Direct Interaction between Selenoprotein P and Tubulin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening an Interacting Protein of SelP from the Human Fetal-Brain cDNA Library
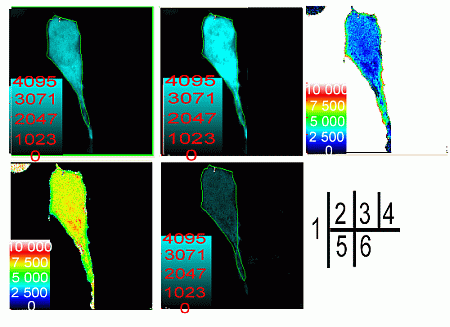
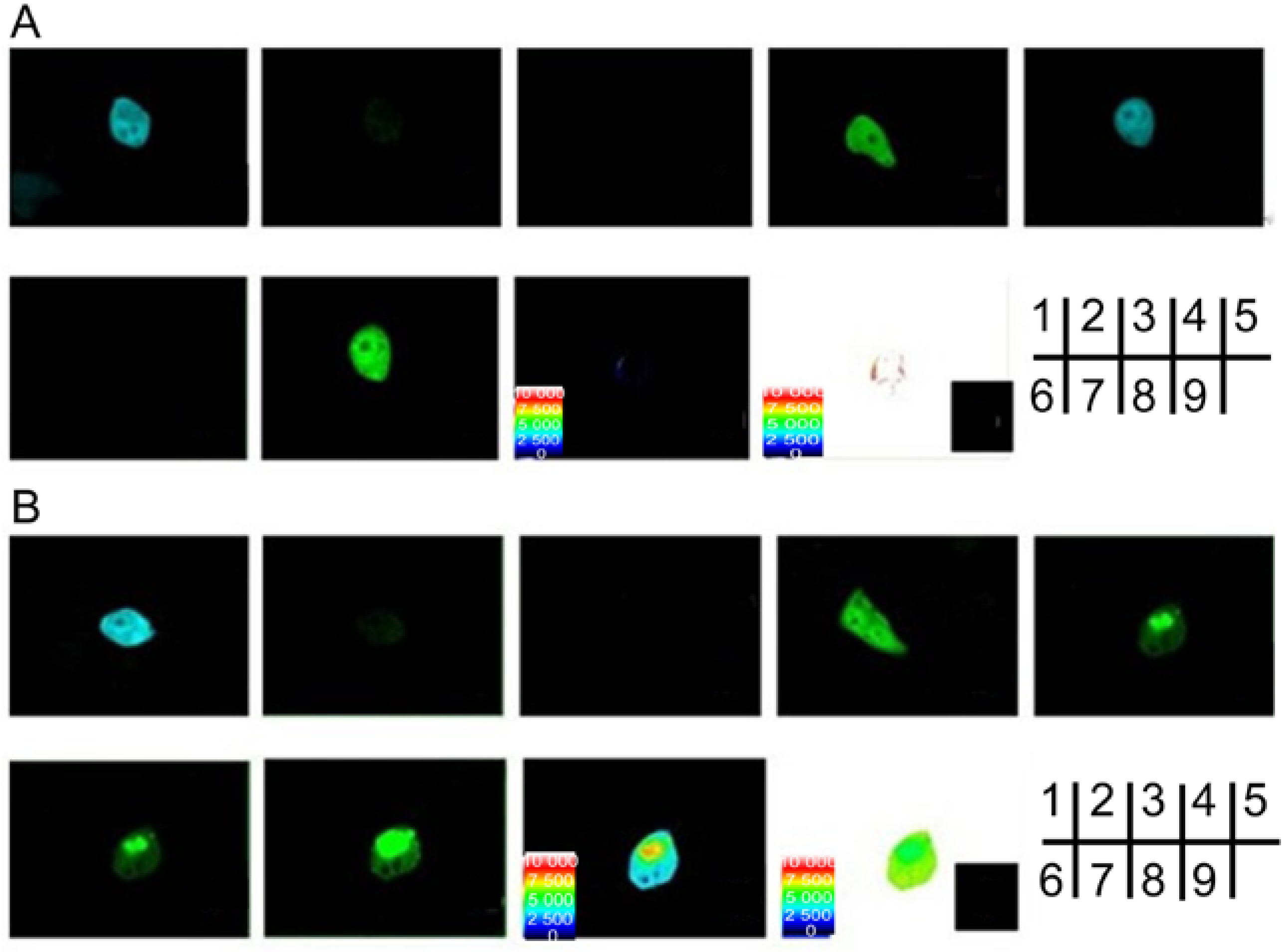
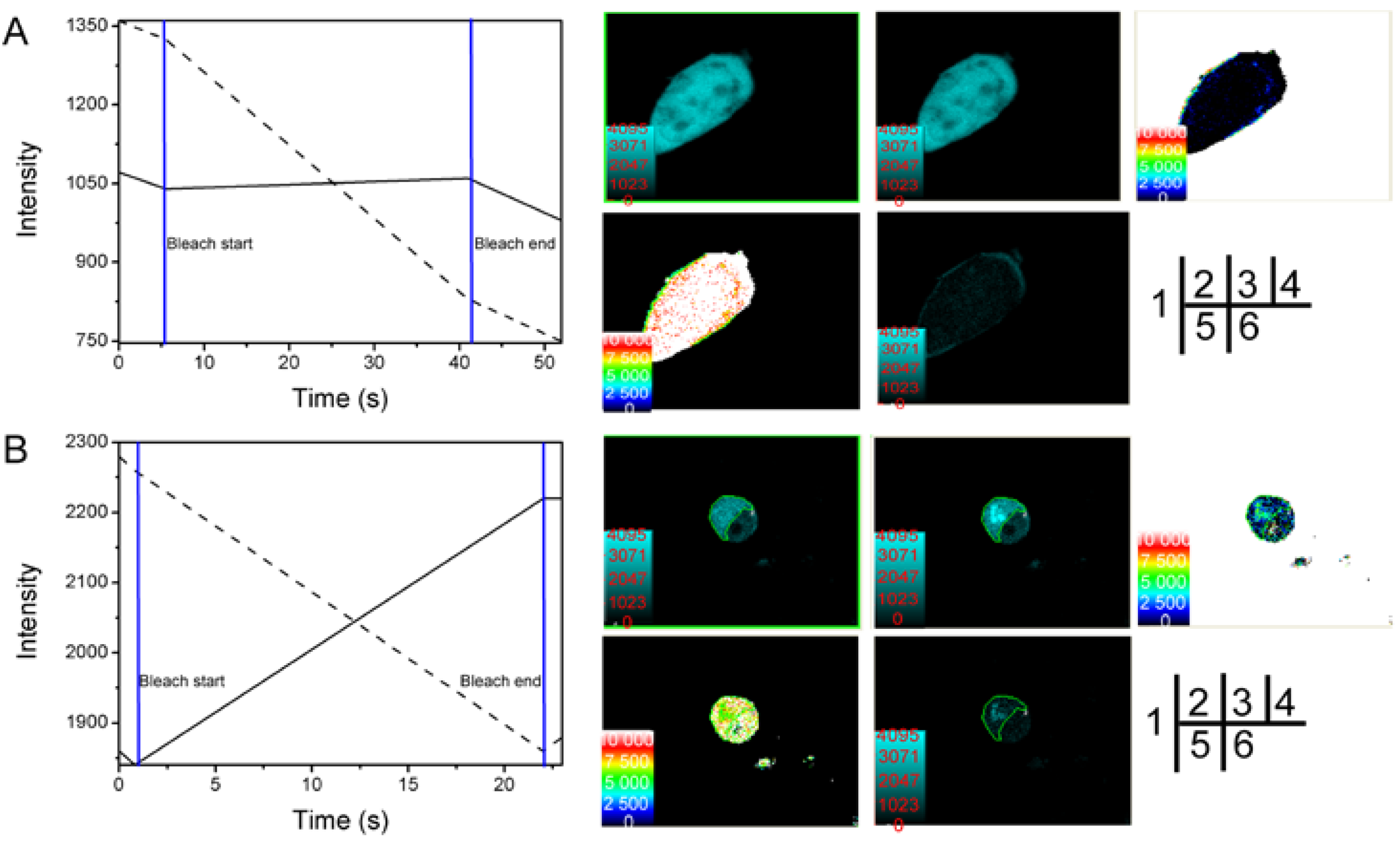
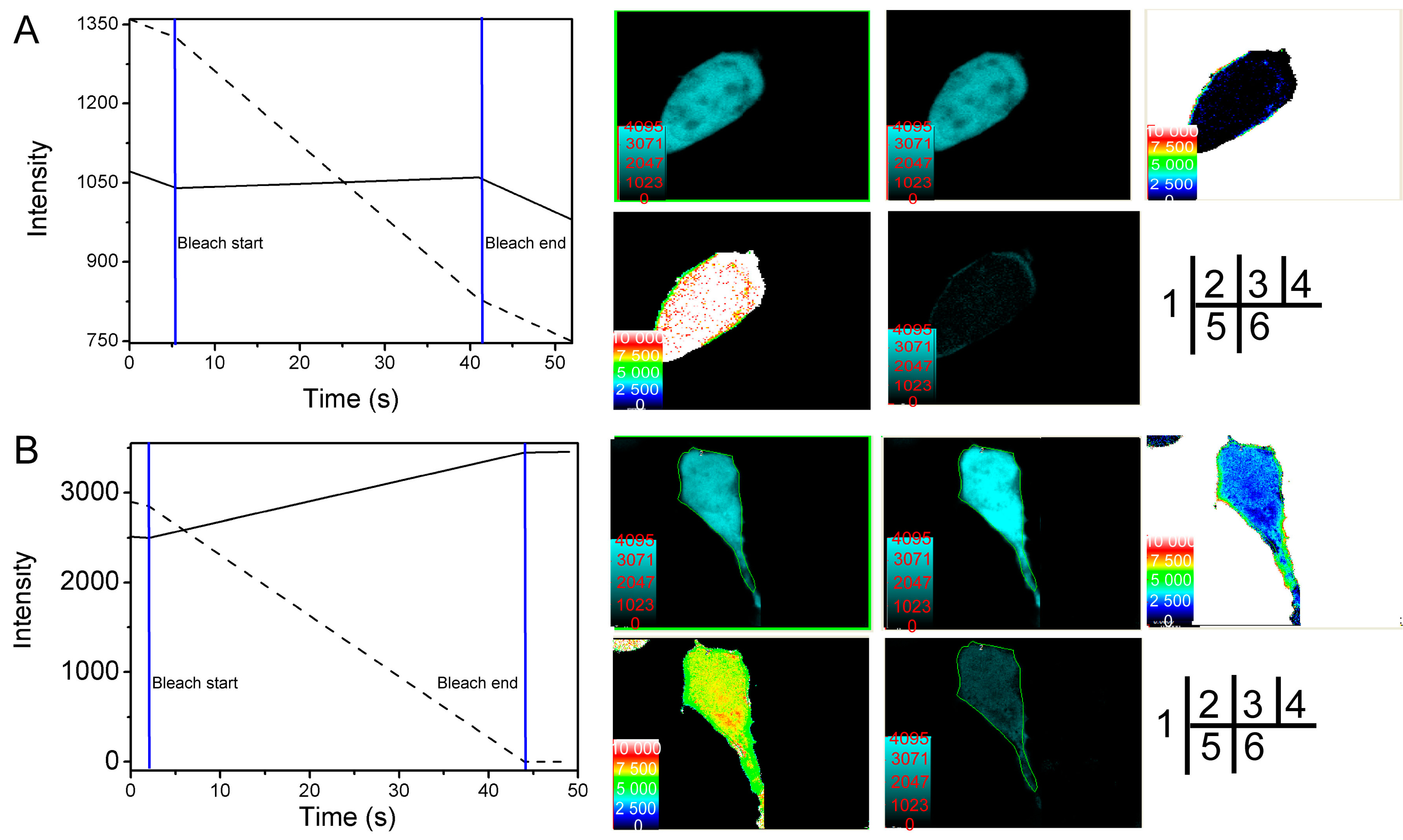
2.2. Verification of the Interaction between SelP' and Tubulin by Fluorescence Resonance Energy Transfer (FRET)

| Cell Number | FRET’s Efficiency E (%) | Distance between Donor and Acceptor r (nm) |
|---|---|---|
| 1 | 19.7 | 6.981 |
| 2 | 18.7 | 6.884 |
| 3 | 32.6 | 6.095 |
| 4 | 17.7 | 7.222 |
| 5 | 18.0 | 6.849 |
| Average | 21.34 | 6.806 |
| Cell Number | FRET Efficiency E (%) | Distance between Donor and Acceptor r (nm) |
|---|---|---|
| 1 | 17.6 | 7.794 |
| 2 | 14.1 | 8.239 |
| 3 | 12.2 | 8.816 |
| 4 | 31.5 | 6.634 |
| 5 | 20.0 | 7.595 |
| 6 | 26.7 | 7.293 |
| 7 | 19.2 | 7.845 |
| 8 | 17.9 | 7.744 |
| 9 | 35.6 | 6.341 |
| 10 | 20.2 | 7.551 |
| 11 | 25.0 | 6.856 |
| Average | 21.81 | 7.519 |
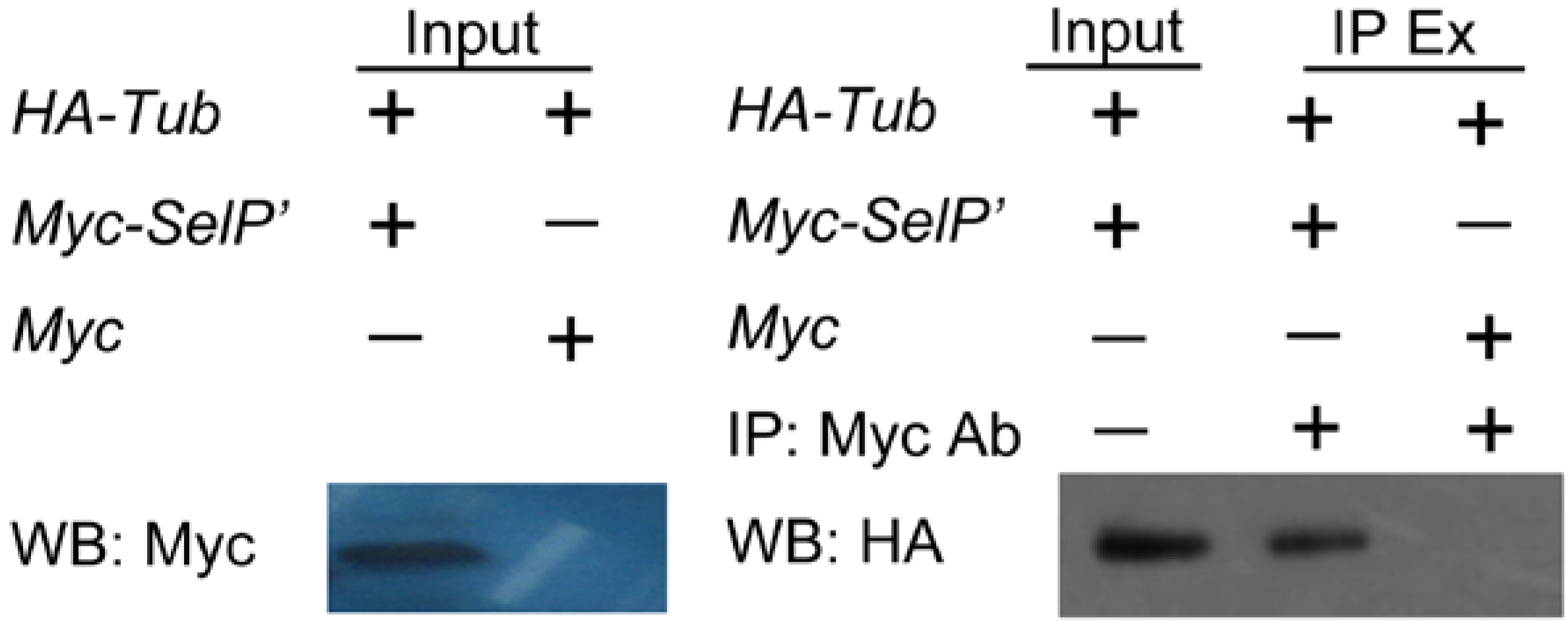
2.3. Verification of the Interaction between SelP' and Tubulin by Co-Immunoprecipitation Assay
2.4. Studying the Interactive Domains between SelP' and Tubulin by FRET
