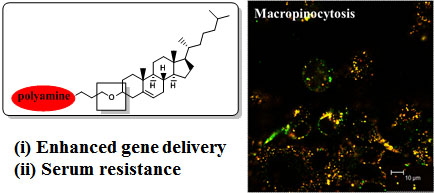
Efficient Delivery of Plasmid DNA Using Cholesterol-Based Cationic Lipids Containing Polyamines and Ether Linkages
Abstract
:1. Introduction
2. Results
2.1. Cationic Lipids
2.2. In Vitro Characterization of Transfection Efficiencies at Different Lipid:DOPE Weight Ratios
2.3. In Vitro Transfection Efficiencies in Response to Changes in N/P Weight Ratio at an Optimized Lipid:DOPE Ratio
2.4. Comparison with Commercial Liposomes
2.5. In Vitro Cytotoxicity of Liposomes
2.6. Liposome/DNA Binding Interaction
2.7. Liposome Formulation in the Presence of Helper-Lipid DOPE in Aqueous Solution
3. Discussion
4. Experimental Section
4.1. General Procedures and Materials
4.2. Liposomes
4.2.1. Preparation of Liposomes
4.2.2. Size Measurement and Zeta Potential Analysis of Liposomes
4.3. Lipoplexes
4.3.1. Preparation of Plasmids DNA
4.3.2. Preparation of Lipoplexes
4.3.3. DNA-Binding Assay
4.4. Cell Biology
4.4.1. In Vitro Transfection
4.4.2. Luciferase Assay
4.4.3. Fluorescence Microscopy: GFP Expression
4.4.4. Cytotoxicity Assay
4.4.5. Statistical Analysis
5. Conclusions
Supplementary Information
Synthesis of Cationic Lipids
Synthesis of 3β-cholest-5-en-3-yl N-(3-aminopropyl) carbamate, lipid A
Synthesis of 2-cyanoethyl-O-β-cholesterol ether 2
General Method for the Synthesis of Intermediates (Compound 3, 6, and 9)
General Method for the Synthesis of Target Lipids (Compound 4, 7, and 10)
Synthesis of 2-cyanoethyl-3-aminopropyl-O-β-cholesterol ether 5 and di-2-cyanoethyl-3-aminopropyl-O-β-cholesterol ether 8
Acknowledgments
Conflicts of Interest
- Author ContributionsBieong-Kil Kim and Young-Bae Seu designed the study, and carried out the synthesis. Yun-Ui Bae, Tae-Won Kwak, Hyungu Kang, and Guen-Bae Hwang did the gene expression experiment. Ik-Jae Moon, So-Young Park and Kyung-Oh Doh designed the study, analyzed data and wrote manuscript.
References
- El-Aneed, A. An overview of current delivery systems in cancer gene therapy. J. Control. Release 2004, 94, 1–14. [Google Scholar]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar]
- Bajaj, A.; Kondiah, P.; Bhattacharya, S. Design, synthesis, and in vitro gene delivery efficacies of novel cholesterol-based gemini cationic lipids and their serum compatibility: A structure-activity investigation. J. Med. Chem 2007, 50, 2432–2442. [Google Scholar]
- Zhang, S.; Zhao, B.; Jiang, H.; Wang, B.; Ma, B. Cationic lipids and polymers mediated vectors for delivery of sirna. J. Control. Release 2007, 123, 1–10. [Google Scholar]
- Ewert, K.; Ahmad, A.; Evans, H.M.; Schmidt, H.W.; Safinya, C.R. Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J. Med. Chem 2002, 45, 5023–5029. [Google Scholar]
- Wang, L.; MacDonald, R.C. New strategy for transfection: Mixtures of medium-chain and long-chain cationic lipids synergistically enhance transfection. Gene Ther 2004, 11, 1358–1362. [Google Scholar]
- Kaneda, Y.; Tabata, Y. Non-viral vectors for cancer therapy. Cancer Sci 2006, 97, 348–354. [Google Scholar]
- Siehl, J.M.; Thiel, E.; Schmittel, A.; Hutter, G.; Deckert, P.M.; Szelenyi, H.; Keilholz, U. Ifosfamide/liposomal daunorubicin is a well tolerated and active first-line chemotherapy regimen in advanced soft tissue sarcoma: Results of a phase ii study. Cancer 2005, 104, 611–617. [Google Scholar]
- Gao, H.; Hui, K.M. Synthesis of a novel series of cationic lipids that can act as efficient gene delivery vehicles through systematic heterocyclic substitution of cholesterol derivatives. Gene Ther 2001, 8, 855–863. [Google Scholar]
- Gao, X.; Huang, L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem. Biophys. Res. Commun 1991, 179, 280–285. [Google Scholar]
