Lyophilized Platelet-Rich Fibrin (PRF) Promotes Craniofacial Bone Regeneration through Runx2
Abstract
:1. Introduction
2. Results
2.1. Lyophilized Platelet-Rich Fibrin (PRF) Featured a Sponge-Like Microstructure with 13.4-Fold Larger Pores Compared to Fresh PRF
2.2. Lyophilized PRF Improved Cell Proliferation and Migration of Periodontal Progenitors in Vitro when Compared to Fresh PRF and DMEM Medium
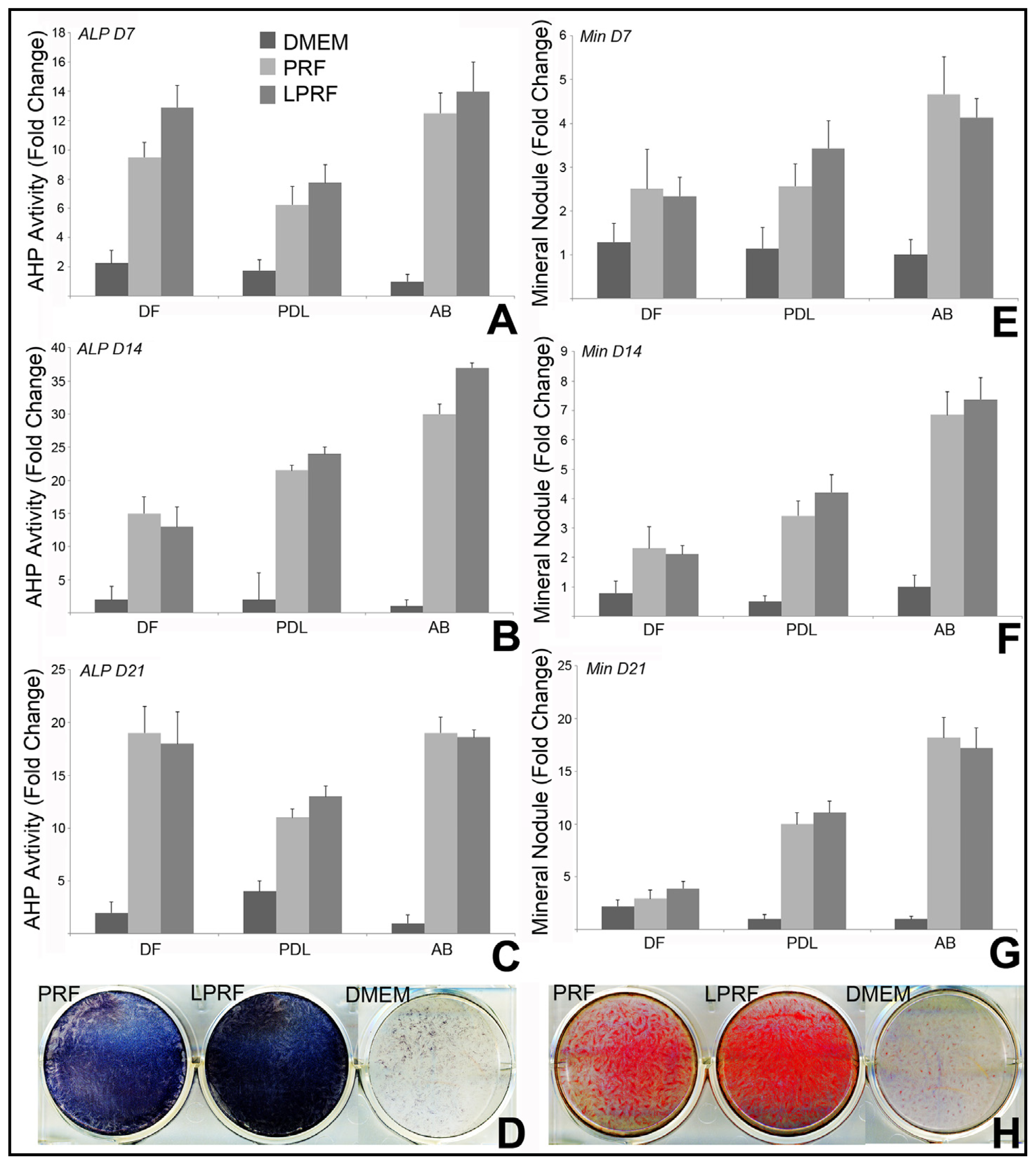
2.3. Lyophilized PRF Enhanced the Mineralization Activity of Periodontal Progenitors in Vitro
2.4. Lyophilized PRF Upregulated RunX2 Expression and Modulated Matrix Gla Protein Gene Expression in Alveolar Bone Cells
2.5. Lyophilized PRF Promoted Cell Homing and Collagen Synthesis in Subcutaneous Implants
2.6. Lyophilized PRF Enhanced Cranial Bone Regeneration
3. Discussion
4. Experimental Section
4.1. Preparation of Fresh PRF, Lyophilized PRF, and Conditioned Medium
4.2. Isolation of Human Dental Mesenchymal Stem Cells
4.3. Tissue Processing
4.4. Scanning Electron Microscopy
4.5. Proliferation Assay
4.6. Chemotaxis Assay
4.7. Alkaline Phosphatase (ALP) Activity Assessment
4.8. Quantification of Mineralization Nodules
4.9. Gene Expression Assay
4.10. Subcutaneous Implant
4.11. Bone Regeneration in the Rat Critical Size Calvarial Defects
4.12. Micro-CT Analysis
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsQi Li, David A. Reed, Xianghong Luan, and Thomas G.H. Diekwisch wrote the manuscript, Liu Min, Gokul Gopinathan, and Smit J. Dangaria performed key experiments, Leo Li, Yajun Geng, Maria-Therese Galang, Praveen Gajendrareddy, and Yanmin Zhou were involved in data analysis, and Thomas G.H. Diekwisch oversaw the entire study.
References
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 2006, 101, e56–e60. [Google Scholar]
- Bensaid, W.; Triffitt, J.T.; Blanchat, C.; Oudina, K.; Sedel, L.; Petite, H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 2003, 24, 2497–2502. [Google Scholar]
- Kang, Y.H.; Jeon, S.H.; Park, J.Y.; Chung, J.H.; Choung, Y.H.; Choung, H.W.; Kim, E.S.; Choung, P.H. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng. Part A 2011, 17, 349–359. [Google Scholar]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A comparative study of Platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 707–713. [Google Scholar]
- Dohan Ehrenfest, D.M.; Diss, A.; Odin, G.; Doglioli, P.; Hippolyte, M.P.; Charrier, J.B. In vitro effects of Choukroun’s PRF (Platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 2009, 108, 341–352. [Google Scholar]
