Searching for “Environmentally-Benign” Antifouling Biocides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Compounds
2.1.1. Known Agricultural Poisons as Anti-Fouling Agents
| Booster biocides [CAS number] | Structure | Mr | tPSA | Log Kow | BIOWIN™ | ECOSAR™ acute toxicity (mg/L) (Exp. values, mg/L) | |
|---|---|---|---|---|---|---|---|
| Chlorothalonil [1897-45-6] |  | 265.90 | 47.58 | 4.0 [15] | 1: 0.51; 2: 0.61; 3: 1.62; 4: 2.67; 5: 0.09; 6: 0.004; 7: −0.77; A: NO B: NO | F: 6.98 (0.012) D: 4.62 (0.06) A: 6.50 (0.6) | |
| Dichlofluanid [1085-98-9] |  | 333.20 | 40.62 | 3.7 [15] | 1: 0.31; 2: 0.005; 3: 1.93; 4: 3.02; 5: −0.15; 6: 0; 7: 0.05; A: NO B: NO | F: 62.02 (0.032) D: 37.63 A: 36.87 | |
| Irgarol 1051 [28159-98-0] |  | 253.37 | 61.14 | 3.95 [15] | 1: 0.44; 2: 0.09; 3: 2.43; 4: 3.33; 5: 0.08; 6: 0.02; 7: −0.02; A: NO B: NO | F: 33.65 (0.7) D: 20.73 (5.3) A: 21.62 (0.002) | |
| TCMS Pyridine [13108-52-6] |  | 294.96 | 47.03 | 1.95 * | 1: −0.28; 2: 0; 3: 1.51; 4: 2.74; 5: −0.17; 6: 0.0007; 7: −0.21; A: NO B: NO | F: 267.43 D: 151.18 A: 110.58 | |
| TCMTB [21564-17-0] |  | 238.34 | 36.15 | 3.3 [15] | 1: 0.63; 2: 0.41; 3: 2.67; 4: 3.50; 5: 0.09; 6: 0.03; 7: 0.48; A: NO B: NO | F: 19.40 (28.8) D: 12.21 A: 13.94 | |
| Diuron [330-54-1] |  | 233.09 | 32.34 | 2.8 [15] | 1: 0.27; 2: 0.01; 3: 2.27; 4: 3.18; 5: 0.06; 6: 0.01; 7: −0.41; A: NO B: NO | F: 47.65 (0.53) D: 28.79 (7.7) A: 27.73 (9.32) | |
| DCOIT [64359-81-5] |  | 282.22 | 20.31 | 2.8 [15] | 1: 0.50; 2: 0.05; 3: 2.53; 4: 3.51; 5: 0.22; 6: 0.02; 7: 0.59; A: NO B: NO | F: 8.65 D: 5.69 A: 7.78 | |
| Zinc pyrithione (Zinc omadine) [13463-41-7] |  | 317.69 | 23.47 ** | 0.9 [15] | 1: 0.69 **; 2: 0.77 **; 3: 2.92 **; 4: 3.66 **; 5: 0.36 **; 6: 0.26 **; 7: 0.54 **; A: YES B: NO ** | F: 12190 ** (0.003) D: 5596 ** (0.008) A: 1731 ** | |
| Copper pyrithione (Copper omadine) [14915-37-8] or [154592-20-8] |  | 315.85 | 23.47 ** | −0.3 ** | 1: 0.69 **; 2: 0.77 **; 3: 2.92 **; 4: 3.66 **; 5: 0.36 **; 6: 0.26 **; 7: 0.54 **; A: YES B: NO ** | F: 12190 ** D: 5596 ** A: 1731 ** (0.035) | |
| Zineb [12122-67-7] |  | 275.73 | 24.06 ** | 0.8 | 1: 0.64 **; 2: 0.50 **; 3: 2.73 **; 4: 3.54 **; 5: 0.18 **; 6: 0.06 **; 7: 0.89 **; A: NO B: NO ** | F: 3036 ** (>180) D: 1517 ** (0.6–1.8) A: 667 ** |
2.1.1.1. Metabolites
| Booster biocides | Aerobic conditions | Anaerobic conditions | ||||
|---|---|---|---|---|---|---|
| BIOWIN™ 3-predicted time for ultimate biodegradation | BIOWIN™ 4-predicted time for primary biodegradation | Experimental half-life [6,16,17,21] | Predicted readily biodegradable (Y) or not readily biodegradable (N) | BIOWIN™ 7-predicted probability for fast biodegradation Fast (Y) or Slow (N) | Experimental half-life [6,16,17,21] | |
| Chlorothalonil | Recalcitrant | Weeks to months | Hours to days | N | N | Hours |
| Dichlofluanid | Months | Weeks | Hours | N | N | Hours to days |
| TCMTB | Weeks to months | Days to weeks | Days | N | N | Days |
| Diuron | Weeks to months | Weeks | Months | N | N | Weeks |
| DCOIT | Weeks to months | Days to weeks | Hours | N | Y | Hours |
| Irgarol 1051 | Weeks to months | Days to weeks | Months | N | N | Persistent |
| Zinc/copper pyrithione | Weeks * | Days to weeks * | Hours | N * | Y * | Hours |
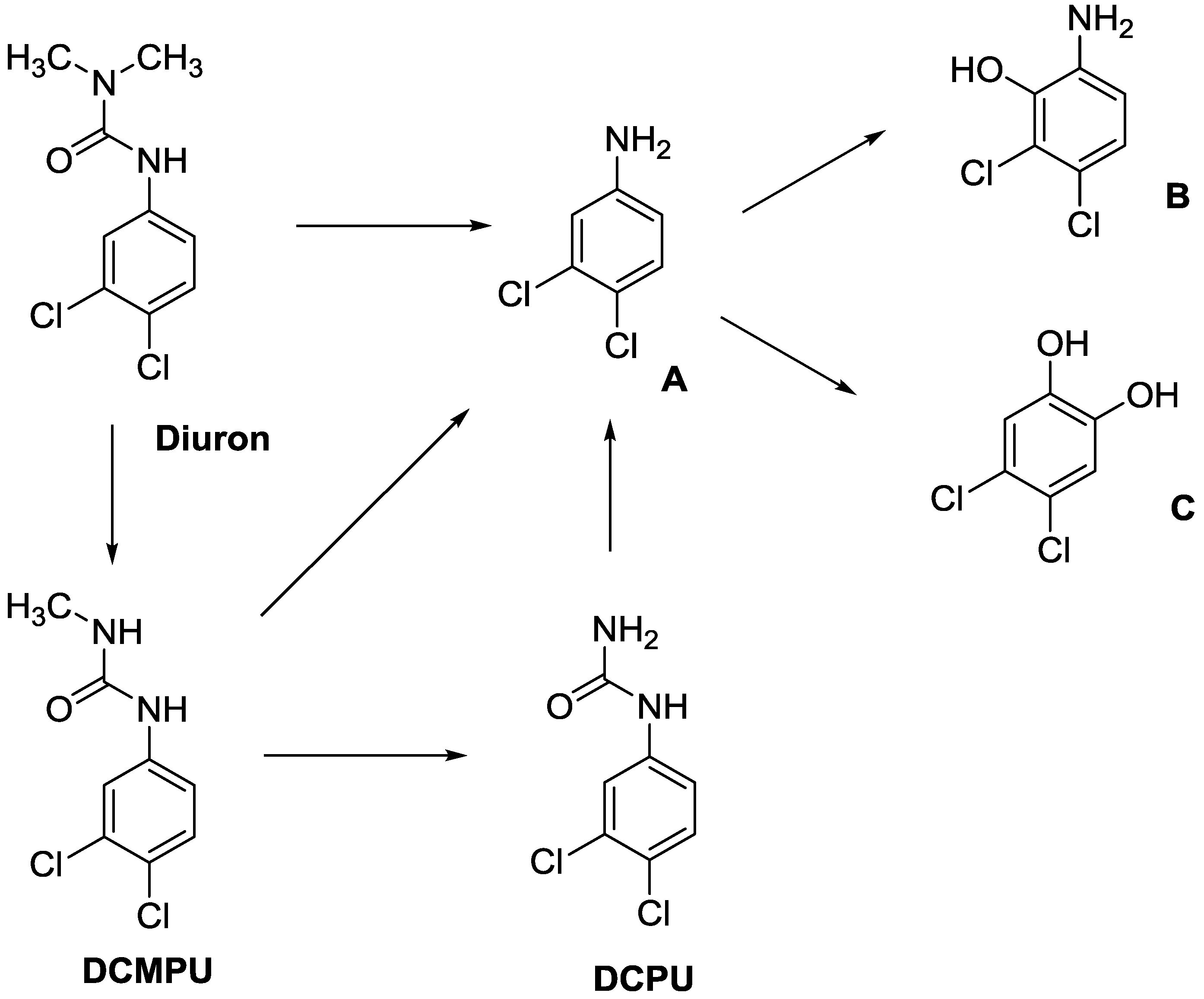
| Structure | Mr | tPSA | Log Kow | BIOWIN™ |
|---|---|---|---|---|
