Synthesis, Characterization and Photocatalytic Activity of New Photocatalyst ZnBiSbO4 under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
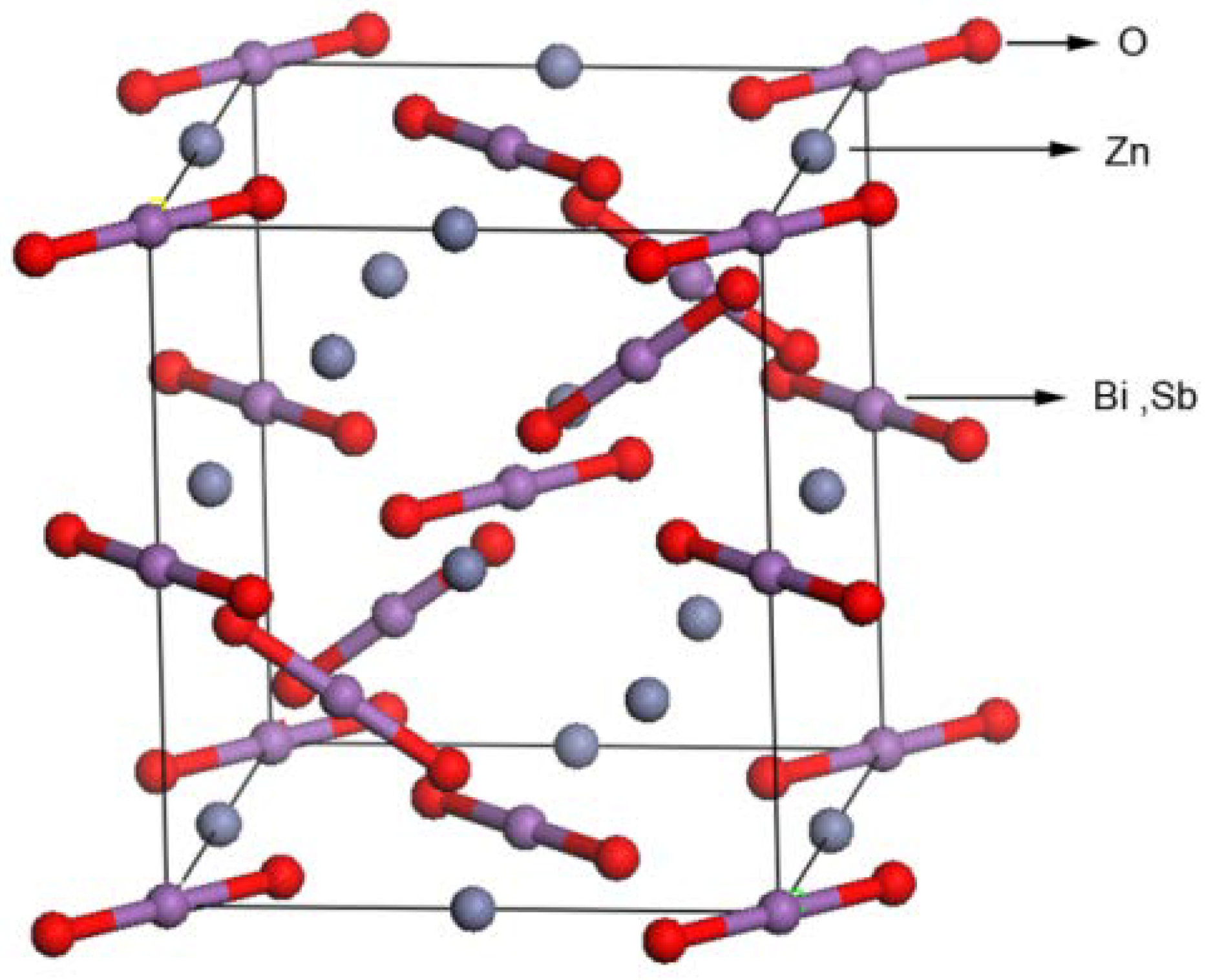
2.1. Crystal Structure and Optical Properties
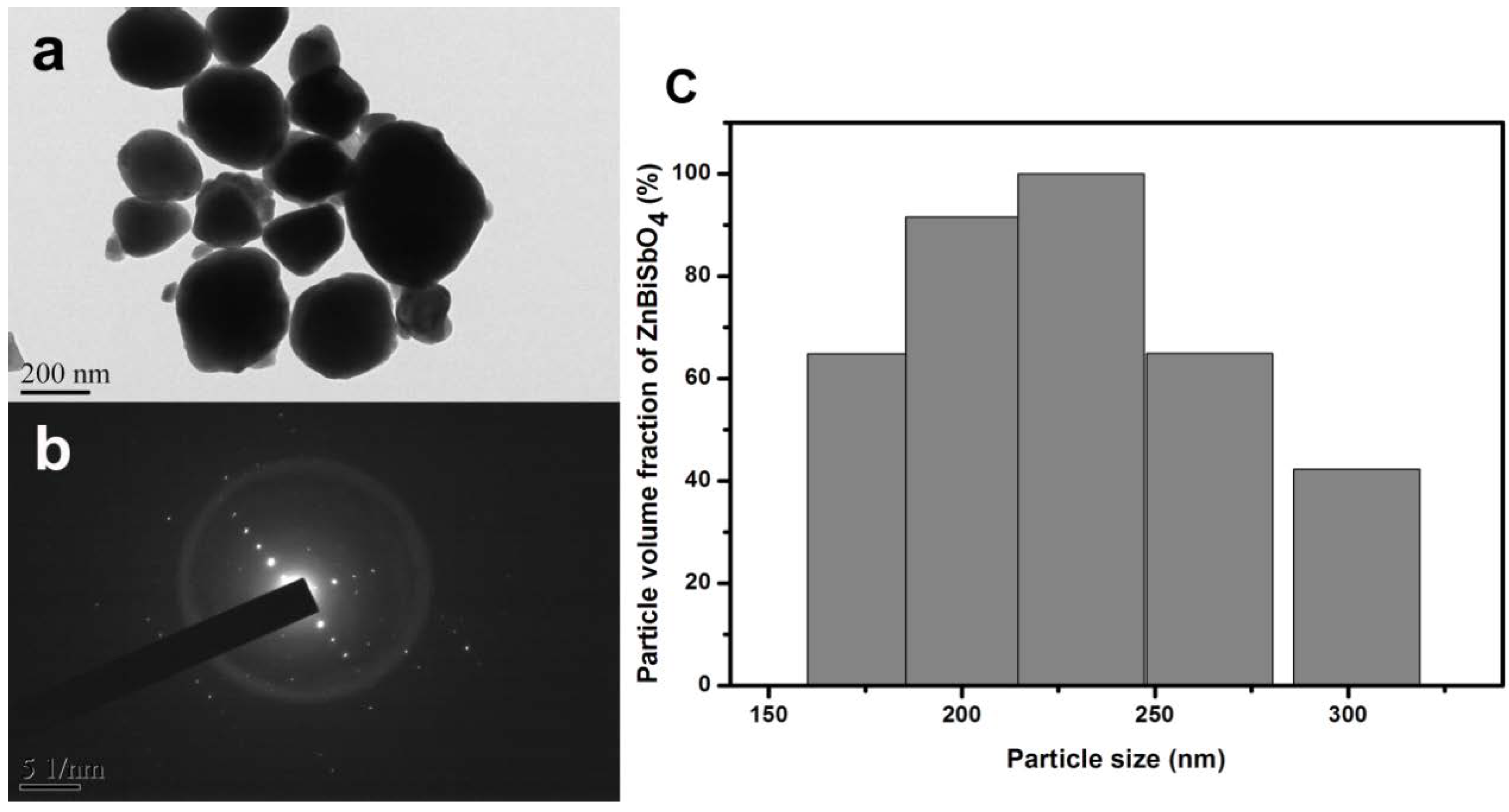
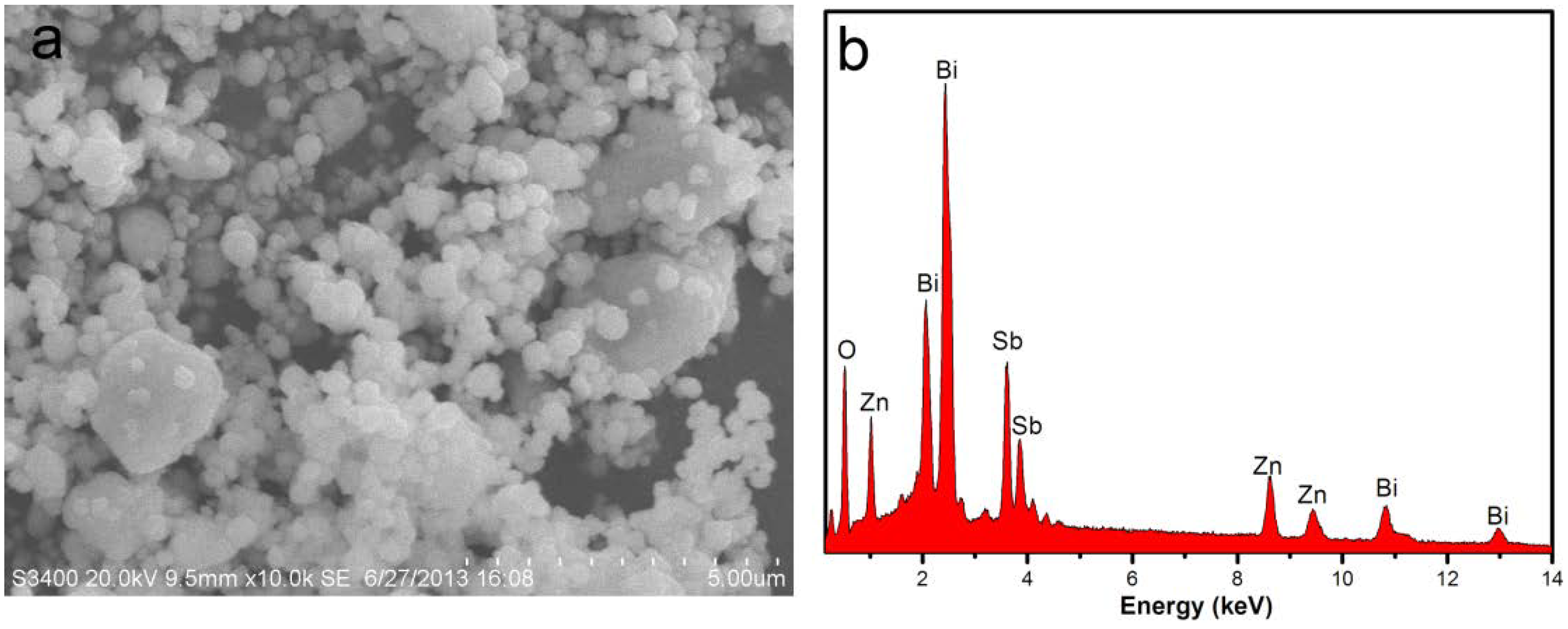