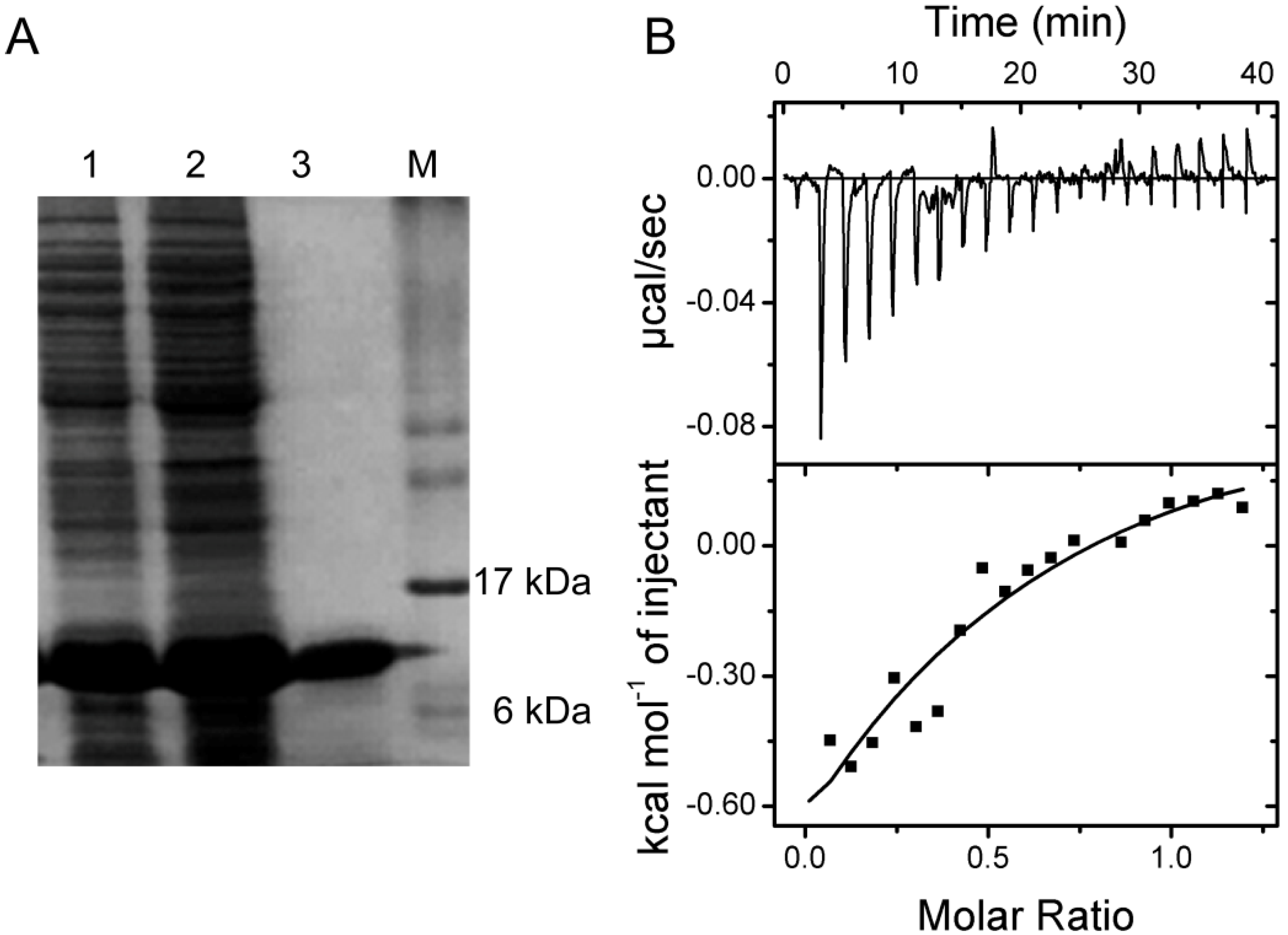
2.5. Verification of the Interaction between SelP-H and Tub-C by Isothermal Titration Calorimetry (ITC)
3. Experimental Section
3.1. Materials
3.2. Gene Amplification and Plasmid Construction
| Number | Sequence |
|---|---|
| F1 | 5'-ATGGCCATTACGGCCGAGAGCCAGGACCAAAGCTCCTTATGTAAGCAACC-3' |
| R1 | 5'-ATGGCCGAGGCGGCCTTAGTTTGAAGGGCATTCGCACTTTTTTGCCTGATTC-3' |
| F2 | 5'-TCTTCAAGCCAGCTGCTACCTGTGCATACTGC-3' |
| R2 | 5'-GCAGCTGGCTTGAAGAAGAGCAACCACAGTCAC-3' |
| F3 | 5'-TGGCTCCTAGGAGCTGCTGCTGCCATTGTCGAC-3' |
| R3 | 5'-GCAGCTCCTAGGAGCCAACTCTGAATCTGTGG-3' |
| F4 | 5'-TCTGCAATCACCTGCCAGTGTAAAGAAAACC-3' |
| R4 | 5'-GCAGGTGATTGCAGACCCTGTTTTTTCAAAT-3' |
| F5 | 5'-ATCTTTATGTAGCTGCCAGGGACTTCGGGCAG-3' |
| R5 | 5'-GCAGCTACATAAAGATGGGAGGTTTTCTTTAC-3' |
| F6 | 5'-TGCCGTTTGCCTCCAGCAGCCTGCCAAATAAGTCAGCAGCTTAT-3' |
| R6 | 5'-GCAGGCTGCTGGAGGCAAACGGCACTGACAAGATTCAGTTATG-3' |
| F7 | 5'-AGTGCCAGT TGCCGCTGCAAGAATCAGGC-3' |
| R7 | 5'-GCAGCGGCAACTGGCACTGGCTTCTGTGG-3' |
3.3. Library Screening via the Yeast Two-Hybrid System
3.4. Mammalian Cell Culture and Plasmid Transfection
3.5. Fluorescence Resonance Energy Transfer (FRET) Analyses
3.5.1. Acceptor Bleaching Method
3.5.2. Sensitized Emission Method
3.6. Verification by Co-Immunoprecipitation Assay
