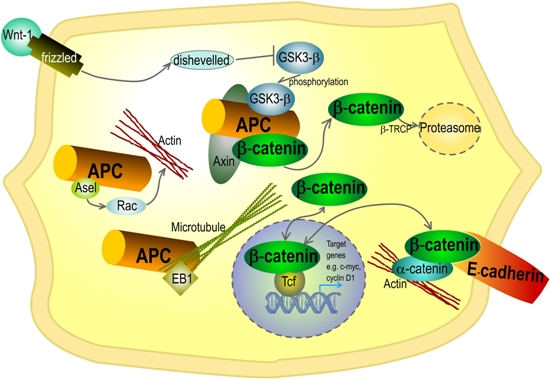
Brain Metastases from Lung Cancer Show Increased Expression of DVL1, DVL3 and Beta-Catenin and Down-Regulation of E-Cadherin
Abstract
:1. Introduction
2. Results
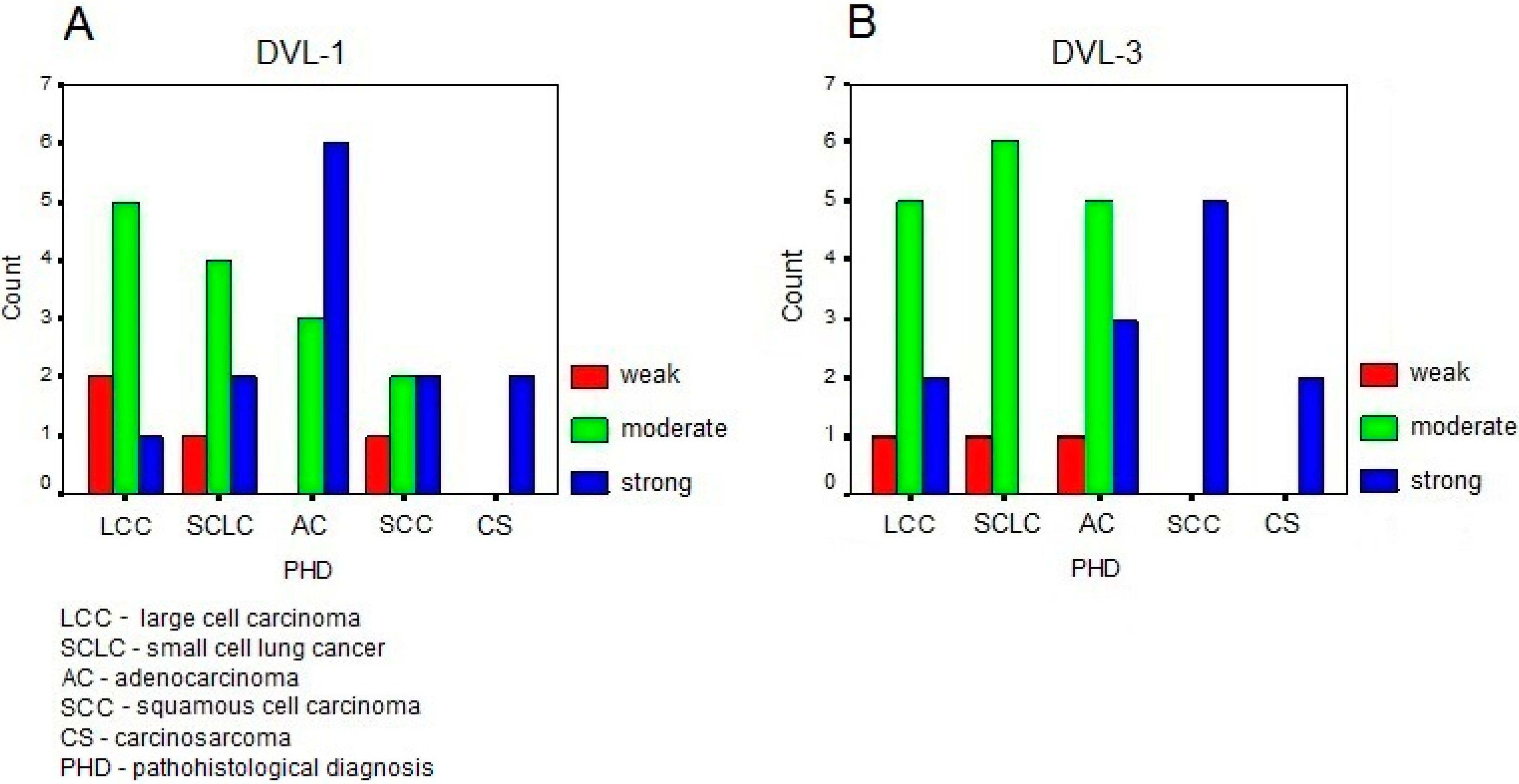
| Patient No. | Location | CDH1 D16S752&D16S265&D16S398 | E-Cadherin | Beta-Catenin | Dishevelled-1 | Dishevelled-3 | Primary Tumor |
|---|---|---|---|---|---|---|---|
| 1 | Cerebellum | LOH D16S265 | 0 | C++ N+ | ++ | +++ | Large cell carcinoma |
| 2 | Cerebellum | HETERO | +++ | C++ | ++ | ++ | Large cell carcinoma |
| 3 | Frontal region | HETERO | ++ | 0 | + | ++ | Large cell carcinoma |
| 4 | Parietal region | HETERO | +++ | C+ | +++ | ++ | Large cell carcinoma |
| 5 | Occipital region | HETERO | + | C++ | ++ | +++ | Large cell carcinoma |
| 6 | Frontal region | HETERO | + | N++ | ++ | + | Large cell carcinoma |
| 7 | Occipital region | HETERO | ++ | C+N+++ | ++ | ++ | Large cell carcinoma |
| 8 | Frontal region | HETERO | + | C++ | + | ++ | Large cell carcinoma |
| 9 | Parietal region | HETERO | + | C+ | ++ | ++ | SCLC |
| 10 | Frontal region | LOH D16S398 | 0 | C+ | ++ | ++ | SCLC |
| 11 | Parietal region | LOH all | 0 | C++N+++ | ++ | ++ | SCLC |
| 12 | Parietooccipital region | LOH D16S265 | ++ | C+ | +++ | ++ | SCLC |
| 13 | Parietal | ND | ND | ND | ++ | ++ | SCLC |
| 14 | Parietal | ND | ND | ND | + | + | SCLC |
| 15 | Cerebellum | ND | ND | ND | +++ | ++ | SCLC |
| 16 | Cerebellum | MSI D16S265 | 0 | C+ | +++ | +++ | Adenocarcinoma |
| 17 | Temporal region | HETERO | + | C+ | ++ | ++ | Adenocarcinoma |
| 18 | Parietal region | LOH D16S752 | ++ | C+ | +++ | +++ | Adenocarcinoma |
| 19 | PRFrontal region | NDLOH all | ++++++ | C+++C++N+ | ++++ | ++++ | Adenocarcinoma |
| 20 | Temporal region | LOH D16S398 | ++ | C+ | ++ | ++ | Adenocarcinoma |
| 21 | PRCerebellum | NDLOH D16S752 | ++++ | 0C+ | +++++ | ++++ | Adenocarcinoma |
| 22 | Temporal | ND | ND | ND | +++ | + | Adenocarcinoma |
| 23 | Frontoparietal | ND | ND | ND | ++ | ++ | Adenocarcinoma |
| 24 | Frontoparietal | ND | ND | ND | +++ | ++ | Adenocarcinoma |
| 25 | Parietal region | LOH D16S398 | 0 | C++ | + | +++ | Squamous cell carcinoma |
| 26 | Parietal region | HETERO | +++ | C+N++ | +++ | +++ | Squamous cell carcinoma |
| 27 | Parietal region | HETERO | +++ | C+++ | ++ | +++ | Squamous cell carcinoma |
| 28 | Multiple metastases | HETERO | ++ | C++N+ | ++ | +++ | Squamous cell carcinoma |
| 29 | Cerebellum | HETERO | + | C+N++ | +++ | +++ | Squamous cell carcinoma |
| 30 | PRTemporal region | NDHETERO | ++++ | C++C+++N+ | +++++ | ++++++ | Carcinosarcoma |
| 31 | PRParietal region | NDHETERO | ++++ | 0C+ | ++++ | +++++ | Carcinosarcoma |

3. Discussion
4. Experimental Section
