The Metallothionein Gene, TaMT3, from Tamarix androssowii Confers Cd2+ Tolerance in Tobacco
Abstract
:1. Introduction
2. Results
2.1. Isolation of cDNA Encoding Type-II Metallothionein

2.2. Expression of TaMT3 Gene in Response to CdCl2 Stress

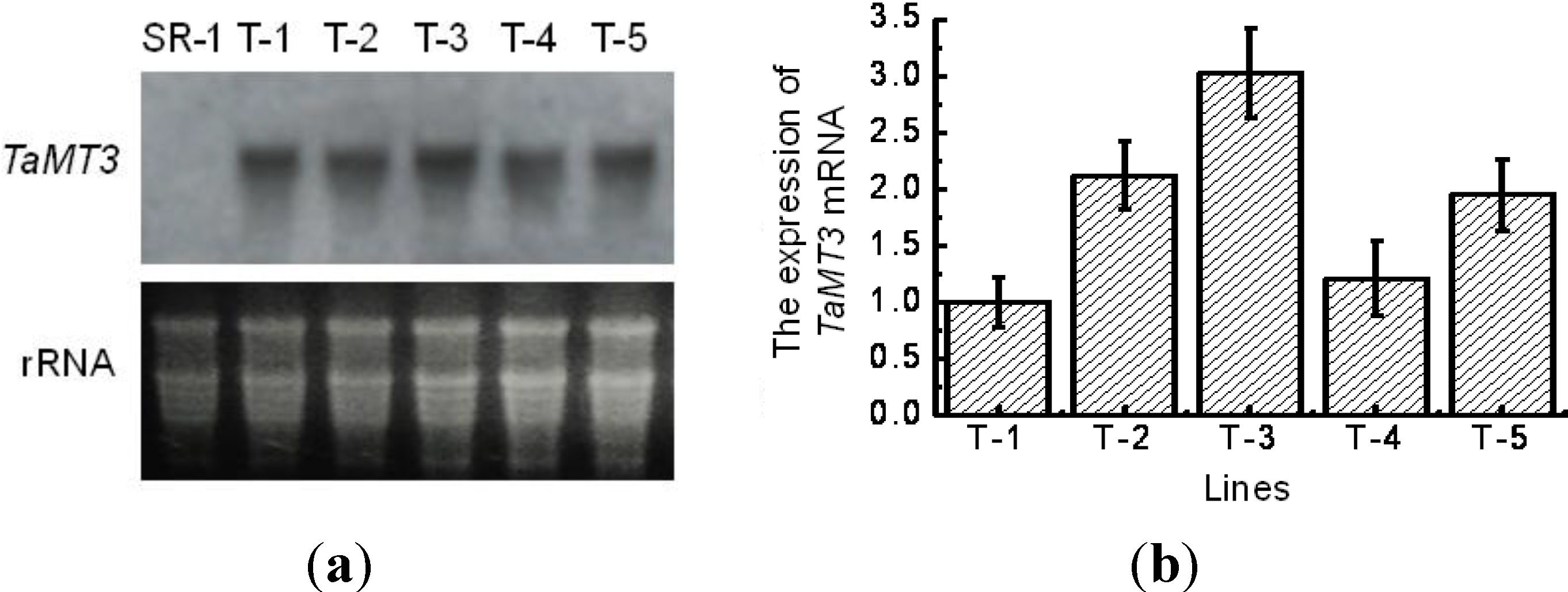
2.3. Expression of TaMT3 Gene in Transgenic Tobacco Plants
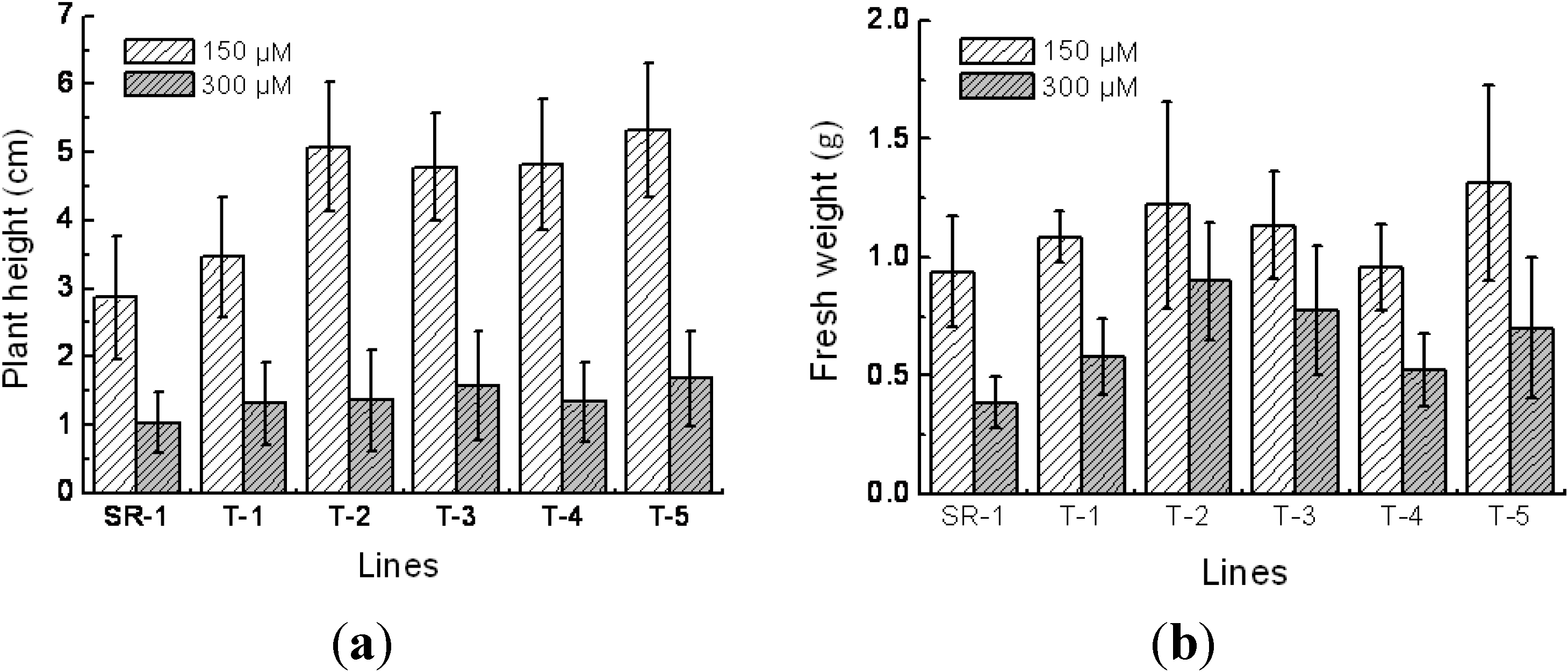
2.4. Responses to Cd2+ Resistance in Transgenic Tobacco
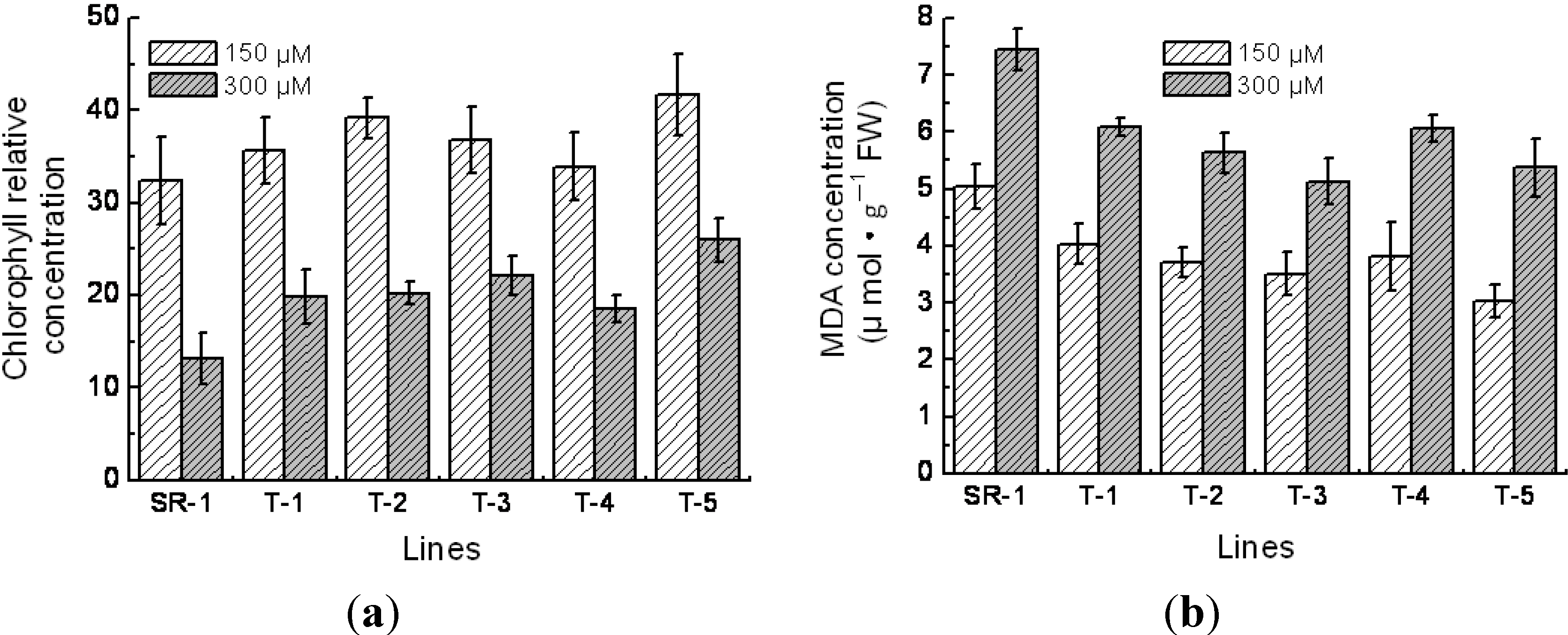
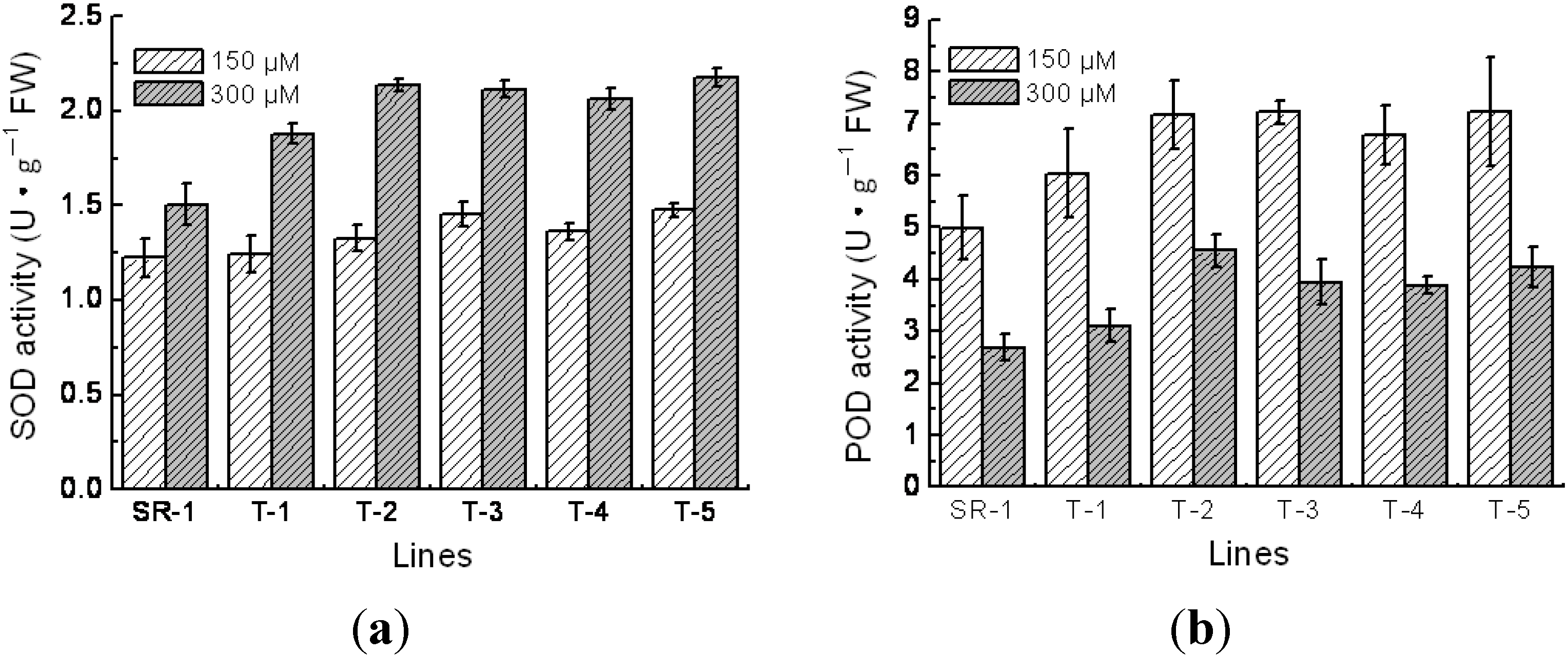
2.5. Physiological Character of Transgenic Tobacco
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
4.3. Northern Blotting Analysis
4.4. Characterization of Growth Traits
4.5. Measurements of Superoxide Dismutase (SOD), Peroxidase (POD) Activity, Chlorophyll Content and Malondialdehyde (MDA) Content
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marlowe, M.; Errera, J.; Jacobs, J. Increased lead and cadmium burdens among mentally retarded children and children with borderline intelligence. Am. J. Ment. Defic. 1983, 87, 477–483. [Google Scholar]
- Roelfzems, W.H.; Zahm-breidenbach, U.; Copius, J.H.J. Light and electron microscopic investigation of the rat placenta after cadmium administration during pregnancy. Anat. Em. Bryol. 1988, 178, 345–351. [Google Scholar] [CrossRef]
- Sugiyama, M. Role of cellular antioxidants in metal induced damage. Cell Biol. Toxicol. 1994, 10, 1–22. [Google Scholar] [CrossRef]
- Luo, Y.M. Phytoremediation of soil heavy metal pollution. Soils 1999, 31, 261–265. (In Chinese) [Google Scholar]
- Shi, Y.; He, Y.K. Molecular mechanism for phytoremediation of heavy metal polluted environment. J. Plant Physiol. Mol. Biol. 2003, 29, 267–274. (In Chinese) [Google Scholar]