- Takeuchi, K.; Ishihara, M.; Kawaura, C.; Noji, M.; Furuno, T.; Nakanishi, M. Effect of zeta potential of cationic liposomes containing cationic cholesterol derivatives on gene transfection. FEBS Lett 1996, 397, 207–209. [Google Scholar]
- Kisoon, N.; Ariatti, M.; Moodley, T. A novel cationic cholesterol derivative, its formulation into liposomes, and the efficient transfection of the transformed human cell lines hepg2 and hela. Drug Deliv 2002, 9, 161–167. [Google Scholar]
- Reynier, P.; Briane, D.; Coudert, R.; Fadda, G.; Bouchemal, N.; Bissieres, P.; Taillandier, E.; Cao, A. Modifications in the head group and in the spacer of cholesterol-based cationic lipids promote transfection in melanoma b16-f10 cells and tumours. J. Drug Target 2004, 12, 25–38. [Google Scholar]
- Wu, G.Y.; Wu, C.H. Receptor-mediated gene delivery and expression in vivo. J. Biol. Chem. 1988, 263, 14621–14624. [Google Scholar]
- Dong, Y.H.; Skoultchi, A.I.; Pollard, J.W. Efficient DNA transfection of quiescent mammalian-cells using poly-l-ornithine. Nucleic Acids Res 1993, 21, 771–772. [Google Scholar]
- Ghosh, Y.K.; Visweswariah, S.S.; Bhattacharya, S. Nature of linkage between the cationic headgroup and cholesteryl skeleton controls gene transfection efficiency. FEBS Lett 2000, 473, 341–344. [Google Scholar]
- Kim, B.K.; Doh, K.O.; Nam, J.H.; Kang, H.; Park, J.G.; Moon, I.J.; Seu, Y.B. Synthesis of novel cholesterol-based cationic lipids for gene delivery. Bioorg. Med. Chem. Lett 2009, 19, 2986–2989. [Google Scholar]
- Kim, B.K.; Bae, Y.U.; Doh, K.O.; Hwang, G.B.; Lee, S.H.; Kang, H.; Seu, Y.B. The synthesis of cholesterol-based cationic lipids with trimethylamine head and the effect of spacer structures on transfection efficiency. Bioorg. Med. Chem. Lett 2011, 21, 3734–3737. [Google Scholar]
- Kim, B.K.; Doh, K.O.; Bae, Y.U.; Seu, Y.B. Synthesis and optimization of cholesterol-based diquaternary ammonium gemini surfactant (chol-gs) as a new gene delivery vector. J. Microbiol. Biotechnol 2011, 21, 93–99. [Google Scholar]
- Kim, B.K.; Doh, K.O.; Hwang, G.B.; Seu, Y.B. Transfection property of a new cholesterol-based cationic lipid containing tri-2-hydroxyethylamine as gene delivery vehicle. J. Microbiol. Biotechnol 2012, 22, 866–871. [Google Scholar]
- Tagami, T.; Barichello, J.M.; Kikuchi, H.; Ishida, T.; Kiwada, H. The gene-silencing effect of sirna in cationic lipoplexes is enhanced by incorporating pdna in the complex. Int. J. Pharm 2007, 333, 62–69. [Google Scholar]
- Maestrelli, F.; Gonzalez-Rodriguez, M.L.; Rabasco, A.M.; Mura, P. Effect of preparation technique on the properties of liposomes encapsulating ketoprofen-cyclodextrin complexes aimed for transdermal delivery. Int. J. Pharm 2006, 312, 53–60. [Google Scholar]
- Al-Jamal, W.T.; Kostarelos, K. Construction of nanoscale multicompartment liposomes for combinatory drug delivery. Int. J. Pharm 2007, 331, 182–185. [Google Scholar]
- Simberg, D.; Weiss, A.; Barenholz, Y. Reversible mode of binding of serum proteins to dotap/cholesterol lipoplexes: A possible explanation for intravenous lipofection efficiency. Hum. Gene Ther 2005, 16, 1087–1096. [Google Scholar]
- Yang, J.P.; Huang, L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther 1997, 4, 950–960. [Google Scholar]
- Zelphati, O.; Uyechi, L.S.; Barron, L.G.; Szoka, F.C., Jr. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim. Biophys. Acta 1998, 1390, 119–133. [Google Scholar]
- Audouy, S.; Molema, G.; de Leij, L.; Hoekstra, D. Serum as a modulator of lipoplex-mediated gene transfection: Dependence of amphiphile, cell type and complex stability. J. Gene Med 2000, 2, 465–476. [Google Scholar]