3.7. Isothermal Titration Calorimetry (ITC)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Pub. Health Nutr. 2001, 4, 593–599. [Google Scholar]
- Liu, Q.; Tian, J.; Chen, P.; Yang, S.L.; Song, Y. Selenium deficiency and Alzheimer’s disease. Chin. Bull. Life Sci. 2012, 24, 892–900. [Google Scholar]
- Forchhammer, K.; Leinfelder, W.; Bock, A. Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein. Nature 1989, 342, 453–456. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P—Expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447. [Google Scholar] [CrossRef]
- Olson, G.E.; Winfrey, V.P.; NagDas, S.K.; Hill, K.E.; Burk, R.F. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J. Biol. Chem. 2007, 282, 12290–12297. [Google Scholar]
- Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Burk, R.F. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J. Biol. Chem. 2008, 283, 6854–6860. [Google Scholar]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Austin, L.M.; Norsworthy, B.K. Deletion of selenoprotein P upregulates urinary selenium excretion and depresses whole-body selenium content. Biochim. Biophys. Acta 2006, 1760, 1789–1793. [Google Scholar] [CrossRef]
- Hill, K.E.; Zhou, J.; McMahan, W.J.; Motley, A.K.; Austin, L.M.; Gesteland, R.F.; Burk, R.F. Deletion of selenoprotein P alters distribution of selenium in the mouse. J. Biol. Chem. 2003, 278, 13640–13646. [Google Scholar]
- Hill, K.E.; Zhou, J.; Austin, L.M.; Motley, A.K.; Ham, A.J.; Olson, G.E.; Atkins, J.F.; Gesteland, R.F.; Burk, R.F. The selenium-rich C-terminal domain of mouse selenoprotein P is necessary for the supply of selenium to brain and testis but not for the maintenance of whole body selenium. J. Biol. Chem. 2007, 282, 10972–10980. [Google Scholar] [CrossRef]
- Kurokawa, S.E.S.; Rose, K.L.; Wu, S.; Motley, A.K.; Hill, S.; Winfrey, V.P.; McDonald, W.H.; Capecchi, M.R.; Atkins, J.F.; Arnér, E.S.; et al. Sepp1UF forms are N-terminal selenoprotein P truncations that have peroxidase activity when coupled with thioredoxin reductase-1. Free Radic. Biol. Med. 2014, 69, 67–76. [Google Scholar] [CrossRef]
- Du, X.B.; Li, H.P.; Wang, Z.; Qiu, S.; Liu, Q.; Ni, J.Z. Selenoprotein P and selenoprotein M block Zn2+-mediated aβ42 aggregation and toxicity. Metallomics 2013, 5, 861–870. [Google Scholar] [CrossRef]
- Du, X.B.; Wang, Z.; Zheng, Y.B.; Li, H.P.; Ni, J.Z.; Liu, Q. Inhibitory effect of selenoprotein p on Cu+/Cu2+-induced aβ42 aggregation and toxicity. Inorg. Chem. 2014, 53, 1672–1678. [Google Scholar] [CrossRef]