- Chaney, R.L.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J.M. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef]
- Robinson, N.J.; Tommey, A.M.; Kuske, C.; Jackson, P.J. Plant metallothioneins. J. Biochem. 1993, 295, 1–10. [Google Scholar]
- Murphy, A.; Zhou, J.; Goldsbrough, P.B.; Taiz, L. Purification and immunological identification of metallothioneins 1 and 2 from Arabidopsis thaliana. Plant Physiol. 1997, 113, 1293–1301. [Google Scholar]
- Murphy, A.; Taiz, L. Comparison of metallothionein gene expression and nonprotein thiols in ten Arabidopsis ecotypes (Correlation with copper tolerance). Plant Physiol. 1995, 109, 945–954. [Google Scholar]
- Lane, B.; Kajoik, R.; Kennedy, T. The wheat-germ Ec protein is a zinc-containing metallothionein. Biochem. Cell Biol. 1987, 65, 1001–1005. [Google Scholar] [CrossRef]
- Kawashima, I.; Kennedy, T.D.; Chino, M.; Lane, B.G. Wheat Ec metallothionein genes. Eur. J. Biochem. 1992, 209, 971–976. [Google Scholar] [CrossRef]
- Kawashima, I.; Inokuchi, Y.; Chino, M.; Kimura, M.; Shimizu, N. Isolation of a gene for a metallothionein protein from soybean. Plant Cell Physiol. 1991, 32, 913–916. [Google Scholar]
- Giritch, A.; Ganal, M.; Stephan, U.W.; Baumlein, H. Structure, expression and chromosomal localization of the metallothionein-like gene family of tomato. Plant Mol. Biol. 1998, 37, 701–714. [Google Scholar] [CrossRef]
- Ma, M.; Lau, P.S.; Jia, Y.T.; Tsang, W.K.; Lam, S.K.S.; Tam, N.F.Y.; Wong, Y.S. The isolation and characterzation of type 1 metallothionein (MT) cDNA from a heavy-metal-tolerant plant, Festuca rubra cv. Merlin. Plant Sci. 2003, 164, 51–60. [Google Scholar] [CrossRef]