 Diuron | 233.09 | 32.34 | 2.68 | 1: 0.27; 2: 0.01; 3: 2.27; 4: 3.18; 5: 0.05; 6: 0.01; 7: −0.41; A: NO B: NO |
 DCMPU | 219.07 | 41.13 | 2.94 | 1: 0.28; 2: 0.02; 3: 2.30; 4: 3.20; 5: 0.10; 6: 0.02; 7: −0.33; A: NO B: NO |
 DCPU | 205.04 | 55.12 | 2.65 | 1: 0.28; 2: 0.02; 3: 2.33; 4: 3.22; 5: 0.14; 6: 0.03; 7: −0.25; A: NO B: NO |
 A | 162.01 | 26.02 | 2.69 | 1: 0.07; 2: 0.01; 3: 2.29; 4: 3.17; 5: 0.11; 6: 0.03; 7: −0.53; A: NO B: NO |
 B | 178.01 | 46.25 | 1.88 * | 1: 0.18; 2: 0.01; 3: 2.31; 4: 3.19; 5: 0.12; 6: 0.03; 7: −0.36; A: NO B: NO |
 C | 179.00 | 40.46 | 2.32 * | 1: 0.53; 2: 0.15; 3: 2.50; 4: 3.34; 5: 0.34; 6: 0.13; 7: 0.002; A: NO B: NO |
2.1.1.2. Ecological Effects
2.1.2. Pharmaceuticals/Veterinary Medicines as Anti-Fouling Agents
2.1.3. Miscellaneous
| Pharmaceuticals/Veterinary medicines | Name [CAS number] | Biological activity | Mr | Log Kow | tPSA | BIOWIN™ | ||
|---|---|---|---|---|---|---|---|---|
| EC50 (Cyprid) µg/mL | LC50 (Naupliar) µg/mL | |||||||
 | Norvasc [111470-99-6] | 0.32 | 0.71 | 567.05 (salt) 409.89 (ionised form of drug) | 0.14 (ionised form of drug) | 155.87 (ionised form of drug) | 1: 0.58; 2: 0.83; 3: 2.31; 4: 3.51; 5: 0.43; 6: 0.09; 7: −0.34; A: YES B: NO | |
 | Cardepine/Nicardipine [54527-84-3] | 0.18 | 0.05 | 515.99 (salt) 480.54 (ionised form of drug) | 2.39 (ionised form of drug) | 120.88 (ionised form of drug) | 1: 0.90; 2: 1.00; 3: 2.21; 4: 3.48; 5: −0.09; 6: 0.001; 7: −0.29; A: YES B: NO | |
 | Inderal [318-98-9] | 2.5 | 0.040 | 295.81 (salt) 260.36 (ionised form of drug) | 0.74 (ionised form of drug) | 46.07 (ionised form of drug) | 1: 0.91; 2: 0.93; 3: 2.72; 4: 3.68; 5: 0.34; 6: 0.29; 7: −0.40; A: YES B: NO | |
 | Sibelium [30484-77-6] | 0.2 | 3.4 | 477.42 (salt) 404.50 (ionised form of drug) | 4.91 (ionised form of drug) | 8.88 (ionised form of drug) | 1: −0.94; 2: 0; 3: 1.50; 4: 3.29; 5: −0.24; 6: 0; 7: −1.19; A: NO B: NO | |
 | Zyrtec [83881-52-1] | 0.04 | 25 | 461.81 (salt) 390.91 (ionised form of drug) | 2.90 (ionised form of drug) | 55.41 (ionised form of drug) | 1: 0.23; 2: 0.004; 3: 2.50; 4: 3.50; 5: 0.01; 6: 0.02; 7: −1.08; A: NO B: NO | |
 | Clarityne/Loratidine [79794-75-5] | 0.5 | 0.62 | 382.89 (neutral) | 5.20 (neutral) | 41.9 (neutral) | 1: 0.42; 2: 0.02; 3: 1.74; 4: 3.17; 5: −0.43; 6: 0.001; 7: −0.27; A: NO B: NO | |
 | Cyclizine [82-92-8] | 0.04 | 0.04 | 266.39 (neutral) | 2.97 (neutral) | 6.48 (neutral) | 1: 0.47; 2: 0.16; 3: 2.14; 4: 2.90; 5: −0.10; 6: 0.01; 7: −2.36; A: NO B: NO | |
 | Celebrex/Celecoxib [169590-42-5] | 4 | 4 | 381.37 (neutral) | 3.47 (neutral) | 75.76 (neutral) | 1: 0.10; 2: 0.0006; 3: 1.77; 4: 2.95; 5: −0.25; 6: 0.00; 7: −0.39; A: NO B: NO | |
 | Diclomelan/Diclofenac [15307-79-6] | 0.2 | 1.6 | 318.13 (salt) 295.14 (ionised form of drug) | 4.51 (ionised form of drug) | 52.16 (ionised form of drug) | 1: 0.13; 2: 0.003; 3: 2.29; 4: 3.30; 5: −0.13; 6: 0.003; 7: −0.85; A: NO B: NO | |
 | Ponstan/Mefenamic acid[61-68-7] | 0.2 | 5.2 | 241.29 (neutral) | 5.12 (neutral) | 49.33 (neutral) | 1: 0.67; 2: 0.78; 3: 2.47; 4: 3.26; 5: 0.35; 6: 0.15; 7: −0.59; A: YES B: NO | |
 | Prozac/Fluoxetine [56296-78-7] | 1.5 | 0.2 | 345.79 (salt) 310.34 (ionised form of drug) | 2.79 (ionised form of drug) | 25.84 (ionised form of drug) | 1: 0.34; 2: 0.05; 3: 1.96; 4: 3.21; 5: 0.19; 6: 0; 7: −0.06; A: NO B: NO | |
 | Haldol/Haloperidol [52-86-8] | <0.04 | 0.26 | 375.87 (neutral) | 4.30 (neutral) | 40.54 (neutral) | 1: −0.81; 2: 0; 3: 1.27; 4: 2.69; 5: 0.01; 6: 0.0001; 7: −2.53; A: NO B: NO | |