- Lu, T.; Pan, Y.; Kao, S.Y.; Li, C.; Kohane, I.; Chan, J.; Yankner, B.A. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883–891. [Google Scholar] [CrossRef]
- Bellinger, F.P.; He, Q.P.; Bellinger, M.T.; Lin, Y.; Raman, A.V.; White, L.R.; Berry, M.J. Association of selenoprotein P with Alzheimer’s pathology in human cortex. J. Alzheimers Dis. 2008, 15, 465–472. [Google Scholar]
- Takemoto, A.S.; Berry, M.J.; Bellinger, F.P. Role of selenoprotein P in Alzheimer’s disease. Ethn. Dis. 2010, 20, 92–95. [Google Scholar]
- Lefevre, J.; Lefèvre, J.; Chernov, K.G.; Joshi, V.; Delga, S.; Toma, F.; Pastré, D.; Curmi, P.A.; Savarin, P. The C terminus of tubulin, a versatile partner for cationic molecules: Binding of Tau, polyamines, and calcium. J. Biol. Chem. 2011, 286, 3065–3078. [Google Scholar] [CrossRef]
- Hill, K.E.; Wu, S.; Motley, A.K.; Stevenson, T.D.; Winfrey, V.P.; Capecchi, M.R.; Atkins, J.F.; Burk, R.F. Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J. Biol. Chem. 2012, 287, 40414–40424. [Google Scholar] [CrossRef]