- Zenk, M.H. Heavy metal detoxification in higher plants: A review. Gene 1996, 179, 21–30. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Zhao, K.F. Mechanism of heavy metal injury and resistance of plants. Chin. J. Appl. Environ. Biol. 2001, 7, 92–99. (In Chinese) [Google Scholar]
- De Borne, F.D.; Elmayan, T.; de Roton, C.; de Hys, L.; Tepfer, M. Cadmium partitioning in transgenic tobacco plants expressing a mammalian metallothionein gene. Mol. Breed. 1998, 4, 83–90. [Google Scholar]
- Li, W.; Zhang, J.; Zhang, X.Y.; Shan, L.; Ru, B.G. Pb tolerance and accumulation of petunia transformed by metallothionein recombinantαα gene. Prog. Biochem. Biophys. 2001, 28, 405–409. (In Chinese) [Google Scholar]
- Zhang, X.Y.; Zuo, X.F.; Shan, L.; Ru, B.G. Genetic analysis of T1 progeny of transgenic tobacco with metallothionein gene and metallothionein domain mutant alpha alpha gene. Acta Genet. Sin. 2001, 28, 877–883. (In Chinese) [Google Scholar]
- Wang, Y.C.; Yang, C.P.; Liu, G.F.; Jiang, J.; Wu, J.H. Generation and analysis of expressed sequence tags from a cDNA library of Tamarix androssowii. Plant Sci. 2006, 170, 28–36. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Fridovich, I. Superoxide dismutase. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef]
- Chris, B.; Marc, V.H.; Dirk, I. Superoxidedismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83. [Google Scholar] [CrossRef]
- Freisinger, E. The metal-thiolate clusters of plant metallothioneins. Chimia 2010, 64, 217–224. [Google Scholar] [CrossRef]