 | Zyprexa/Olanzapine [132539-06-1] | 0.04 | 0.2 | 312.44 (neutral) | 3.00 (neutral) | 30.87 (neutral) | 1: 0.21; 2: 0.007; 3: 2.04; 4: 2.93; 5: −0.30; 6: 0.001; 7: −1.92; A: NO B: NO | |
 | Exelon/Rivastigmine [129101-54-8] | 25 | 0.026 | 400.43 (salt) 252.36 (ionised form of drug) | 1.31 (ionised form of drug) | 35.18 (ionised form of drug) | 1: 0.63; 2: 0.35; 3: 2.64; 4: 3.48; 5: 0.02; 6: 0.04; 7: −0.32; A: NO B: NO | |
 | Aricept/Donepezic [884740-09-4] | 0.28 | 0.52 | 415.96 (salt) 380.51 (ionised form of drug) | 3.35 (ionised form of drug) | 39.97 (ionised form of drug) | 1: 1.02; 2: 0.98; 3: 2.16; 4: 3.37; 5: 0.18; 6: 0.05; 7: −0.75; A: NO B: NO | |
 | Imodium/Loperamide [34552-83-5] | >5 | 0.24 | 513.50 (salt) 478.05 (ionised form of drug) | 3.63 (ionised form of drug) | 44.98 (ionised form of drug) | 1: 0.44; 2: 0.05; 3: 1. 50; 4: 2.90; 5: −0.16; 6: 0.002; 7: −2.73; A: NO B: NO | |
 | Phentolamine [73-05-2] | MIC 0.28 MTC 28.1 | 317.82 (salt) 282.37 (ionised form of drug) | 2.10 (ionised form of drug) | 52.44 (ionised form of drug) | 1: 0.58; 2: 0.15; 3: 2.30; 4: 3.12; 5: 0.04; 6: 0.03; 7: −1.95; A: NO B: NO | ||
 | Prazosin [19237-84-4] | MIC 0.38 MTC 1.53 | 419.87 (salt) 384.42 (ionised form of drug) | 2.16 (ionised form of drug) | 103.6 (ionised form of drug) | 1: 0.83; 2: 0.92; 3: 1.92; 4: 3.36; 5: 0.11; 6: 0.02; 7: −2.00; A: NO B: NO | ||
 | Medetomidine [145108-58-3] | MIC 0.0002 MTC 20.029 | 236.74 (salt) 201.29 (ionised form of drug) | −0.15 (ionised form of drug) | 28.97 (ionised form of drug) | 1: 0.82; 2: 0.87; 3: 2.53; 4: 3.35; 5: 0.23; 6: 0.14; 7: −0.55; A: YES B: NO | ||
 | Clonidine [4205-91-8] | MIC 0.00023 MTC 34.51 | 266.55 (salt) 231.10 (ionised form of drug) | 0 (ionised form of drug) | 41 (ionised form of drug) | 1: 0.04; 2: 0.002; 3: 2.14; 4: 3.07; 5: −0.07; 6: 0.009; 7: −1.21; A: NO B: NO | ||
| Structure | Name [CAS number] | ECOSAR™ acute toxicity (mg/L) | MSDS ecological toxicity (mg/L) | ||||
|---|---|---|---|---|---|---|---|
| Fish LC50 | Daphnid EC50 | Green algae EC50 | Fish LC50 | Daphnid EC50 | Green algae EC50 | ||
 | Norvasc [111470-99-6] | 15,904 | 7602 | 2779 | 14 | 9.9 | 0.28 |
 | Celebrex/Celecoxib [169590-42-5] | 14.858 | 9.667 | 12.642 | 1.2 | 1.5 | – |
 | Prozac/Fluoxetine [56296-78-7] | 49.31 | 30.13 | 30.39 | 1.57 | 0.94 | – |
 | Medetomidine [145108-58-3] | 14,042.98 | 6539.26 | 2145.39 | 30 | 4.5 | 0.34 |
| Class of compound | Structure | Antifouling activity EC50 (Cyprid) µg/mL [REF] | Mr | Log Kow | tPSA | BIOWIN™ | ECOSAR™ acute toxicity (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| Diphenyl ethers |  | 0.81 [30] | 186.21 | 3.35 | 29.46 | 1: 1.05; 2: 1.00; 3: 2.82; 4: 3.70; 5: 0.49; 6: 0.51; 7: 0.45; A: YES B: NO | F: 5.96 D: 3.91 A: 5.31 | |
| Diphenyl ethers |  | 0.31 [30] | 204.65 | 4.70 | 9.23 | 1: 0.73; 2: 0.89; 3: 2.50; 4: 3.47; 5: 0.39; 6: 0.23; 7: −0.03; A: YES B: NO | F: 0.64 D: 0.47 A: 0.97 | |
| Diketopiperazines |  | 1.5 [26] | 291.31 | 1.20 | 70.23 | 1: 1.03; 2: 1.00; 3: 2.45; 4: 3.84; 5: 0.18; 6: 0.05; 7: −1.16; A: YES B: NO | F: 1252.82 D: 660.67 A: 362.55 | |
| Diketopiperazines |  | 0.034 [26] | 291.31 | 1.20 | 70.23 | 1: 1.03; 2: 0.99; 3: 2.45; 4: 3.84; 5: 0.18; 6: 0.05; 7: −1.16; A: YES B: NO | F: 1252.82 D: 660.67 A: 362.55 | |