- Scharpf, M.; Schweizer, U.; Arzberger, T.; Roggendorf, W.; Schomburg, L.; Köhrle, J. Neuronal and ependymal expression of selenoprotein P in the human brain. J. Neural. Transm. 2007, 114, 877–884. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Alili, L.; Bilgic, E.; Sies, H.; Brenneisen, P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic. Biol. Med. 2006, 40, 1513–1523. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Winfrey, V.P.; Kurokawa, S.; Mitchell, S.L.; Zhang, W. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014, 2014. [Google Scholar] [CrossRef]
- Perez, E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009, 8, 2086–2095. [Google Scholar] [CrossRef]
- Tanemura, K.; Murayama, M.; Akagi, T.; Hashikawa, T.; Tominaga, T.; Ichikawa, M.; Yamaguchi, H.; Takashima, A. Neurodegeneration with Tau accumulation in a transgenic mouse expressing V337M human Tau. J. Neurosci. 2002, 22, 133–141. [Google Scholar]
- Ishihara, T.; Higuchi, M.; Zhang, B.; Yoshiyama, Y.; Hong, M.; Trojanowski, J.Q.; Lee, V.M. Attenuated neurodegenerative disease phenotype in Tau transgenic mouse lacking neurofilaments. J. Neurosci. 2001, 21, 6026–6035. [Google Scholar]
- Zhang, B.; Maiti, A.; Shively, S.; Lakhani, F.; McDonald-Jones, G.; Bruce, J.; Lee, E.B.; Xie, S.X.; Joyce, S.; Li, C.; et al. Microtubule-binding drugs offset Tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl. Acad. Sci. USA 2005, 102, 227–231. [Google Scholar] [CrossRef]