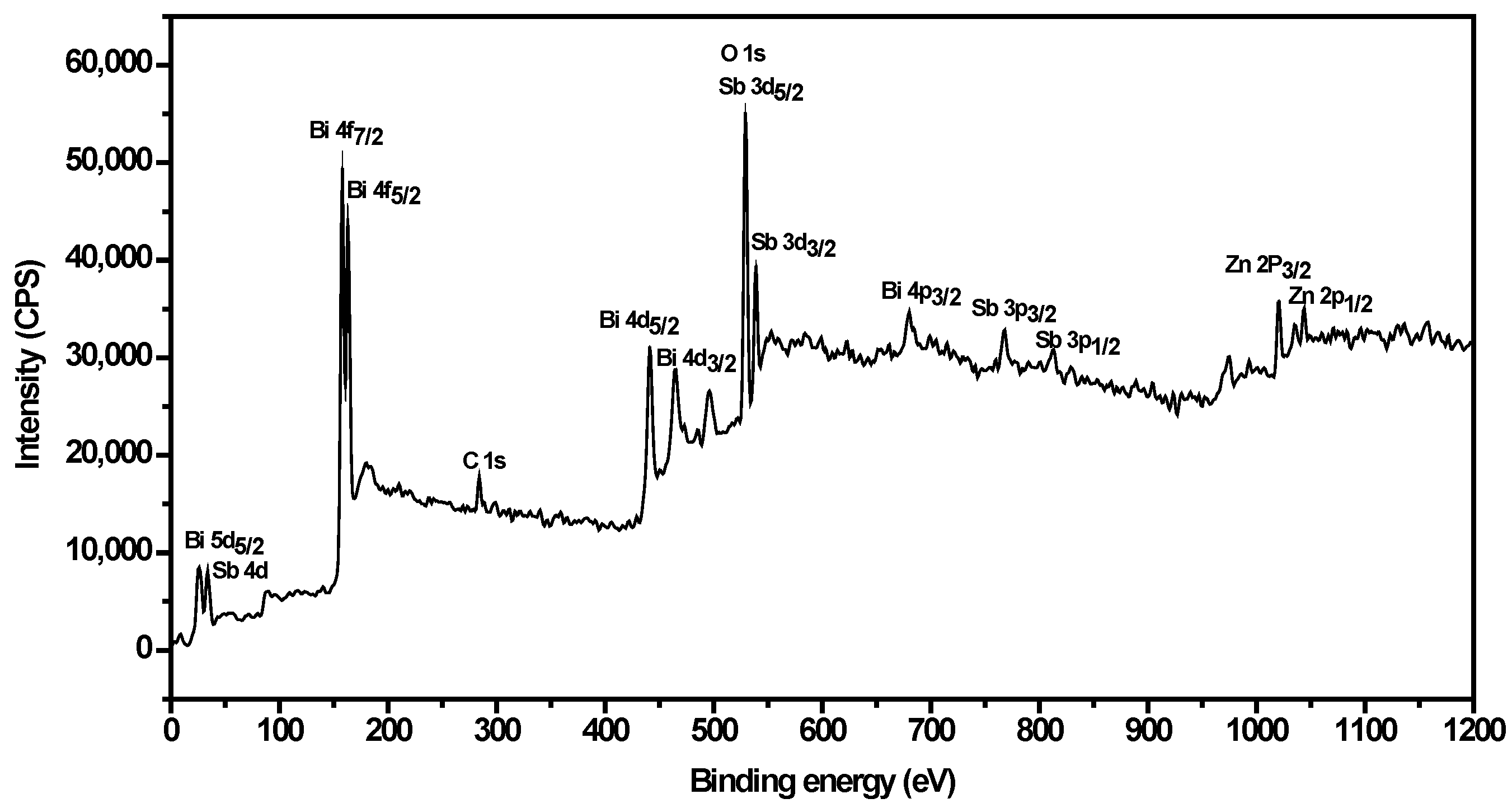
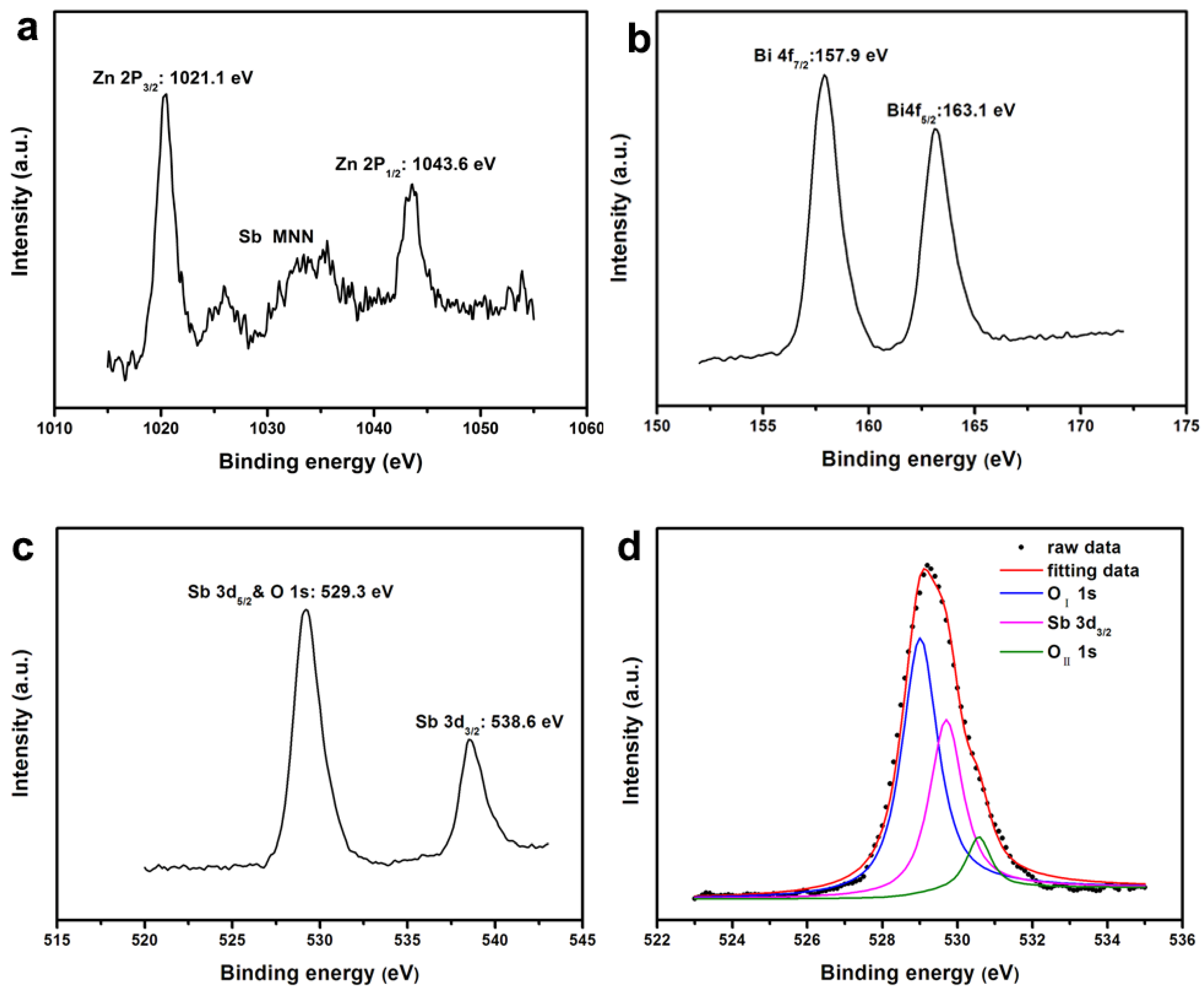
| Element | Sb 3d5/2 | O 1s | |
|---|---|---|---|