| Butenolides |  | 0.69 [31] | 311.42 | 3.82 | 64.63 | 1: 0.67; 2: 0.88; 3: 2.39; 4: 3.67; 5: 0.57; 6: 0.55; 7: 0.53; A: YES B: NO | F: 5.94 D: 3.99 A: 5.95 | |
| Butenolides |  | 7.62 [31] | 211.31 | 1.86 | 52.32 | 1: 0.97; 2: 0.99; 3: 2.90; 4: 3.82; 5: 0.88; 6: 0.88; 7: 1.29; A: YES B: YES | F: 230.25 D: 129.10 A: 91.28 | |
| Butenolides |  | 3.55 [31] | 211.26 | 0.33 | 55.4 | 1: 1.03; 2: 0.99; 3: 2.82; 4: 4.00; 5: 0.83; 6: 0.86; 7: 0.38; A: YES B: YES | F: 5458.77 D: 2657.20 A: 1047.22 | |
| Butenolides |  | 2.39 [31] | 265.23 | 1.38 | 55.4 | 1: 0.49; 2: 0.59; 3: 2.19; 4: 3.63; 5: 0.72; 6: 0; 7: 0.46; A: NO B: NO | F: 777.89 D: 417.29 A: 245.76 | |
| Butenolides |  | 1.22 [31] | 299.37 | 2.32 | 55.4 | 1: 1.12; 2: 1.00; 3: 2.65; 4: 3.86; 5: 0.62; 6: 0.49; 7: 0.06; A: YES B: NO | F: 127.86 D: 74.75 A: 62.83 | |
| Butenolides |  | 1.5 [32] | 370.60 | 7.02 | 55.76 | 1: 0.49; 2: 0.56; 3: 2.39; 4: 3.50; 5: 0.45; 6: 0.19; 7: −0.56; A: YES; B: NO | F: 0.01 D: 0.009 A: 0.043 | |
| Butenolides |  | 3.0 [32] | 402.67 | 5.95 | 64.99 | 1: 0.18; 2: 0.02; 3: 1.81; 4: 3.03; 5: 0.13; 6: 0.01; 7: −1.05; A: NO B: NO | F: 0.094 D: 0.077 A: 0.258 | |
| Butenolides |  | 3.0 [32] | 268.39 | 5.63 | 46.53 | 1: 0.72; 2: 0.97; 3: 2.83; 4: 3.81; 5: 0.73; 6: 0.82; 7: 0.12; A: YES B: YES | F: 0.122 D: 0.097 A: 0.288 | |
| Phenyl ethers |  | 10 [33] | 220.31 | 3.52 | 26.3 | 1: 0.94; 2: 0.98; 3: 2.85; 4: 3.80; 5: 0.55; 6: 0.61; 7: −0.25; A: YES B: YES | F: 7.84 D: 5.1 A: 6.81 | |
| Phenyl ethers |  | 10.4 [33] | 236.31 | 4.13 | 35.53 | 1: 1.10; 2: 1.00; 3: 2.98; 4: 4.01; 5: 0.72; 6: 0.83; 7: 0.31; A: YES B: YES | F: 2.39 D: 1.65 A: 2.77 | |
| Pyridyl ethers |  | 0.02 [34] | 247.38 | 5.62 | 21.59 | 1: 0.66; 2: 0.66; 3: 2.31; 4: 3.48; 5: 0.36; 6: 0.20; 7: 0.62; A: YES B: NO | F: 0.11 D: 0.09 A: 0.27 | |
| Juvenoid |  | 0.01 [34] | 264.41 | 6.50 | 26.3 | 1: 0.61; 2: 0.83; 3: 2.54; 4: 3.54; 5: 0.49; 6: 0.31; 7: −0.11; A: YES B: NO | F: 0.02 D: 0.02 A: 0.07 | |
| Capsaicin derivative |  | 5.19 [35] | 293.41 | 3.79 | 58.56 | 1: 1.17; 2: 1.00; 3: 2.79; 4: 4.02; 5: 0.54; 6: 0.51; 7: −0.06; A: YES B: YES | F: 5.90 D: 3.95 A: 5.84 | |
| N-benzoyl monoethanolamine benzoate |  | 6.92 [35] | 269.30 | 2.69 | 55.4 | 1: 1.26; 2: 1.00; 3: 2.73; 4: 3.90; 5: 0.56; 6: 0.54; 7: −0.03; A: YES B: NO | F: 53.02 D: 32.08 A: 31.12 |
2.2. Natural Products as Anti-Foulants
| Class of compound | Structure | Name | Antifouling activity EC50 (Cyprid) µg/mL [REF] | Mr | Log Kow | tPSA | BIOWIN™ | ECOSAR™ acute toxicity (mg/L) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Diterpenoids |  | Pukalide | 0.019 [46] | 372.42 | 3.67 | 74.36 | 1: 0.44; 2: 0.77; 3: 2.36; 4: 3.54; 5: 0.41; 6: 0.10; 7: −0.34; A: YES B: NO | F: 9.66 D: 6.40 A: 9.02 | ||
| Diterpenoids |  | Epoxypukalide | 0.055 [46] | 388.42 | 3.28 | 86.89 | 1: −0.10; 2: 0.02; 3: 2.10; 4: 3.35; 5: 0.44; 6: 0.07; 7: −0.81; A: NO B: NO | F: 22.79 D: 14.56 A: 17.65 | ||
| Terpenoids |  | Kalihinol A | 0.087 [47] | 392.97 | 3.91 | 77.04 | 1: −0.02; 2: 0.004; 3: 1.14; 4: 2.43; 5: 0.14; 6: 0.001; 7: −1.69; A: NO B: NO | F: 6.26 D: 4.24 A: 6.54 | ||
| Terpene |  | Bromo tetrasphaerol | 0.38 [48] | 385.39 | 5.32 | 40.46 | 1: 0.13; 2: 0; 3: 1.90; 4: 3.00; 5: 0.17; 6: 0.002; 7: −0.64; A: NO B: NO | F: 0.33 D: 0.26 A: 0.68 | ||
| Sesquiterpene carbonimide dichloride |  | Axinyssimide A | 1.2 [49] | 352.72 | 7.63 | 24.89 | 1: −0.29; 2: 0; 3: 1.68; 4: 2.87; 5: −0.04; 6: 0.0007; 7: −0.25; A: NO B: NO | F: 0.003 D: 0.002 A: 0.02 | ||