- Brunden, K.R.; Zhang, B.; Carroll, J.; Yao, Y.; Potuzak, J.S.; Hogan, A.M.; Iba, M.; James, M.J.; Xie, S.X.; Ballatore, C.; et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci. 2010, 30, 13861–13866. [Google Scholar] [CrossRef]
- Van Eersel, J.; Ke, Y.D.; Liu, X.; Delerue, F.; Kril, J.J.; Götz, J.; Ittner, L.M. Sodium selenate mitigates Tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc. Natl. Acad. Sci. USA 2010, 107, 13888–13893. [Google Scholar] [CrossRef]
- Song, G.L.; Zhang, Z.H.; Wen, L.; Chen, C.; Shi, Q.X.; Zhang, Y.; Ni, J.Z.; Liu, Q. Selenomethionine ameliorates cognitive decline, reduces Tau hyperphosphorylation, and reverses synaptic deficit in the triple transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2014, 2014. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell Longev. 2013, 2013. [Google Scholar] [CrossRef]
- Gamblin, T.C.; King, M.E.; Kuret, J.; Berry, R.W.; Binder, L.I. Oxidative regulation of fatty acid-induced Tau polymerization. Biochemistry 2000, 39, 14203–14210. [Google Scholar] [CrossRef]
- Eckers, J.C.; Kalen, A.L.; Xiao, W.S.; Sarsour, E.H.; Goswami, P.C. Selenoprotein P inhibits radiation-induced late reactive oxygen species accumulation and normal cell injury. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 619–625. [Google Scholar] [CrossRef]