| OI | OII | ||
| Binding energy (eV) | 529.7 | 529.0 | 530.6 |
| Element | Zn 2p3/2 | Bi 4f7/2 | Sb 3d5/2 | O 1s |
|---|---|---|---|---|
| Binding energy (eV) | 1021.1 | 157.9 | 529.7 | 529.0 |
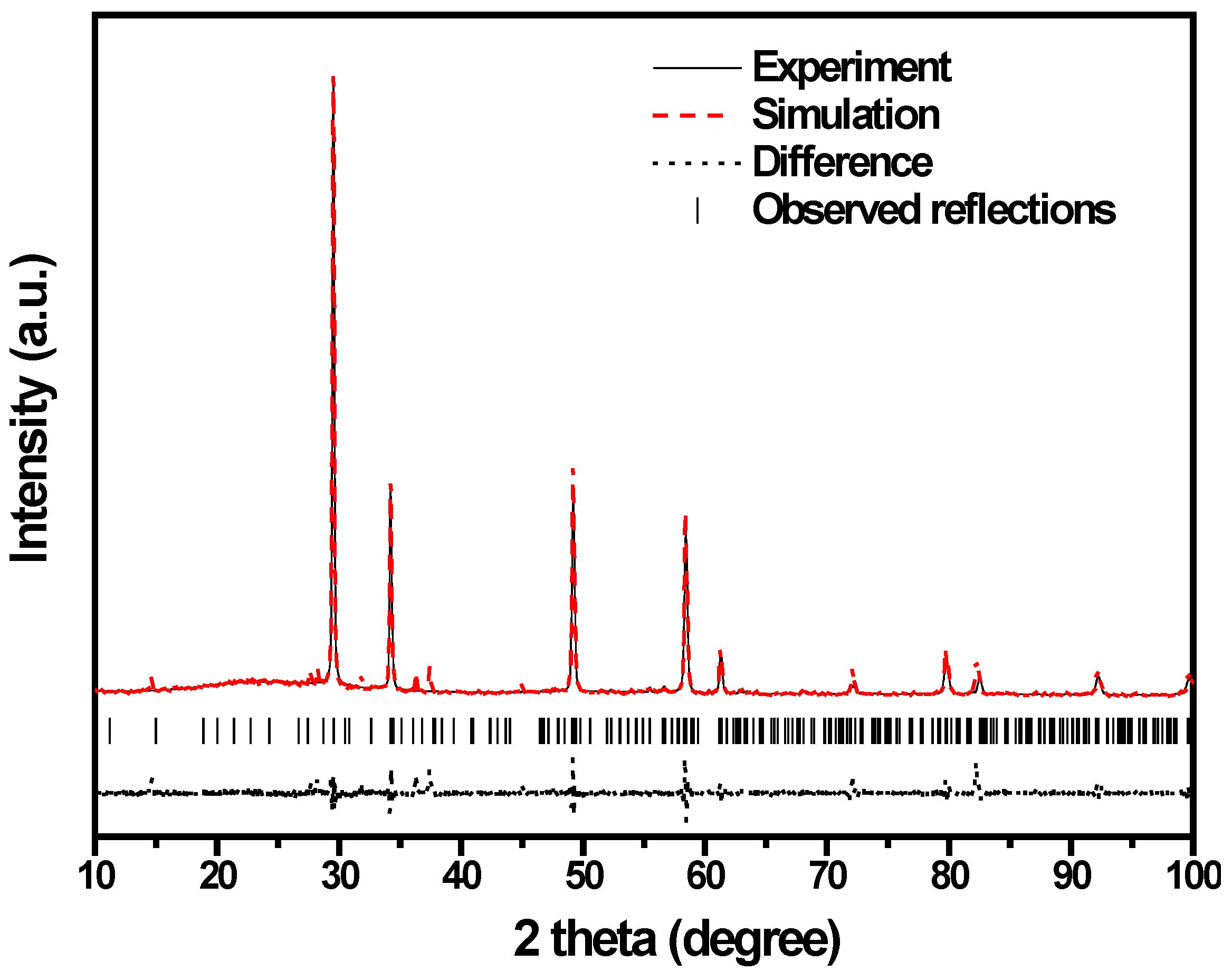
| Atom | x | y | z | Occupation factor |
|---|---|---|---|---|