- Crook, K.; Stevenson, B.J.; Dubouchet, M.; Porteous, D.J. Inclusion of cholesterol in dotap transfection complexes increases the delivery of DNA to cells in vitro in the presence of serum. Gene Ther 1998, 5, 137–143. [Google Scholar]
- Han, S.E.; Kang, H.; Shim, G.Y.; Suh, M.S.; Kim, S.J.; Kim, J.S.; Oh, Y.K. Novel cationic cholesterol derivative-based liposomes for serum-enhanced delivery of sirna. Int. J. Pharm 2008, 353, 260–269. [Google Scholar]
- Soutschek, J.; Akinc, A.; Bramlage, B.; Charisse, K.; Constien, R.; Donoghue, M.; Elbashir, S.; Geick, A.; Hadwiger, P.; Harborth, J.; et al. Therapeutic silencing of an endogenous gene by systemic administration of modified sirnas. Nature 2004, 432, 173–178. [Google Scholar]
- Ish-I, T.I.R.; Snip, E.; Ikeda, M.; Shinkai, S. [60]fullerene can reinforce the organogel structure of porphyrin-appended cholesterol derivatives: Novel odd-even effect of the (ch2)n spacer on the organogel stability. Langmuir 2001, 17, 5825–5833. [Google Scholar]
- Hassani, Z.; Lemkine, G.F.; Erbacher, P.; Palmier, K.; Alfama, G.; Giovannangeli, C.; Behr, J.P.; Demeneix, B.A. Lipid-mediated sirna delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J. Gene Med 2005, 7, 198–207. [Google Scholar]
- Vijayanathan, V.; Thomas, T.; Thomas, T.J. DNA nanoparticles and development of DNA delivery vehicles for gene therapy. Biochemistry 2002, 41, 14085–14094. [Google Scholar]
- Manosroi, A.; Thathang, K.; Manosroi, J.; Werner, R.G.; Schubert, R.; Peschka-Suss, R. Expression of luciferase plasmid (pcmvluc) entrapped in dppc/cholesterol/ddab liposomes in hela cell lines. J. Liposome Res 2009, 19, 131–140. [Google Scholar]
- Huang, Q.D.; Ou, W.J.; Chen, H.; Feng, Z.H.; Wang, J.Y.; Zhang, J.; Zhu, W.; Yu, X.Q. Novel cationic lipids possessing protonated cyclen and imidazolium salt for gene delivery. Eur. J. Pharm. Biopharm 2011, 78, 326–335. [Google Scholar]
- Biswas, J.; Bajaj, A.; Bhattacharya, S. Membranes of cationic gemini lipids based on cholesterol with hydroxyl headgroups and their interactions with DNA and phospholipid. J. Phys. Chem. B 2011, 115, 478–486. [Google Scholar]
- Islam, R.U.; Hean, J.; van Otterlo, W.A.; de Koning, C.B.; Arbuthnot, P. Efficient nucleic acid transduction with lipoplexes containing novel piperazine- and polyamine-conjugated cholesterol derivatives. Bioorg. Med. Chem. Lett 2009, 19, 100–103. [Google Scholar]
- Dabkowska, A.P.; Barlow, D.J.; Hughes, A.V.; Campbell, R.A.; Quinn, P.J.; Lawrence, M.J. The effect of neutral helper lipids on the structure of cationic lipid monolayers. J. R. Soc. Interface 2012, 9, 548–561. [Google Scholar]
- Zhang, Y.; Bradshaw-Pierce, E.L.; Delille, A.; Gustafson, D.L.; Anchordoquy, T.J. In vivo comparative study of lipid/DNA complexes with different in vitro serum stability: Effects on biodistribution and tumor accumulation. J. Pharm. Sci 2008, 97, 237–250. [Google Scholar]
- Duarte, S.; Faneca, H.; de Lima, M.C. Non-covalent association of folate to lipoplexes: A promising strategy to improve gene delivery in the presence of serum. J. Control. Release 2011, 149, 264–272. [Google Scholar]
- Faneca, H.; Simoes, S.; de Lima, M.C. Evaluation of lipid-based reagents to mediate intracellular gene delivery. Biochim. Biophys. Acta 2002, 1567, 23–33. [Google Scholar]
- Koster, F.; Finas, D.; Schulz, C.; Hauser, C.; Diedrich, K.; Felberbaum, R. Additive effect of steroids and cholesterol on the liposomal transfection of the breast cancer cell line t-47d. Int. J. Mol. Med 2004, 14, 769–772. [Google Scholar]