| Isocyano-sesquiterpene |  | (1S, 4R, 7S, 10R)-10-Isocyano-5-cadinen-4-ol | 0.17 [49] | 247.38 | 3.75 | 44.02 | 1: 0.57; 2: 0.67; 3: 2.15; 4: 3.12; 5: 0.24; 6: 0.05; 7: −0.79; A: NO B: NO | F: 5.40 D: 3.60 A: 5.25 | ||
| Sesquiterpene hydroquinone |  | Avarol | 0.65 [50] | 314.47 | 6.92 | 40.46 | 1: 0.33; 2: 0.01; 3: 1.90; 4: 2.94; 5: 0.18; 6: 0.04; 7: −1.32; A: NO B: NO | F: 0.010 D: 0.009 A: 0.04 | ||
| Sesquiterpene |  | 3-isocyanotheonellin | 0.13 [51] | 231.38 | 5.99 | 23.79 | 1: 0.77; 2: 0.93; 3: 2.39; 4: 3.30; 5: 0.22; 6: 0.07; 7: −0.64; A: YES B: NO | F: 0.05 D: 0.04 A: 0.14 | ||
| Bromotyramine |  | Moloka’iamine A | 4.3 [52] | 352.07 | 2.71 | 61.27 | 1: 0.85; 2: 0.43; 3: 2.07; 4: 3.13; 5: 0.42; 6: 0.11; 7: 1.16; A: NO B: NO | F: 66.63 D: 40.40 A: 39.46 | ||
| Polyketide |  | Sterigmatocystin | <0.125 [53] | 324.29 | 4.15 | 74.22 | 1: 0.82; 2: 0.94; 3: 2.26; 4: 3.55; 5: 0.56; 6: 0.28; 7: 0.15; A: YES B: NO | F: 3.13 D: 2.17 A: 3.67 | ||
| Polyacetylene |  | Callytriol C | 0.24 [50] | 348.44 | 4.50 | 60.69 | 1: 1.06; 2: 0.80; 3: 2.91; 4: 3.73; 5: 0.43; 6: 0.17; 7: 0.79; A: YES B: NO | F: 1.61 D: 1.15 A: 2.23 | ||
| Flavone |  | 5,4'-dihydroxy-3,6,7-trimethoxy flavone | 2.5 [54] | 344.32 | 2.00 | 94.45 | 1: 0.87; 2: 0.94; 3: 2.35; 4: 3.63; 5: 0.56; 6: 0.25; 7: 0.17; A: YES B: NO | F: 280.30 D: 159.23 A: 118.80 | ||
| Polyhydroxyl benzylalcohol |  | 3-chloro-2,5-dihydroxybenzyl alcohol | 3.19–3.81 [55] | 174.58 | 0.76 | 60.69 | 1: 0.87; 2: 0.81; 3: 2.88; 4: 3.64; 5: 0.45; 6: 0.35; 7: 0.53; A: YES B: NO | F: 1864.70 D: 944.21 A: 438.04 | ||
| Aaptamine |  | Isoaaptamine | 2.65 [56] | 228.25 | 1.88 | 45.06 | 1: 0.68; 2: 0.67; 3: 2.44; 4: 3.35; 5: 0.25; 6: 0.07; 7: −0.57; A: YES B: NO | F: 237.95 D: 133.68 A: 95.29 | ||
| Butenolide |  | Brominated furanone | 0.02 [41] | 388.88 | 2.96 | 26.3 | 1: 0.71; 2: 0; 3: 2.87; 4: 3.89; 5: 0.34; 6: 0.001; 7: 1.77; A: NO B: NO | F: 44.27 D: 27.46 A: 29.46 | ||
| Capsaicin |  | Capsaicin | 4.18 [35] | 305.42 | 4.00 | 58.56 | 1: 1.06; 2: 0.99; 3: 2.47; 4: 3.73; 5: 0.32; 6: 0.16; 7: −0.21; A: YES B: NO | F: 4.04 D: 2.76 A: 4.40 | ||
| Miscellaneous |  | Subergorgic acid | 1.2 [57] | 218.34 | 4.38 | 17.07 | 1: 0.28; 2: 0.02; 3: 2.27; 4: 3.207; 5: 0.42; 6: 0.15; 7: −0.86; A: NO B: NO | F: 1.31 D: 0.93 A: 1.72 | ||
3. Experimental Section
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Anti-Fouling Systems. Available online: http://www.imo.org/OurWork/Environment/Anti-foulingSystems/Pages/Default.aspx (accessed on 15 May 2014).
- Omae, I. Organotin antifouling paints and their alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Laughlin, R.J. Bioaccumulation of TBT by aquatic organisms. In Organotin; Champ, M., Seligman, P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1996; pp. 331–355. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice, 1st ed.; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Evans, S.M.; Birchenough, A.C.; Brancato, M.S. The TBT ban: Out of the frying pan into the fire? Mar. Pollut. Bull. 2000, 40, 204–211. [Google Scholar] [CrossRef]
- Thomas, K.V.; Brooks, S. The environmental fate and effects of antifouling paint biocides. Biofouling 2010, 26, 73–88. [Google Scholar] [CrossRef]
- Okamura, H.; Aoyama, I.; Liu, D.; Maguire, R.J.; Pacepavicius, G.J.; Lau, Y.L. Fate and ecotoxicity of the new antifouling compound irgarol 1051 in the aquatic environment. Water Res. 2000, 34, 3523–3530. [Google Scholar] [CrossRef]