| Zn | 0 | 0 | 0.5 | 1 |
| Bi | 0 | 0 | 0 | 1 |
| Sb | 0 | 0 | 0 | 1 |
| O | 0.76731 | 0.14013 | 0.08188 | 1 |
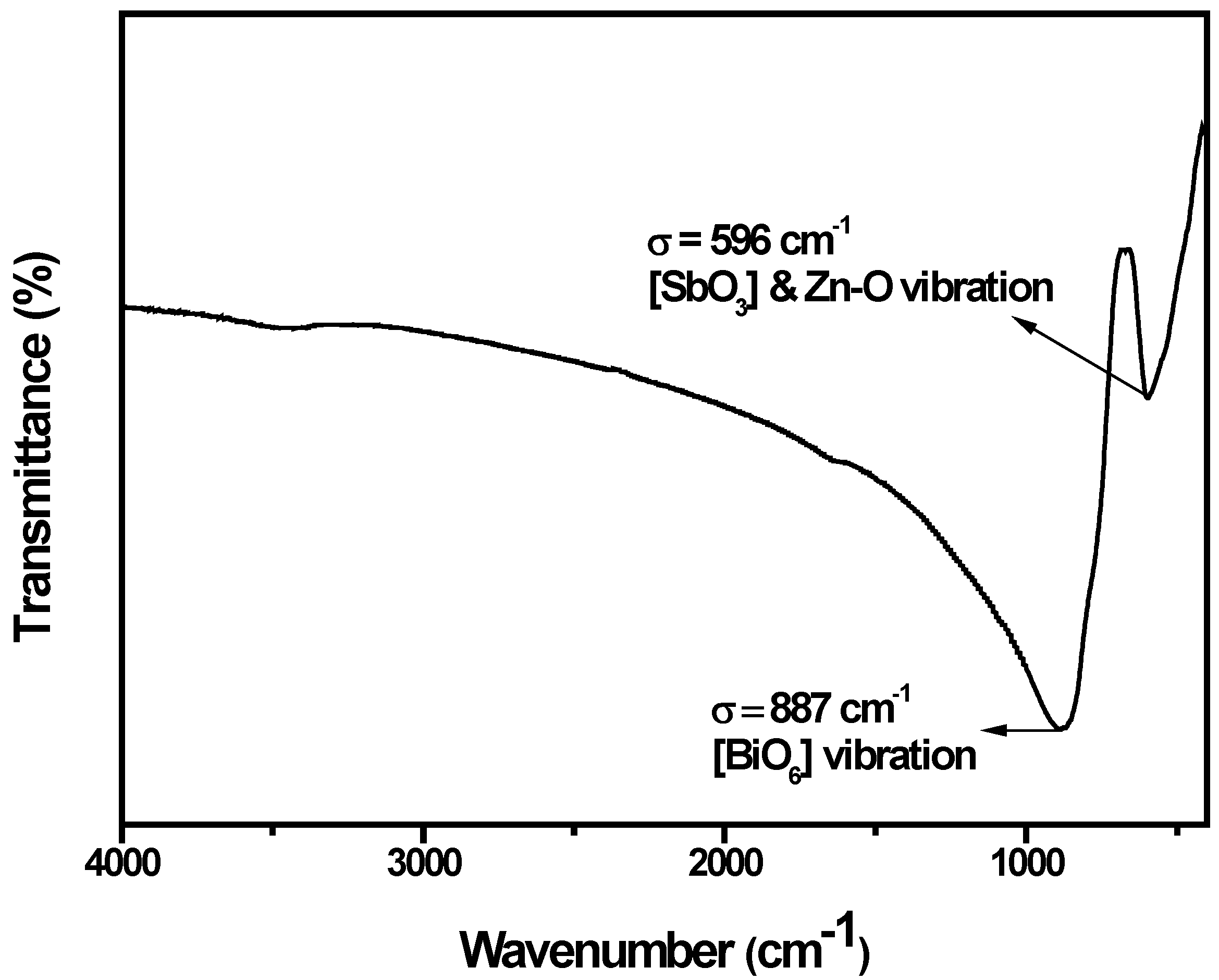
