodegradation and biocompatibility of a new poly(ester amide) derived from l-alanine. J. Appl. Polym. Sci 1998, 69, 1537–1549. [Google Scholar]
- Rodríguez-Galán, A.; Pelfort, J.E.; Aceituno, J.; Puiggalí, J. Comparative studies on the degradability of poly(ester amides) derived from l-and l,d-alanine. J. Appl. Polym. Sci 1999, 74, 2312–2320. [Google Scholar]
- Rodríguez-Galán, A.; Fuentes, L.; Puiggalí, J. Studies on the degradability of a poly(ester amide) derived from l-alanine, 1,12-dodecanediol and 1,12-dodecanedioic acid. Polymer 2000, 41, 5967–5970. [Google Scholar]
- Paredes, N.; Casas, M.T.; Puiggalí, J. Poly(ester amide)s derived from glycine, even-numbered diols, and dicarboxylic acids: considerations on the packing. J. Polym. Sci. B 2001, 39, 1036–1045. [Google Scholar]
- Montane, J.; Armelin, E.; Asín, L.; Rodríguez-Galán, A.; Puiggalí, J. Comparative degradation data of polyesters and related poly(ester amide)s derived from 1,4-butanediol, sebacic acid, and α-amino acids. J. Appl. Polym. Sci 2002, 85, 1815–1824. [Google Scholar]
- Karimi, P.; Rizkalla, A.S.; Mequanint, K. Versatile biodegradable poly(ester amide)s derived from α-amino acids for vascular tissue engineering. Materials 2010, 3, 2346–2368. [Google Scholar]
- Turnell, W.G.; Gomurashvili, Z.D.; Anderl, J.N. Biodegradable Proline- Based Polymers. U.S. Patent 20120027859, 2 February 2012. [Google Scholar]
- Omay, D.; Katsarava, R. University of Yalova, Yalova, Turkey. Unpublished work. 2014.
- Cleaver, C.; Pratt, B. Polyamides from 2,2′-bis-[5(4H)-oxazolones]. J. Am. Chem. Soc 1955, 77, 1541–1543. [Google Scholar]
- Katsarava, R.; Tugushi, D.; Zavradashvili, N.; Kobauri, S.; Dgebuadze, M. New biodegradable copoly(amide/ester amide)s obtained via bis-azlacton chemistry. Proceedings of POLYCHAR-19 World Forum on Advanced Materials, Kathmandu, Nepal, 20–24 March 2011.
- Kobauri, S.; Zavradashvili, N.; Kviria, T.; Machaidze, M.; Katsarava, R. New hydrophobic biodegradable polymers on the basis of bis-azlactones. Ga. Chem. J 2010, 10, 295–305. [Google Scholar]
- Guo, K.; Chu, C.C. Synthesis and characterization of novel biodegradable unsaturated poly(ester amide)/poly(ethylene glycol) diacrylate hydrogels. J. Polym. Sci. A 2005, 43, 3932–3944. [Google Scholar]