- Grennan, A.K. Metallothioneins, a diverse protein family. Plant Physiol. 2011, 155, 1750–1751. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W.; Dai, W.; He, Z.; Ma, M. Functional characterization of cadmium-responsive garlic gene AsMT2b: A new member of metallothionein family. Chin. Sci. Bull. 2006, 51, 409–416. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Attipalli, R.R.; Kolluru, V.C.; Munusamy, V. Drought-induced responses of photosynthesis and antioxidant metalbolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Penuelas, J. Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Sci. 2004, 166, 1105–1110. [Google Scholar] [CrossRef]
- Van Assche, F.; Clijsters, H. Effects of metal on enzyme activity in plant. Plant Cell Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, L.P. Effects of antioxidant enzymes activities in hordeum vulgar seedling under Cd2+, Pb2+, Hg2+, Ni2+ stresses. J. Agro-Environ. Sci. 2005, 24, 217–221. (In Chinese) [Google Scholar]
- Wang, C.C.; Shen, Z.G. Uptake of Cd2+ by three species of plants and responses of mung bean to Cd2+ toxicity. J. Nanjing Agric. Univ. 2001, 24, 9–13. (In Chinese) [Google Scholar]
- Plekhanov, S.H.; Chemeris, Y.K. Early toxic effects of zinc, cobalt and cadmium on photosynthetic activity of the green alga chlorella pyrenoidosa chick Chick S239. Biol. Bull. 2003, 30, 506–511. [Google Scholar] [CrossRef]
- Wierzbicka, M. The effect of lead on the ultrastructure changes in the root tip of onion. Cytobiology 1986, 24, 340–341. [Google Scholar]
- Shen, Z.G.; Shen, Q.R.; Guan, H.Y.; Wang, Z.Y.; Shen, K. Relationship between nitrogen nutrition and growth of barley seedlings as well as asion balance under NaCl stress. J. Nanjing Agric. Univ. 1994, 17, 22–26. [Google Scholar]