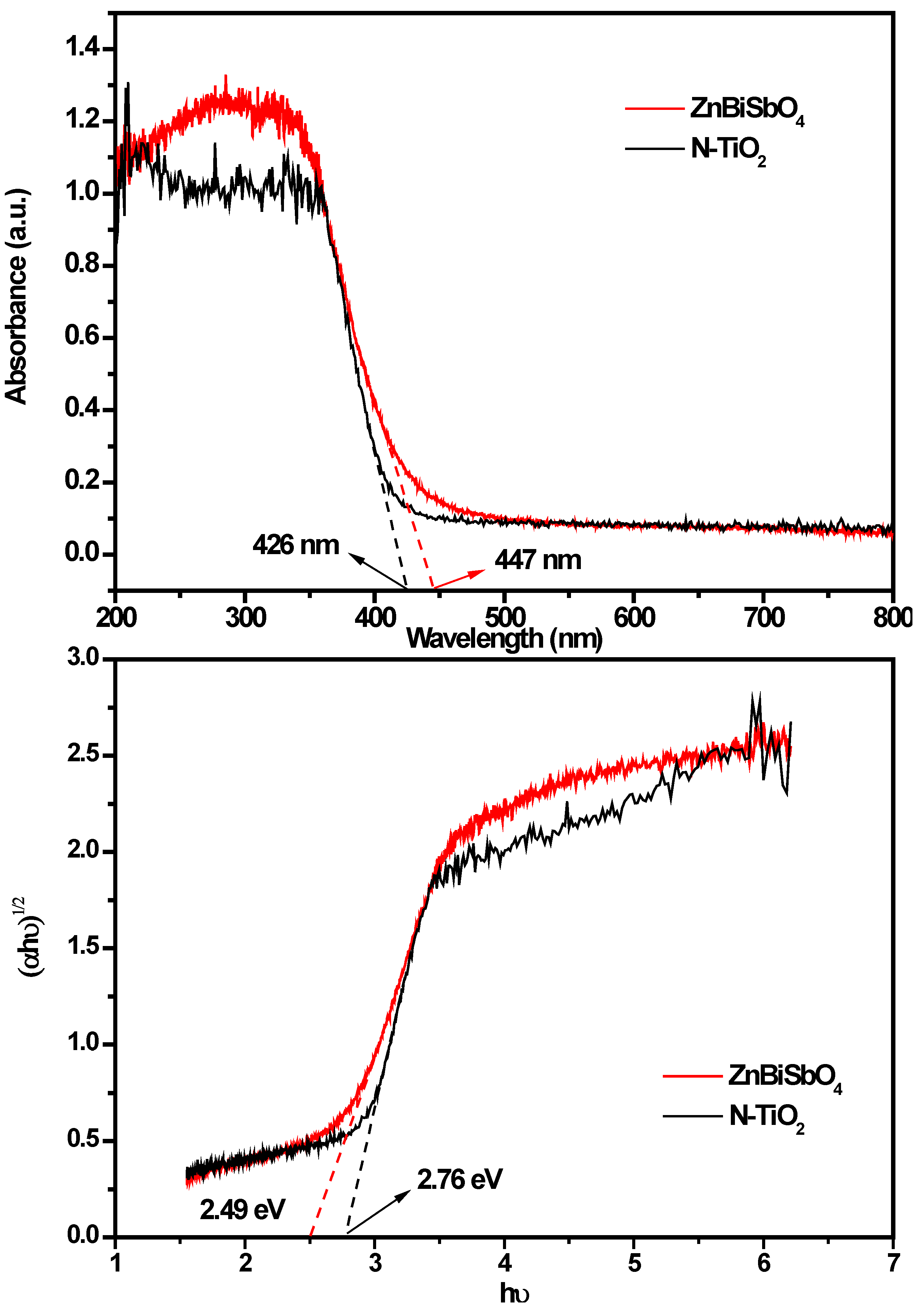
2.2. Photocatalytic Properties
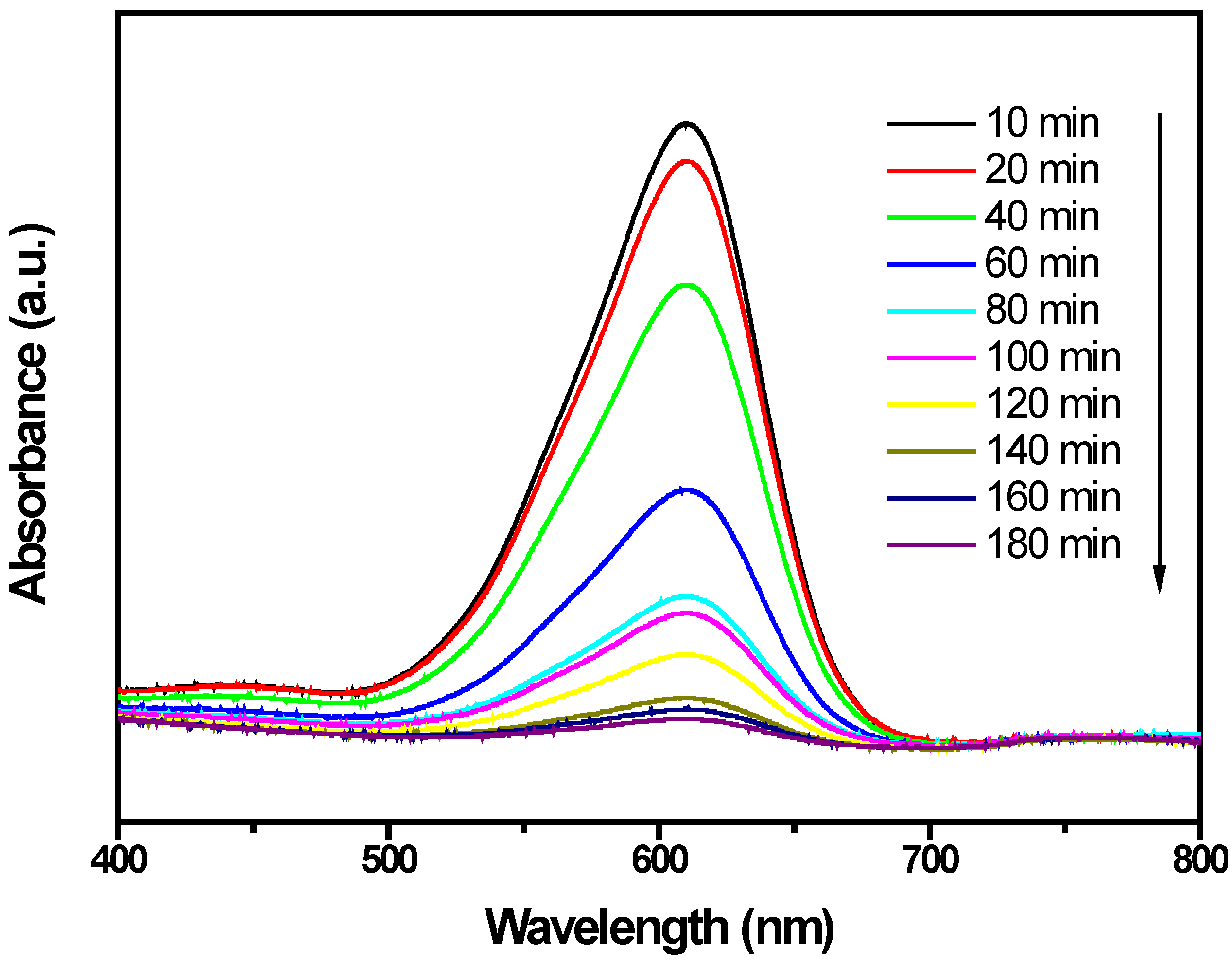