- Katsarava, R.; Alavidze, Z. Polymer Blends as Biodegradable Matrices for Preparing Biocomposites. U.S. Patent 6703040, 9 March 2004. [Google Scholar]
- Market Wired. Available online: http://www.marketwire.com/press-release/Dsm-NV-959896.html accessed on 11 April 2014.
- Trollsas, M.; Maslanka, B.; Pham, N.; Lin, Q.; Hossainy, S.; Hsu, L.S.; Ngo, M.H. Polyesteramide coatings for drug eluting stents: Controlling drug release by polymer engineering. In Active Implants and Scaffolds for Tissue Regeneration; Springer: Berlin, Germany, 2011; pp. 127–143. [Google Scholar]







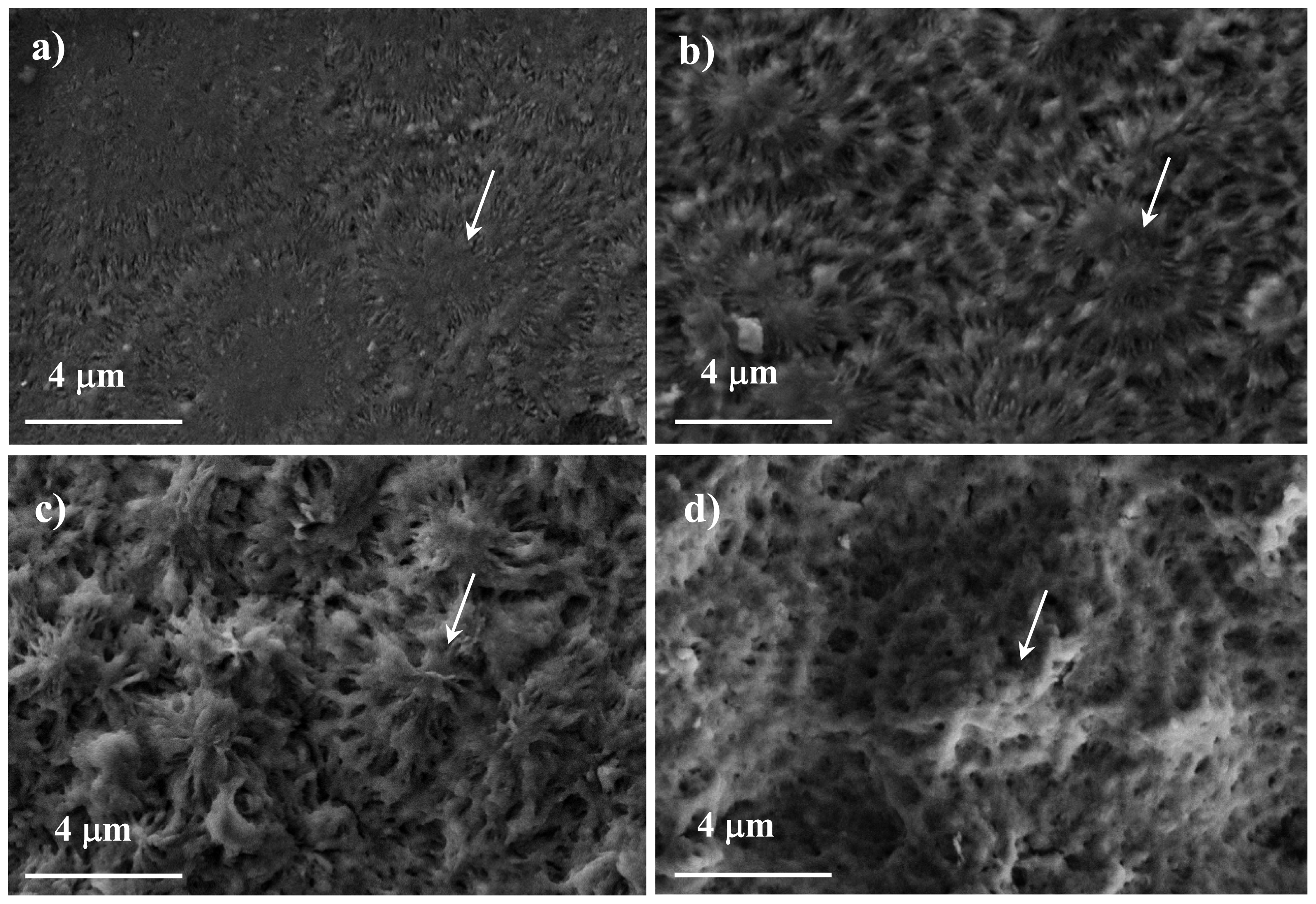



























| PEA | Yield (%) | MW (Da) | Mn (Da) | PDI |
|---|---|---|---|---|
| 8-Ala-8-Sol | 67 | 51,400 | 36,600 | 1.40 |
| 8-Ala-8-Int | 68 | 62,500 | 45,100 | 1.39 |
| 8-Phe-4-Sol | 85 | 103,000 | 53,500 | 1.93 |
| 8-Phe-4-Int | 78 | 168,000 | 63,600 | 2.64 |
| 8-Phe-8-Sol | 61 | 63,300 | 44,400 | 1.43 |
| 8-Phe-8-Int | 60 | 111,000 | 71,800 | 1.55 |
| Polymer a | [η] b (dL/g) | |
|---|---|---|
| Interfacial Polycondensation | Thermal Polycondensation | |
| PGHGT | 0.68 | 0.74 |
| PGDGT | 0.60 | 0.68 |
| PGHG0 | c | 0.60 |
| PGDG0 | c | 0.73 |
| PGHG2 | 0.23 | 0.38 |
| PGDG2 | 0.20 | 0.40 |
| PGHG8 | 0.37 | 0.51 |
| PGDG8 | 0.41 | 0.48 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Díaz, A.; Katsarava, R.; Puiggalí, J. Synthesis, Properties and Applications of Biodegradable Polymers Derived from Diols and Dicarboxylic Acids: From Polyesters to Poly(ester amide)s. Int. J. Mol. Sci. 2014, 15, 7064-7123. https://doi.org/10.3390/ijms15057064
Díaz A, Katsarava R, Puiggalí J. Synthesis, Properties and Applications of Biodegradable Polymers Derived from Diols and Dicarboxylic Acids: From Polyesters to Poly(ester amide)s. International Journal of Molecular Sciences. 2014; 15(5):7064-7123. https://doi.org/10.3390/ijms15057064
Chicago/Turabian StyleDíaz, Angélica, Ramaz Katsarava, and Jordi Puiggalí. 2014. "Synthesis, Properties and Applications of Biodegradable Polymers Derived from Diols and Dicarboxylic Acids: From Polyesters to Poly(ester amide)s" International Journal of Molecular Sciences 15, no. 5: 7064-7123. https://doi.org/10.3390/ijms15057064