4.1. Tumor Specimen
4.2. Immunohistochemistry
4.3. Genetic Analyses of E-Cadherin and Beta-Catenin
4.3.1. Polymerase Chain Reaction (PCR)
4.3.2. Loss of Heterozygosity, Microsatellite Instability (MSI)
4.3.3. Heteroduplex Analysis
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, G.P.; Minn, A.J.; Kang, Y.; Siegel, P.M.; Serganova, I.; Cordon-Cardo, C.; Olshen, A.B.; Gerald, W.L.; Massague, J. Identifying site-specific metastasis genes and functions. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 149–158. [Google Scholar]
- Albini, A.; Mirisola, V.; Pfeffer, U. Metastasis signatures: Genes regulating tumor–microenvironment interactions predict metastatic behavior. Cancer Metastasis Rev. 2008, 27, 75–83. [Google Scholar]
- Cliffe, A.; Hamada, F.; Bienz, M. A role of Dishevelled in relocating AXIN to the plasma membrane during wingless signaling. Curr. Biol. 2003, 13, 960–966. [Google Scholar]
- Novak, A.; Dedhar, S. Signaling through β-catenin and Lef/Tcf. Cell. Mol. Life Sci. 1999, 56, 523–537. [Google Scholar]
- Brantjes, H.; Barker, N.; van Es, J.; Clevers, H. TCF: Lady justice casting the final verdict on the outcome of Wnt signalling. Biol. Chem. 2002, 383, 255–261. [Google Scholar]
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar]
- Shitashige, M.; Hirohashi, S.; Yamada, T. Wnt signaling inside the nucleus. Cancer Sci. 2008, 99, 631–637. [Google Scholar]
- Pecina-Slaus, N. Wnt signal transduction pathway and apoptosis: A review. Cancer Cell Int. 2010, 10, 22. [Google Scholar]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar]
- Li, X.; Xu, Y.; Chen, Y.; Chen, S.; Jia, X.; Sun, T.; Liu, Y.; Li, X.; Xiang, R.; Li, N. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of Wnt/β-catenin signal network. Cancer Lett. 2013, 336, 379–389. [Google Scholar]
- Stamos, J.L.; Weis, W.I. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar]
- Lee, N.Y.; Gao, Y.; Wang, H.Y. Differential mediation of the wnt canonical pathway by mammalian Dishevelleds-1, -2, -3. Cell Signal. 2008, 20, 443–452. [Google Scholar]
- Gao, C.; Chen, Y.G. Dishevelled: The hub of wnt signaling. Cell Signal. 2010, 22, 717–727. [Google Scholar]
- Pulvirenti, T.; van der Heijden, M.; Droms, L.A.; Huse, J.T.; Tabar, V.; Hall, A. Dishevelled 2 signaling promotes self-renewal and tumorigenicity in human gliomas. Cancer Res. 2011, 71, 7280–7290. [Google Scholar]
- Nagahata, T.; Shimada, T.; Harada, A.; Nagai, H.; Onda, M.; Yokoyama, S.; Shiba, T.; Jin, E.; Kawanami, O.; Emi, M. Amplification, up-regulation and over expression of DVL1, the human counterpart of the Drosophila disheveled gene, in primary breast cancer. Cancer Sci. 2003, 90, 515–518. [Google Scholar]