- Wheeler, C.J.; Felgner, P.L.; Tsai, Y.J.; Marshall, J.; Sukhu, L.; Doh, S.G.; Hartikka, J.; Nietupski, J.; Manthorpe, M.; Nichols, M.; et al. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung. Proc. Natl. Acad. Sci. USA 1996, 93, 11454–11459. [Google Scholar]
- Ren, T.; Song, Y.K.; Zhang, G.; Liu, D. Structural basis of dotma for its high intravenous transfection activity in mouse. Gene Ther 2000, 7, 764–768. [Google Scholar]
- Hattori, Y.; Suzuki, S.; Kawakami, S.; Yamashita, F.; Hashida, M. The role of dioleoylphosphatidylethanolamine (dope) in targeted gene delivery with mannosylated cationic liposomes via intravenous route. J. Control. Release 2005, 108, 484–495. [Google Scholar]
- Kim, W.J.; Christensen, L.V.; Jo, S.; Yockman, J.W.; Jeong, J.H.; Kim, Y.H.; Kim, S.W. Cholesteryl oligoarginine delivering vascular endothelial growth factor sirna effectively inhibits tumor growth in colon adenocarcinoma. Mol. Ther 2006, 14, 343–350. [Google Scholar]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc 2010, 132, 5761–5768. [Google Scholar]
- Capriotti, A.L.; Caracciolo, G.; Caruso, G.; Foglia, P.; Pozzi, D.; Samperi, R.; Lagana, A. Differential analysis of “protein corona” profile adsorbed onto different nonviral gene delivery systems. Anal. Biochem 2011, 419, 180–189. [Google Scholar]
- Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Amenitsch, H.; Lagana, A. Evolution of the protein corona of lipid gene vectors as a function of plasma concentration. Langmuir 2011, 27, 15048–15053. [Google Scholar]
- Wong, A.W.; Scales, S.J.; Reilly, D.E. DNA internalized via caveolae requires microtubule-dependent, rab7-independent transport to the late endocytic pathway for delivery to the nucleus. J. Biol. Chem 2007, 282, 22953–22963. [Google Scholar]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar]
- Cardarelli, F.; Pozzi, D.; Bifone, A.; Marchini, C.; Caracciolo, G. Cholesterol-dependent macropinocytosis and endosomal escape control the transfection efficiency of lipoplexes in cho living cells. Mol. Pharm 2012, 9, 334–340. [Google Scholar]
- Zhang, X.X.; Allen, P.G.; Grinstaff, M. Macropinocytosis is the major pathway responsible for DNA transfection in cho cells by a charge-reversal amphiphile. Mol. Pharm 2011, 8, 758–766. [Google Scholar]
- Bae, Y.U.; Kim, B.K.; Park, J.W.; Seu, Y.B.; Doh, K.O. Endocytic pathway and resistance to cholesterol depletion of cholesterol derived cationic lipids for gene delivery. Mol. Pharm 2012, 9, 3579–3585. [Google Scholar]







| Liposome composition | Size (nm) | ζ-potential (mV) |
|---|---|---|
| Lipid A only | 87 ± 5 | 42 ± 8 |
| Lipid B/DOPE = 3/1 | 176 ± 9 | 50 ± 5 |
| Lipid C/DOPE = 1/1 | 142 ± 1 | 55 ± 1 |
| Lipid D/DOPE = 1/1 | 162 ± 7 | 59 ± 1 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, B.-K.; Seu, Y.-B.; Bae, Y.-U.; Kwak, T.-W.; Kang, H.; Moon, I.-J.; Hwang, G.-B.; Park, S.-Y.; Doh, K.-O. Efficient Delivery of Plasmid DNA Using Cholesterol-Based Cationic Lipids Containing Polyamines and Ether Linkages. Int. J. Mol. Sci. 2014, 15, 7293-7312. https://doi.org/10.3390/ijms15057293
Kim B-K, Seu Y-B, Bae Y-U, Kwak T-W, Kang H, Moon I-J, Hwang G-B, Park S-Y, Doh K-O. Efficient Delivery of Plasmid DNA Using Cholesterol-Based Cationic Lipids Containing Polyamines and Ether Linkages. International Journal of Molecular Sciences. 2014; 15(5):7293-7312. https://doi.org/10.3390/ijms15057293
Chicago/Turabian StyleKim, Bieong-Kil, Young-Bae Seu, Yun-Ui Bae, Tae-Won Kwak, Hyungu Kang, Ik-Jae Moon, Guen-Bae Hwang, So-Young Park, and Kyung-Oh Doh. 2014. "Efficient Delivery of Plasmid DNA Using Cholesterol-Based Cationic Lipids Containing Polyamines and Ether Linkages" International Journal of Molecular Sciences 15, no. 5: 7293-7312. https://doi.org/10.3390/ijms15057293