- Xiao, W.S.; Zhu, Y.; Sarsour, E.H.; Kalen, A.L.; Aykin-Burns, N.; Spitz, D.R.; Goswami, P.C. Selenoprotein P regulates 1-(4-Chlorophenyl)-benzo-2,5-quinone-induced oxidative stress and toxicity in human keratinocytes. Free Radic. Biol. Med. 2013, 65, 70–77. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Dong, S.; Qiao, X.F.; Ni, J.Z. A new method for multi-site-directed mutagenesis. Anal. Biochem. 2010, 406, 83–85. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Du, X.; Qiu, S.; Wang, Z.; Wang, R.; Wang, C.; Tian, J.; Liu, Q. Direct Interaction between Selenoprotein P and Tubulin. Int. J. Mol. Sci. 2014, 15, 10199-10214. https://doi.org/10.3390/ijms150610199
Du X, Qiu S, Wang Z, Wang R, Wang C, Tian J, Liu Q. Direct Interaction between Selenoprotein P and Tubulin. International Journal of Molecular Sciences. 2014; 15(6):10199-10214. https://doi.org/10.3390/ijms150610199
Chicago/Turabian StyleDu, Xiubo, Shi Qiu, Zhi Wang, Ruoran Wang, Chao Wang, Jing Tian, and Qiong Liu. 2014. "Direct Interaction between Selenoprotein P and Tubulin" International Journal of Molecular Sciences 15, no. 6: 10199-10214. https://doi.org/10.3390/ijms150610199