- Zhao, Y.; Yang, Z.-Q.; Wang, Y.; Miao, Y.; Liu, Y.; Dai, S.-D.; Han, Y.; Wang, E.-H. Dishevelled-1 and Dishevelled-3 affect cell invasion mainly through canonical and noncanonical Wnt pathway, respectively, and associate with poor prognosis in nonsmall cell lung cancer. Mol. Carcinog. 2010, 49, 760–770. [Google Scholar]
- Li, T.; Hou, S.-C.; Mao, J.-H.; Wang, Y.-C.; Lu, X.-D.; Tan, J.-L.; You, B.; Liu, Y.-P.; Ni, J.; Au, A.; et al. The expression of Dishevelled-3 and glutamine metabolism in malignant pleural mesothelioma. J. Clin. Pathol. 2012, 65, 855–858. [Google Scholar]
- Uematsu, K.; He, B.; You, L.; Xu, Z.; McCormick, F.; Jablons, D.M. Activation of the Wnt pathway in non small cell lung cancer: Evidence of disheveled overexpression. Oncogene 2003, 22, 7218–7221. [Google Scholar]
- Uematsu, K.; Kanazawa, S.; You, L.; He, B.; Xu, Z.; Li, K.; Peterlin, B.M.; McCormick, F.; Jablons, D.M. Wnt pathway activation in Mesothelioma: Evidence of Dishevelled overexpression and transcriptional activity of β-catenin. Cancer Res. 2003, 63, 4547–4551. [Google Scholar]
- Kim, K.; Lu, Z.; Hay, E.D. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol. Int. 2002, 26, 463–476. [Google Scholar]
- Kim, S.H.; Kim, J.M.; Shin, M.H.; Kim, C.W.; Huang, S.M.; Kang, D.W.; Suh, K.S.; Yi, E.S.; Kim, K.H. Correlation of epithelial-mesenchymal transition markers with clinicopathologic parameters in adenocarcinomas and squamous cell carcinoma of the lung. Histol. Histopathol. 2012, 27, 581–591. [Google Scholar]
- Kostic, A.; Lynch, C.D.; Sheetz, M.P. Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLoS One 2009, 4, e6361. [Google Scholar]
- Zeljko, M.; Pecina-Slaus, N.; Nikuseva Martic, T.; Kusec, V.; Beros, V.; Tomas, D. Molecular alterations of E-cadherin and beta-catenin in brain metastases. Front. Biosci. 2011, 3, 616–624. [Google Scholar]
- Pecina-Slaus, N.; Cicvara-Pecina, T.; Kafka, A. Epithelial-to-mesenchymal transition: Possible role in meningiomas. Front. Biosci. 2012, 4, 889–896. [Google Scholar]
- Nguyen, A.; Rosner, A.; Milovanović, T.; Hope, C.; Planutis, K.; Saha, B.; Chaiwun, B.; Lin, F.; Imam, S.A.; Marsh, J.L.; et al. Wnt pathway component LEF1 mediates tumor cell invasion and is expressed in human and murine breast cancers lacking ErbB2 (her-2/neu) over expression. Int. J. Oncol. 2005, 27, 949–956. [Google Scholar]
- Kriegl, L.; Horst, D.; Reiche, J.A.; Engel, J.; Kirchner, T.; Jung, A. LEF-1 and TCF-4 expression correlate inversely with survival in colorectal cancer. J. Transl. Med. 2010, 8, 123. [Google Scholar]
- Nguyen, D.X.; Chiang, A.C.; Zhang, X.H.; Kim, J.Y.; Kris, M.G.; Ladanyi, M.; Gerald, W.L.; Massagué, J. Wnt/Tcf signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 2009, 138, 51–62. [Google Scholar]