- Rittschof, D. Research on practical environmentally benign antifouling coatings. In Biofouling; Wiley-Blackwell: Oxford, UK, 2010; pp. 396–409. [Google Scholar]
- Pavan, M.; Worth, A.P. Review of QSAR Models for Ready Biodegradation; EUR 22355 EN; European Commission Directorate General Joint Research Centre: Ispra, Italy, 2006. [Google Scholar]
- Rucker, C.; Kummerer, K. Modeling and predicting aquatic aerobic biodegradation—A review from a user’s perspective. Green Chem. 2012, 14, 875–887. [Google Scholar] [CrossRef]
- Howard, P.H.; Muir, D.C. Identifying new persistent and bioaccumulative organics among chemicals in commerce II: Pharmaceuticals. Environ. Sci. Technol. 2011, 45, 6938–6946. [Google Scholar] [CrossRef]
- Howard, P.H.; Muir, D.C.G. Identifying new persistent and bioaccumulative organics among chemicals in commerce. III: Byproducts, impurities, and transformation products. Environ. Sci. Technol. 2013, 47, 5259–5266. [Google Scholar] [CrossRef]
- Boethling, R.S.L.; Lynch, D.G.; Jaworska, J.S.; Tunkel, J.L; Thom, G.C.; Webb, S. Using BIOWIN, bayes, and batteries to predict ready biodegradability. Environ. Toxicol. Chem. 2004, 23, 911–920. [Google Scholar] [CrossRef]
- Kelly M.-B.; Kendra, M.; Bill, M.; Peter, R. Methodology Document for the Ecological Structure-Activity Relationship Model (Ecosar) Class Program; Environmental Protection Agency: Washington, DC, USA, 2012; pp. 1–43. [Google Scholar]
- Thomas, K.V. The environmental fate and behaviour of antifouling paint booster biocides: A review. Biofouling 2001, 17, 73–86. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Omae, I. General aspects of tin-free antifouling paints. Chem. Rev. 2003, 103, 3431–3448. [Google Scholar] [CrossRef]
- Harino, H.; Masaaki, K.; Yoshiaki, M.; Kazuhiko, M.; Akira, K.; Satoshi, A. Degradation of antifouling booster biocides in water. J. Mar. Biol. Assoc. U. K. 2005, 85, 33–38. [Google Scholar] [CrossRef]
- Scarlett, A.; Donkin, M.E.; Fileman, T.W.; Donkin, P. Occurrence of the marine antifouling agent irgarol 1051 within the plymouth sound locality: Implications for the green macroalga enteromorpha intestinalis. Mar. Pollut. Bull. 1997, 34, 645–651. [Google Scholar] [CrossRef]
- Liu, D; Maguire, R.J.; Lau, Y.L.; Pacepavicius, G.J.; Okamura, H.; Aoyama, I. Transformation of the new antifouling compound irgarol 1051 by phanerochaete chrysosporium. Water Res. 1997, 31, 2363–2369. [Google Scholar] [CrossRef]
- Callow, M.E.; Willingham, G.L. Degradation of antifouling biocides. Biofouling 1996, 10, 239–249. [Google Scholar] [CrossRef]
- Howard, P.H.; Hueber, A.E.; Boethling, R.S. Biodegradation data evaluation for structure/biodegradability relations. Environ. Toxicol. Chem. 1987, 6, 1–10. [Google Scholar] [CrossRef]
- Sharma, P.; Suri, C.R. Biotransformation and biomonitoring of phenylurea herbicide diuron. Bioresour. Technol. 2011, 102, 3119–3125. [Google Scholar] [CrossRef]
- Jacobson, A.H.; Willingham, G.L. Sea-nine antifoulant: An environmentally acceptable alternative to organotin antifoulants. Sci. Total Environ. 2000, 258, 103–110. [Google Scholar] [CrossRef]
- Rittschof, D.; Lai, C.-H.; Kok, L.-M.; Teo, S.L.-M. Pharmaceuticals as antifoulants: Concept and principles. Biofouling 2003, 19, 207–212. [Google Scholar] [CrossRef]