- Jiang, T.B.; Chen, H.; Tang, X.H.; Ding, B.J.; Li, F.J. Analysis of physiologic characteristics for Cd2+ tolerance on transgenic tobacco expressing metallothionein gene (MT1). Acta Agron. Sin. 2007, 33, 1902–1905. (In Chinese) [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Engler-Blum, G.; Meier, M.; Frank, J.; Müller, G.A. Reduction of background problems in nonradioactive Northern and Southern blot analyses enables higher sensitivity than 32P-based hybridizations. Anal. Biochem. 1993, 210, 235–244. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase I: Occurrencein higher plant. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Mead, J.F. Free radical mechanism of lipid damage and consequences for cellular membranes. In Free Radicals in Biology; Pryor, W.A., Ed.; Academic Press: New York, NY, USA, 1976; pp. 51–68. [Google Scholar]
- Hao, J.J.; Liu, Y.J. Plant Physiological Experiment Technology; Liaoning Agricultural Science and Technology Press: Shenyang, China, 2001; pp. 71–73, 144–145, 180–181. (In Chinese) [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, B.; Yao, W.; Wang, S.; Wang, X.; Jiang, T. The Metallothionein Gene, TaMT3, from Tamarix androssowii Confers Cd2+ Tolerance in Tobacco. Int. J. Mol. Sci. 2014, 15, 10398-10409. https://doi.org/10.3390/ijms150610398
Zhou B, Yao W, Wang S, Wang X, Jiang T. The Metallothionein Gene, TaMT3, from Tamarix androssowii Confers Cd2+ Tolerance in Tobacco. International Journal of Molecular Sciences. 2014; 15(6):10398-10409. https://doi.org/10.3390/ijms150610398
Chicago/Turabian StyleZhou, Boru, Wenjing Yao, Shengji Wang, Xinwang Wang, and Tingbo Jiang. 2014. "The Metallothionein Gene, TaMT3, from Tamarix androssowii Confers Cd2+ Tolerance in Tobacco" International Journal of Molecular Sciences 15, no. 6: 10398-10409. https://doi.org/10.3390/ijms150610398