- Li, X.-Y.; Liu, S.-L.; Cha, N.; Zhao, Y.-J.; Wang, S.-C.; Li, W.-N.; Wang, E.-H.; Wu, G.-P. Transcription expression and clinical significance of Dishevelled-3 mRNA and δ-catenin mRNA in pleural effusions from patients with lung cancer. Clin. Dev. Immunol. 2012, 2012, 904946. [Google Scholar]
- Wei, Q.; Zhao, Y.; Yang, Z.-Q.; Dong, Q.-Z.; Dong, X.-J.; Han, Y.; Zhao, C.; Wang, E.-H. Dishevelled family proteins are expressed in non-small cell lung cancer and function differentially on tumor progression. Lung Cancer 2008, 62, 181–192. [Google Scholar]
- Prasad, C.P.; Gupta, S.D.; Rath, G.; Ralhan, R. Wnt signaling pathway in invasive ductal carcinoma of the breast: Relationship between beta-catenin, dishevelled and cyclin D1 expression. Oncology 2007, 73, 112–117. [Google Scholar]
- Mizutani, K.; Miyamoto, S.; Nagahata, T.; Konishi, N.; Emi, M.; Onda, M. Up-regulation and over expression of DVL1, the human counterpart of the Drosophila dishevelled gene, in prostate cancer. Tumori 2005, 91, 546–551. [Google Scholar]
- Okino, K.; Nagai, H.; Hatta, M.; Nagahata, T.; Yoneyama, K.; Ohta, Y.; Jin, E.; Kawanami, O.; Araki, T.; Emi, M. Up-regulation and overproduction of DVL-1, the human counterpart of the Drosophila dishevelled gene, in cervical squamous cell carcinoma. Oncol. Rep. 2003, 10, 1219–1223. [Google Scholar]
- Sareddy, G.R.; Panigrahi, M.; Challa, S.; Mahadevan, A.; Babu, P.P. Activation of Wnt/β-catenin/Tcf signaling pathway in human astrocytomas. Neurochem. Int. 2009, 55, 307–317. [Google Scholar]
- Dijksterhuis, J.P.; Petersen, J.; Schulte, G. Wnt/Frizzled signaling: Receptor-ligand selectivity with focus on FZD-G protein signaling and its physiological relevance. Br. J. Pharmacol. 2014, 171, 1195–1209. [Google Scholar]
- Stewart, D.J. Wnt signaling pathway in non-small cell lung cancer. J. Natl. Cancer Inst. 2014, 106, djt356. [Google Scholar]
- Gan, X.; Wang, J.; Ying, X.; Wu, Z.; Li, Y.; Li, L. Nuclear Dvl, c-Jun, β-catenin, and Tcf form a complex leading to stabilization of β-catenin-Tcf interaction. J. Cell Biol. 2008, 180, 1087–1100. [Google Scholar]
- Habas, R.; Dawid, I.B. Dishevelled and Wnt signaling: Is the nucleus the final frontier? J. Biol. 2005, 4, 2. [Google Scholar]
- Wang, Y.; Wang, H. Dvl3 translocates IPMK to the cell membrane in response to Wnt. Cell Signal. 2012, 24, 2389–2395. [Google Scholar]
- McDonald, J.M.; Pelloski, C.E.; Ledoux, A.; Sun, M.; Raso, G.; Komaki, R.; Wistuba, I.I.; Bekele, B.N.; Aldape, K. Elevated phospho-S6 expression is associated with metastasis in adenocarcinoma of the lung. Clin. Cancer Res. 2008, 14, 7832–7837. [Google Scholar]