- Liao, H.T.; Marra, K.G.; Rubin, J.P. Application of Platelet-Rich Plasma and Platelet-rich fibrin in fat grafting: Basic science and literature review. Tissue Eng. Part B Rev 2013, in press. [Google Scholar]
- Naik, B.; Karunakar, P.; Jayadev, M.; Marshal, V.R. Role of Platelet rich fibrin in wound healing: A critical review. J. Conserv. Dent 2013, 16, 284–293. [Google Scholar]
- Sclafani, A.P.; Saman, M. Platelet-rich fibrin matrix for facial plastic surgery. Facial. Plast. Surg. Clin. N. Am 2012, 20, 177–186. [Google Scholar]
- Sharma, A.; Pradeep, A.R. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous Platelet-rich fibrin: A randomized controlled clinical trial. J. Periodontol 2011, 82, 1705–1712. [Google Scholar]
- Chang, Y.C.; Zhao, J.H. Effects of Platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust. Dent. J 2011, 56, 365–371. [Google Scholar]
- Zumstein, M.A.; Berger, S.; Schober, M.; Boileau, P.; Nyffeler, R.W.; Horn, M.; Dahinden, C.A. Leukocyte- and Platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: Review, preliminary results and future directions. Curr. Pharm. Biotechnol 2012, 13, 1196–1206. [Google Scholar]
- Matsunaga, D.; Akizuki, S.; Takizawa, T.; Omae, S.; Kato, H. Compact Platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee 2013, 20, 545–550. [Google Scholar]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Scacco, S.; Inchingolo, A.D.; Dipalma, G.; Vermesan, D.; Abbinante, A.; Cagiano, R. Trial with Platelet-rich fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: Clinical and radiological evaluations. Eur. Rev. Med. Pharmacol. Sci 2010, 14, 1075–1084. [Google Scholar]
- Zhang, Y.; Tangl, S.; Huber, C.D.; Lin, Y.; Qiu, L.; Rausch-Fan, X. Effects of Choukroun’s Platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J. Craniomaxillofac. Surg 2012, 40, 321–328. [Google Scholar]
- Bielanski, A.; Bergeron, H.; Lau, P.C.; Devenish, J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology 2003, 46, 146–152. [Google Scholar]
- Morris, G.J. The origin, ultrastructure, and microbiology of the sediment accumulating in liquid nitrogen storage vessels. Cryobiology 2005, 50, 231–238. [Google Scholar]
- Choi, C.W.; Kim, B.S.; Seo, J.H.; Shin, S.W.; Kim, Y.H.; Kim, J.S. Long-term engraftment stability of peripheral blood stem cells cryopreserved using the dump-freezing method in a μ80 °C mechanical freezer with 10% dimethyl sulfoxide. Int. J. Hematol 2001, 73, 245–250. [Google Scholar]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part. C Methods 2010, 16, 887–894. [Google Scholar]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar]