- Sjögren, M.; Johnson, A.-L.; Hedner, E.; Dahlström, M.; Göransson, U.; Shirani, H.; Bergman, J.; Jonsson, P.R.; Bohlin, L. Antifouling activity of synthesized peptide analogs of the sponge metabolite barettin. Peptides 2006, 27, 2058–2064. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Reuschenbach, P.; Silvani, M.; Dammann, M.; Warnecke, D.; Knacker, T. Ecosar model performance with a large test set of industrial chemicals. Chemosphere 2008, 71, 1986–1995. [Google Scholar] [CrossRef]
- Dahlström, M.; Mårtensson, L.G.E.; Jonsson, P.R.; Arnebrant, T.; Elwing, H. Surface active adrenoceptor compounds prevent the settlement of cyprid larvae of balanus improvisus. Biofouling 2000, 16, 191–203. [Google Scholar] [CrossRef]
- Ortlepp, S.; Pedpradap, S.; Dobretsov, S.; Proksch, P. Antifouling activity of sponge-derived polybrominated diphenyl ethers and synthetic analogues. Biofouling 2008, 24, 201–208. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Xu, Y.; Matsumura, K.; Han, Z.; Liu, L.; Lin, W.; Jia, Y.; Qian, P.-Y. Structural optimization and evaluation of butenolides as potent antifouling agents: Modification of the side chain affects the biological activities of compounds. Biofouling 2012, 28, 857–864. [Google Scholar]
- Stewart, M.; Miles, W.; Depree, C. Antifouling activity of synthetic γ-hydroxybutenolides. Int. Biodeter. Biodegrad. 2013, 88, 176–184. [Google Scholar] [CrossRef]
- Braekman, J.-C.; Daloze, D. Chemical and biological aspects of sponge secondary metabolites. Phytochem. Rev. 2004, 3, 275–283. [Google Scholar] [CrossRef]
- Skattebøl, L.; Nilsen, N.O.; Stenstrøm, Y.; Andreassen, P.; Willemsen, P. The antifouling activity of some juvenoids on three species of acorn barnacle, balanus. Pest Manag. Sci. 2006, 62, 610–616. [Google Scholar] [CrossRef]
- Angarano, M.-B.; McMahon, R.F.; Hawkins, D.L.; Schetz, J.A. Exploration of structure-antifouling relationships of capsaicin-like compounds that inhibit zebra mussel (Dreissena polymorpha) macrofouling. Biofouling 2007, 23, 295–305. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Xu, Y.; He, H.; Schulz, S.; Liu, X.; Fusetani, N.; Xiong, H.; Xiao, X.; Qian, P.-Y. Potent antifouling compounds produced by marine streptomyces. Bioresour. Technol. 2010, 101, 1331–1336. [Google Scholar] [CrossRef]
- Schoenfeld, R.C.; Conova, S.; Rittschof, D.; Ganem, B. Cytotoxic, antifouling bromotyramines: A synthetic study on simple marine natural products and their analogues. Bioorg. Med. Chem. Lett. 2002, 12, 823–825. [Google Scholar] [CrossRef]
- Wang, J.; Shi, T.; Yang, X.; Han, W.; Zhou, Y. Environmental risk assessment on capsaicin used as active substance for antifouling system on ships. Chemosphere 2014, 104, 85–90. [Google Scholar] [CrossRef]
- De Nys, R.; Steinberg, P.D.; Willemsen, P.; Dworjanyn, S.A.; Gabelish, C.L.; King, R.J. Broad spectrum effects of secondary metabolites from the red alga delisea pulchra in antifouling assays. Biofouling 1995, 8, 259–271. [Google Scholar] [CrossRef]
- Boethling, R.S.; Sommer, E.; DiFiore, D. Designing small molecules for biodegradability. Chem. Rev. 2007, 107, 2207–2227. [Google Scholar] [CrossRef]