- Saad, A.G.; Yeap, B.Y.; Thunnissen, F.B.; Pinkus, G.S.; Pinkus, J.L.; Loda, M.; Sugarbaker, D.J.; Johnson, B.E.; Chirieac, L.R. Immunohistochemical markers associated with brain metastases in patients with nonsmall cell lung carcinoma. Cancer 2008, 113, 2129–2138. [Google Scholar]
- Arnold, S.M.; Young, A.B.; Munn, R.K.; Patchell, R.A.; Nanayakkara, N.; Markesbery, W.R. Expression of p53, bcl-2, E-cadherin, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 in paired primary tumors and brain metastasis. Clin. Cancer Res. 1999, 5, 4028–4033. [Google Scholar]
- Prudkin, L.; Liu, D.D.; Ozburn, N.C.; Sun, M.; Behrens, C.; Tang, X.; Brown, K.C.; Bekele, B.N.; Moran, C.; Wistuba, I.I. Epithelial-to-mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod. Pathol. 2009, 22, 668–678. [Google Scholar]
- Shabani, H.K.; Kitange, G.; Tsunoda, K.; Anda, T.; Tokunaga, Y.; Shibata, S.; Kaminogo, M.; Hayashi, T.; Ayabe, H.; Iseki, M. Immunohistochemical expression of E-cadherin in metastatic brain tumors. Brain Tumor Pathol. 2003, 20, 7–12. [Google Scholar]
- Kim, C.H.; Park, S.Y.; Yoo, J. Expression of transforming growth factor β1 and E-cadherin proteins in pulmonary adenocarcinoma: Its significance in tumor progression. Cancer Res. Treat. 2013, 45, 118–125. [Google Scholar]
- Zhang, Y.; Zhao, Y.; Jiang, G.; Zhang, X.; Zhao, H.; Wu, J.; Xu, K. Impact of p120-catenin isoforms 1A and 3A on epithelial mesenchymal transition of lung cancer cells expressing E-cadherin in different subcellular locations. PLoS One 2014, 9, e88064. [Google Scholar]
- Blaukovitsch, M.; Halbwedl, I.; Kothmaier, H.; Gogg-Kammerer, M.; Popper, H.H. Sarcomatoid carcinomas of the lung—Are these histogenetically heterogeneous tumors? Virchows Arch 2006, 449, 455–461. [Google Scholar]
- Shi, C.-S.; Huang, N.-N.; Kehrl, J.H. Regulator of G-protein signaling isoform 1 (PDZ-RGS3) enhances canonical Wnt signaling and promotes epithelial mesenchymal transition. J. Biol. Chem. 2012, 287, 33480–33487. [Google Scholar]
- Guldur, M.E.; Kibar, Y.; Deniz, H.; Bakir, K. Comparison of osteopontin, beta-catenin and hnRNP B1 expression in lung carcinomas. Pathol. Oncol. Res. 2010, 16, 55–59. [Google Scholar]
- Rodriguez-Salas, N.; Palacios, J.; de Castro, J.; Moreno, G.; Gonzalez-Barton, M.; Gamallo, C. Beta-catenin expression pattern in small cell lung cancer: Correlation with clinical and evolutive features. Histol. Histopathol. 2001, 16, 353–358. [Google Scholar]
- Nikuseva Martic, T.; Beros, V.; Pecina-Slaus, N.; Pecina, H.I.; Bulic-Jakus, F. Genetic changes of CDH1, APC and CTNNB1 found in human brain tumors. Pathol. Res. Pract. 2007, 203, 779–787. [Google Scholar]
- Mazieres, J.; He, B.; You, L.; Xu, Z.; Jablons, D.M. Wnt signaling in lung cancer. Cancer Lett. 2005, 222, 1–10. [Google Scholar]