- Tsai, C.H.; Shen, S.Y.; Zhao, J.H.; Chang, Y.C. Platelet-rich fibrin modulates cell proliferatation of human periodontally related cells in vitro. J. Dent. Sci. 2009, 4, 103–135. [Google Scholar]
- Dangaria, S.J.; Ito, Y.; Walker, C.; Druzinsky, R.; Luan, X.; Diekwisch, T.G. Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differentiation 2009, 78, 79–90. [Google Scholar]
- Dangaria, S.J.; Ito, Y.; Luan, X.; Diekwisch, T.G. Successful periodontal ligament regeneration by periodontal progenitor preseeding on natural tooth root surfaces. Stem Cells Dev 2011, 20, 1659–1668. [Google Scholar]
- Dangaria, S.J.; Ito, Y.; Yin, L.; Valdre, G.; Luan, X.; Diekwisch, T.G. Apatite microtopographies instruct signaling tapestries for progenitor-driven new attachment of teeth. Tissue Eng. Part A 2011, 17, 279–290. [Google Scholar]
- Pretorius, E.; Briedenhann, S.; Marx, J.; Smit, E.; van der Merwe, C.; Pieters, M.; Franz, C. Ultrastructural comparison of the morphology of three different platelet and fibrin fiber preparations. Anat. Rec. (Hoboken) 2007, 290, 188–198. [Google Scholar]
- Natan, D.; Nagler, A.; Arav, A. Freeze-Drying of mononuclear cells derived from umbilical cord blood followed by colony formation. PLoS One 2009, 4. [Google Scholar] [CrossRef]
- Arnold, P.; Djerassi, I.; Farber, S.; Freeman, G.; Klein, E.; Toch, R. The preparation and clinical administration of lyophilized platelet material to children with acute leukemia and aplastic anemia. J. Pediatr 1956, 49, 517–522. [Google Scholar]
- Bode, A.P.; Fischer, T.H. Lyophilized platelets: Fifty years in the making. Artif. Cells Blood Substit. Immobil. Biotechnol 2007, 35, 125–133. [Google Scholar]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Mueller, R.; Griffith, L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng 2001, 7, 557–572. [Google Scholar]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem 1997, 121, 317–324. [Google Scholar]
- Cao, Y.; Mitchell, G.; Messina, A.; Price, L.; Thompson, E.; Penington, A. The influence of architecture on degradation and tissue ingrowth into three-dimensional poly(lactic-co-glycolic acid) scaffolds in vitro and in vivo. Biomaterials 2006, 27, 2854–2864. [Google Scholar]
- Tablin, F.; Walker, N.J.; Hogle, S.E.; Pratt, S.M.; Norris, J.W. Assessment of platelet growth factors in supernatants from rehydrated freeze-dried equine platelets and their effects on fibroblasts in vitro. Am. J. Vet. Res. 2008, 69, 1512–1519. [Google Scholar]