- Howard, P.H.; Boethling, R.S. Designing for non-persistence. In Handbook of Green Chemistry; Wiley-VCH: Weinheim, Germany, 2012; volume 9, pp. 453–484. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64 (Suppl.), 4–17. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Gerhart, D.; Rittschof, D.; Mayo, S. Chemical ecology and the search for marine antifoulants. J. Chem. Ecol. 1988, 14, 1905–1917. [Google Scholar] [CrossRef]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. Antifouling kalihinenes from the marine sponge acanthella cavernosa. Tetrahedron Lett. 1995, 36, 8637–8640. [Google Scholar] [CrossRef]
- Piazza, V.; Roussis, V.; Garaventa, F.; Greco, G.; Smyrniotopoulos, V.; Vagias, C.; Faimali, M. Terpenes from the red alga sphaerococcus coronopifolius inhibit the settlement of barnacles. Mar. Biotechnol. 2011, 13, 764–772. [Google Scholar] [CrossRef]
- Hirota, H.; Okino, T.; Yoshimura, E.; Fusetani, N. Five new antifouling sesquiterpenes from two marine sponges of the genus axinyssa and the nudibranch phyllidia pustulosa. Tetrahedron 1998, 54, 13971–13980. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Seven new polyacetylene derivatives, showing both potent metamorphosis-inducing activity in ascidian larvae and antifouling activity against barnacle larvae, from the marine sponge callyspongia truncata. J. Nat. Prod. 1997, 60, 126–130. [Google Scholar] [CrossRef]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New antifouling sesquiterpenes from four nudibranchs of the family phyllidiidae. Tetrahedron 1996, 52, 9447–9454. [Google Scholar] [CrossRef]
- Sjögren, M.; Göransson, U.; Johnson, A.-L.; Dahlström, M.; Andersson, R.; Bergman, J.; Jonsson, P.R.; Bohlin, L. Antifouling activity of brominated cyclopeptides from the marine sponge geodia barretti. J. Nat. Prod. 2004, 67, 368–372. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wu, H.-X.; Xu, Y.; Shao, C.-L.; Wang, C.-Y.; Qian, P.-Y. Antifouling activity of secondary metabolites isolated from chinese marine organisms. Mar. Biotechnol. 2013, 15, 552–558. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, Y.; Jin, C.; He, H.; Hao, X.; Qian, P.-Y. Flavone and isoflavone derivatives of terrestrial plants as larval settlement inhibitors of the barnacle balanus amphitrite. Biofouling 2009, 25, 69–76. [Google Scholar] [CrossRef]
- Kwong, T.; Miao, L.; Li, X.; Qian, P. Novel antifouling and antimicrobial compound from a marine-derived fungus ampelomyces sp. Mar. Biotechnol. 2006, 8, 634–640. [Google Scholar] [CrossRef]
- Diers, J.; Bowling, J.; Duke, S.; Wahyuono, S.; Kelly, M.; Hamann, M. Zebra mussel antifouling activity of the marine natural product aaptamine and analogs. Mar. Biotechnol. 2006, 8, 366–372. [Google Scholar] [CrossRef]
- Qi, S.H.; Zhang, S.; Yang, L.H.; Qian, P.Y. Antifouling and antibacterial compounds from the gorgonians subergorgia suberosa and scripearia gracillis. Nat. Prod. Res. 2008, 22, 154–166. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, Y.T.; Teo, S.L.M.; Leong, W.; Chai, C.L.L. Searching for “Environmentally-Benign” Antifouling Biocides. Int. J. Mol. Sci. 2014, 15, 9255-9284. https://doi.org/10.3390/ijms15069255
Cui YT, Teo SLM, Leong W, Chai CLL. Searching for “Environmentally-Benign” Antifouling Biocides. International Journal of Molecular Sciences. 2014; 15(6):9255-9284. https://doi.org/10.3390/ijms15069255
Chicago/Turabian StyleCui, Yan Ting, Serena L. M. Teo, Wai Leong, and Christina L. L. Chai. 2014. "Searching for “Environmentally-Benign” Antifouling Biocides" International Journal of Molecular Sciences 15, no. 6: 9255-9284. https://doi.org/10.3390/ijms15069255