- Hughes, T.A.; Brady, H.J. Regulation of AXIN2 expression at the levels of transcription, translation and protein stability in the lung and colon cancer. Cancer Lett. 2006, 233, 338–347. [Google Scholar]
- Tseng, R.-C.; Lin, R.-K.; Wen, C.-K.; Tseng, C.; Hsu, H.-S.; Hsu, W.-H.; Wang, Y.-C. Epigenetic silencing of AXIN2/βTrCP and deregulation of p53-mediated control lead to wild-type β-catenin nuclear accumulation in lung tumorigenesis. Oncogene 2008, 27, 4488–4496. [Google Scholar]
- Cooper, C.A.; Bubb, V.J.; Smithson, N.; Carter, R.L.; Gledhill, S.; Lamb, D.; Wyllie, A.H.; Carey, F.A. Loss of heterozygosity at 5q21 in non-small cell lung cancer: A frequent event but without evidence of APC mutation. J. Pathol. 1996, 180, 33–37. [Google Scholar]
- Pecina-Slaus, N.; Nikuseva Martic, T.; Zeljko, M.; Bulat, S. Brain metastases exhibit gross deletions of the APC gene. Brain Tumor Pathol. 2011, 28, 223–228. [Google Scholar]
- Pecina-Slaus, N.; Majic, Z.; Musani, V.; Zeljko, M.; Cupic, H. Report on mutation in exon 15 of the APC gene in a case of brain metastasis. J. Neurooncol. 2010, 97, 143–148. [Google Scholar]
- Salmaggi, A.; Maderna, E.; Calatozzolo, C.; Gaviani, P.; Canazza, A.; Milanesi, I.; Silvani, A.; DiMeco, F.; Carbone, A.; Pollo, B. CXCL12, CXCR4 and CXCR7 expression in brain metastases. Cancer Biol. Ther. 2009, 8, 1608–1614. [Google Scholar]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. WHO Classification of Tumours of the Central Nervous System; International Agency for Research on Cancer: Lyon, France, 2007. [Google Scholar]
- Sarrio, D.; Palacios, J.; Hergueta-Redondo, M.; Gomez-Lopez, G.; Cano, P.; Moreno-Bueno, G. Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer 2009, 9, 74. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kafka, A.; Tomas, D.; Beroš, V.; Pećina, H.I.; Zeljko, M.; Pećina-Šlaus, N. Brain Metastases from Lung Cancer Show Increased Expression of DVL1, DVL3 and Beta-Catenin and Down-Regulation of E-Cadherin. Int. J. Mol. Sci. 2014, 15, 10635-10651. https://doi.org/10.3390/ijms150610635
Kafka A, Tomas D, Beroš V, Pećina HI, Zeljko M, Pećina-Šlaus N. Brain Metastases from Lung Cancer Show Increased Expression of DVL1, DVL3 and Beta-Catenin and Down-Regulation of E-Cadherin. International Journal of Molecular Sciences. 2014; 15(6):10635-10651. https://doi.org/10.3390/ijms150610635
Chicago/Turabian StyleKafka, Anja, Davor Tomas, Vili Beroš, Hrvoje Ivan Pećina, Martina Zeljko, and Nives Pećina-Šlaus. 2014. "Brain Metastases from Lung Cancer Show Increased Expression of DVL1, DVL3 and Beta-Catenin and Down-Regulation of E-Cadherin" International Journal of Molecular Sciences 15, no. 6: 10635-10651. https://doi.org/10.3390/ijms150610635