- McCarrel, T.; Fortier, L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J. Orthop. Res 2009, 27, 1033–1042. [Google Scholar]
- Li, Q.; Pan, S.; Dangaria, S.J.; Gopinathan, G.; Kolokythas, A.; Chu, S.; Geng, Y.; Zhou, Y.; Luan, X. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. Biomed. Res. Int 2013, 2013. [Google Scholar] [CrossRef]
- Greco, F.; de Palma, L.; Specchia, N.; Lisai, P. Experimental investigation into reparative osteogenesis with fibrin adhesive. Arch. Orthop. Trauma Surg 1998, 107, 99–104. [Google Scholar]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Young, G.; Shortkroff, S.; Louie, L.K.; Sledge, C.B.; Yannas, I.V.; Spector, M. Matrix collagen type and pore size influence behavior of seeded canine chondrocytes. Biomaterials 1997, 18, 769–776. [Google Scholar]
- Szpalski, C.; Barr, J.; Wetterau, M.; Saadeh, P.B.; Warren, S.M. Cranial bone defects: Current and future strategies. Neurosurg. Focus 2010, 29. [Google Scholar] [CrossRef]
- Dangaria, S.J.; Ito, Y.; Luan, X.; Diekwisch, T.G. Differentiation of neural-crest-derived intermediate pluripotent progenitors into committed periodontal populations involves unique molecular signature changes, cohort shifts, and epigenetic modifications. Stem Cells Dev 2011, 20, 39–52. [Google Scholar]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, J.A.; Kasper, F.K.; Mikos, A. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Prot 2012, 7, 1918–1929. [Google Scholar]





© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Q.; Reed, D.A.; Min, L.; Gopinathan, G.; Li, S.; Dangaria, S.J.; Li, L.; Geng, Y.; Galang, M.-T.; Gajendrareddy, P.; et al. Lyophilized Platelet-Rich Fibrin (PRF) Promotes Craniofacial Bone Regeneration through Runx2. Int. J. Mol. Sci. 2014, 15, 8509-8525. https://doi.org/10.3390/ijms15058509
Li Q, Reed DA, Min L, Gopinathan G, Li S, Dangaria SJ, Li L, Geng Y, Galang M-T, Gajendrareddy P, et al. Lyophilized Platelet-Rich Fibrin (PRF) Promotes Craniofacial Bone Regeneration through Runx2. International Journal of Molecular Sciences. 2014; 15(5):8509-8525. https://doi.org/10.3390/ijms15058509
Chicago/Turabian StyleLi, Qi, David A. Reed, Liu Min, Gokul Gopinathan, Steve Li, Smit J. Dangaria, Leo Li, Yajun Geng, Maria-Therese Galang, Praveen Gajendrareddy, and et al. 2014. "Lyophilized Platelet-Rich Fibrin (PRF) Promotes Craniofacial Bone Regeneration through Runx2" International Journal of Molecular Sciences 15, no. 5: 8509-8525. https://doi.org/10.3390/ijms15058509