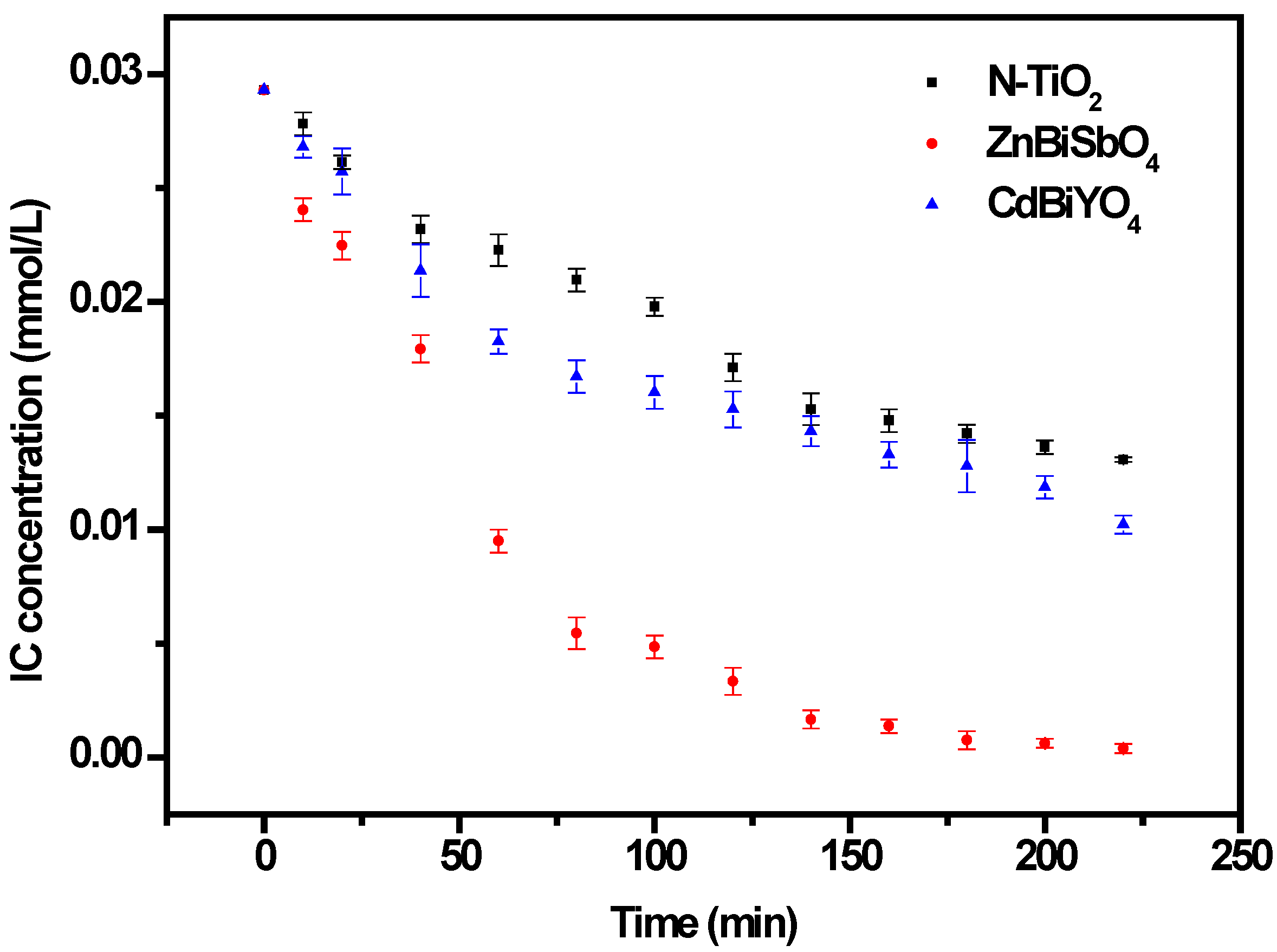
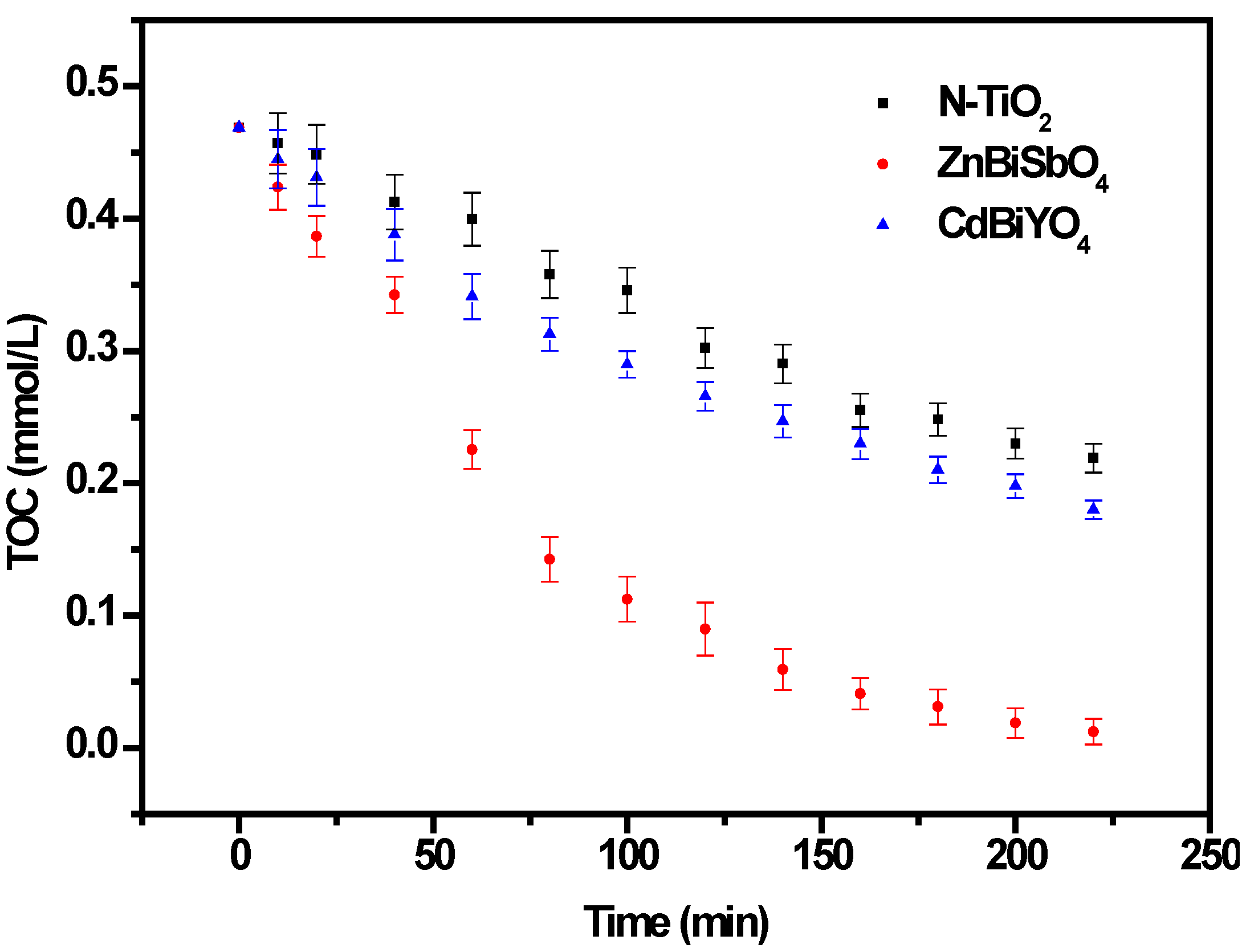

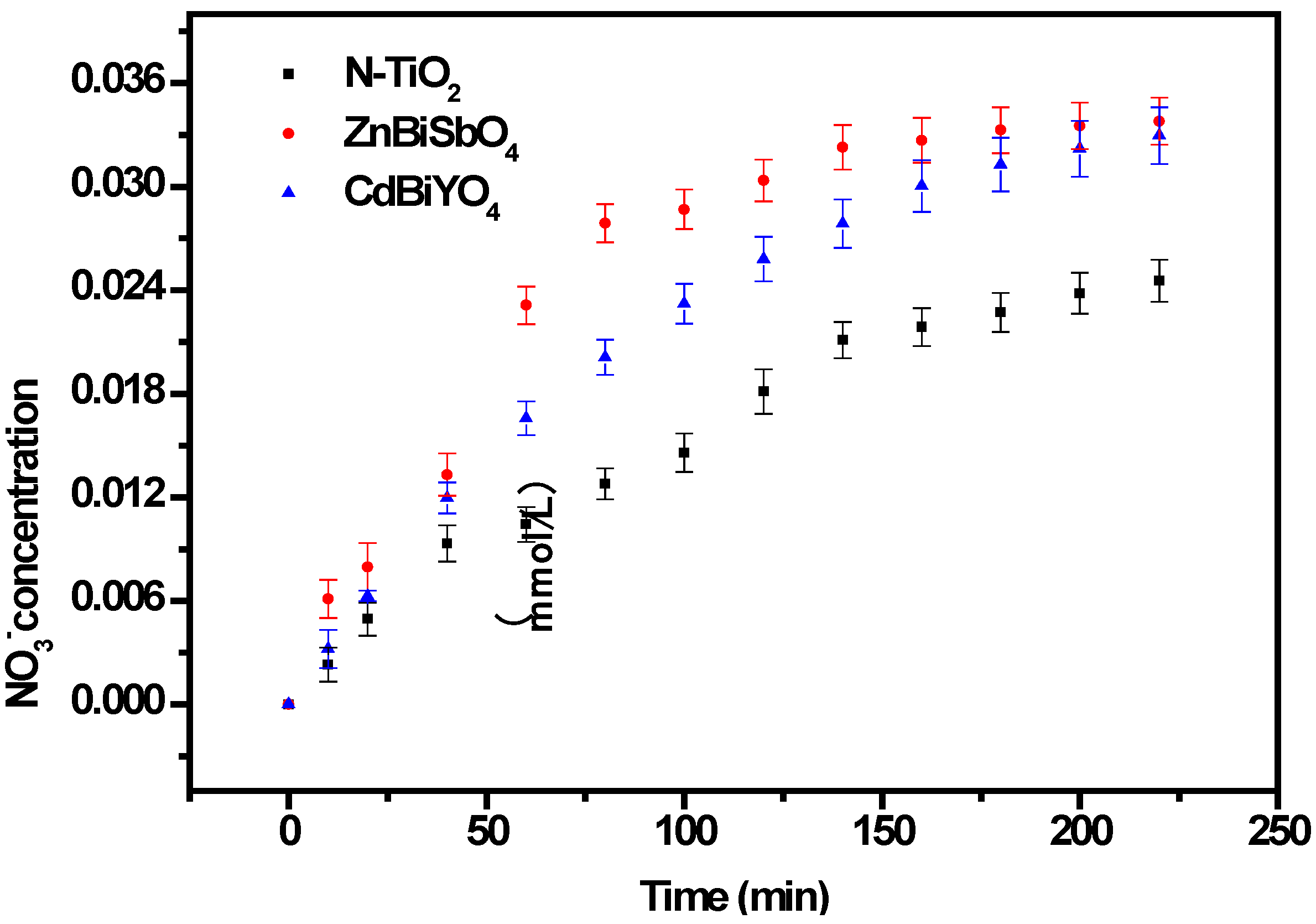
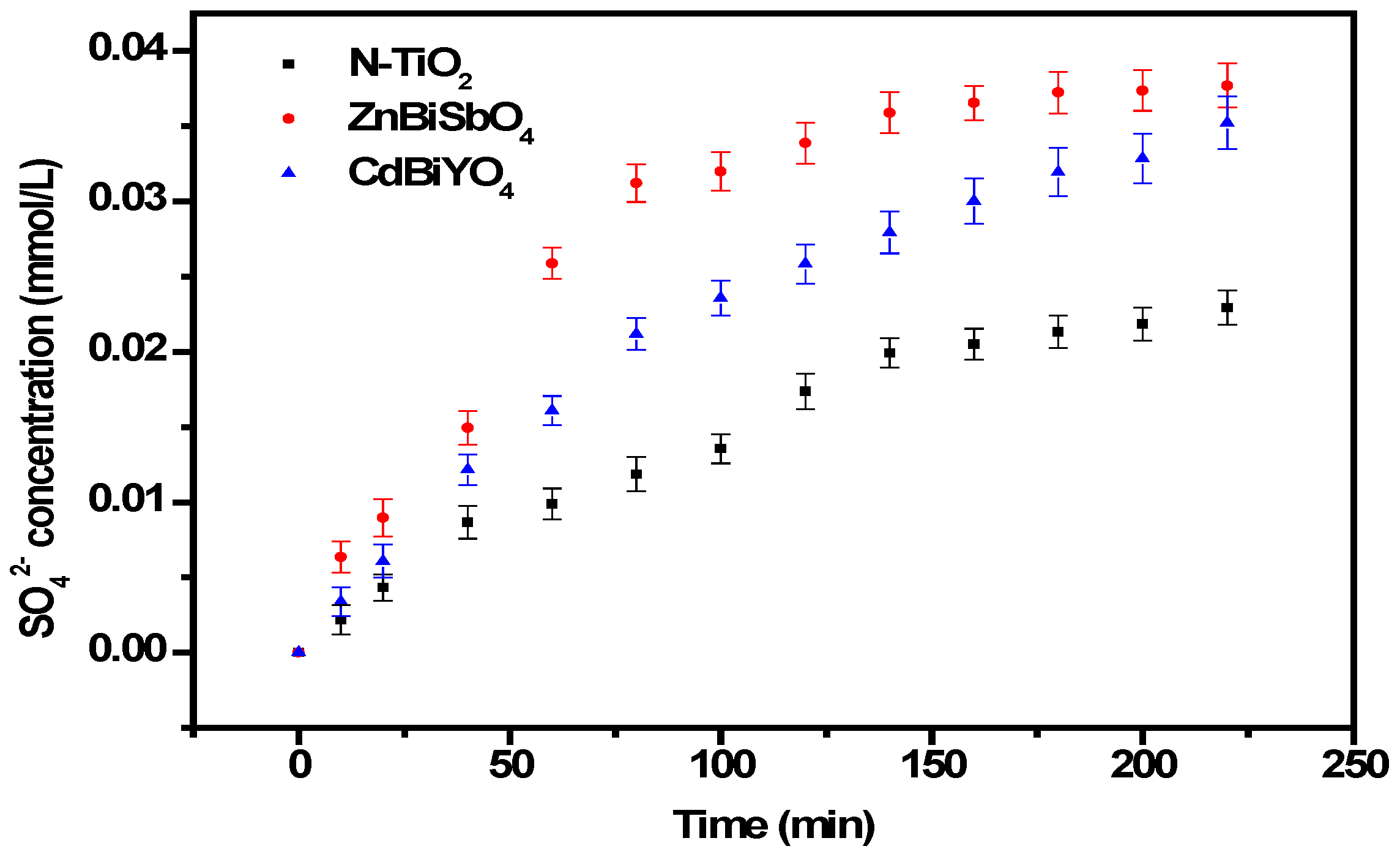
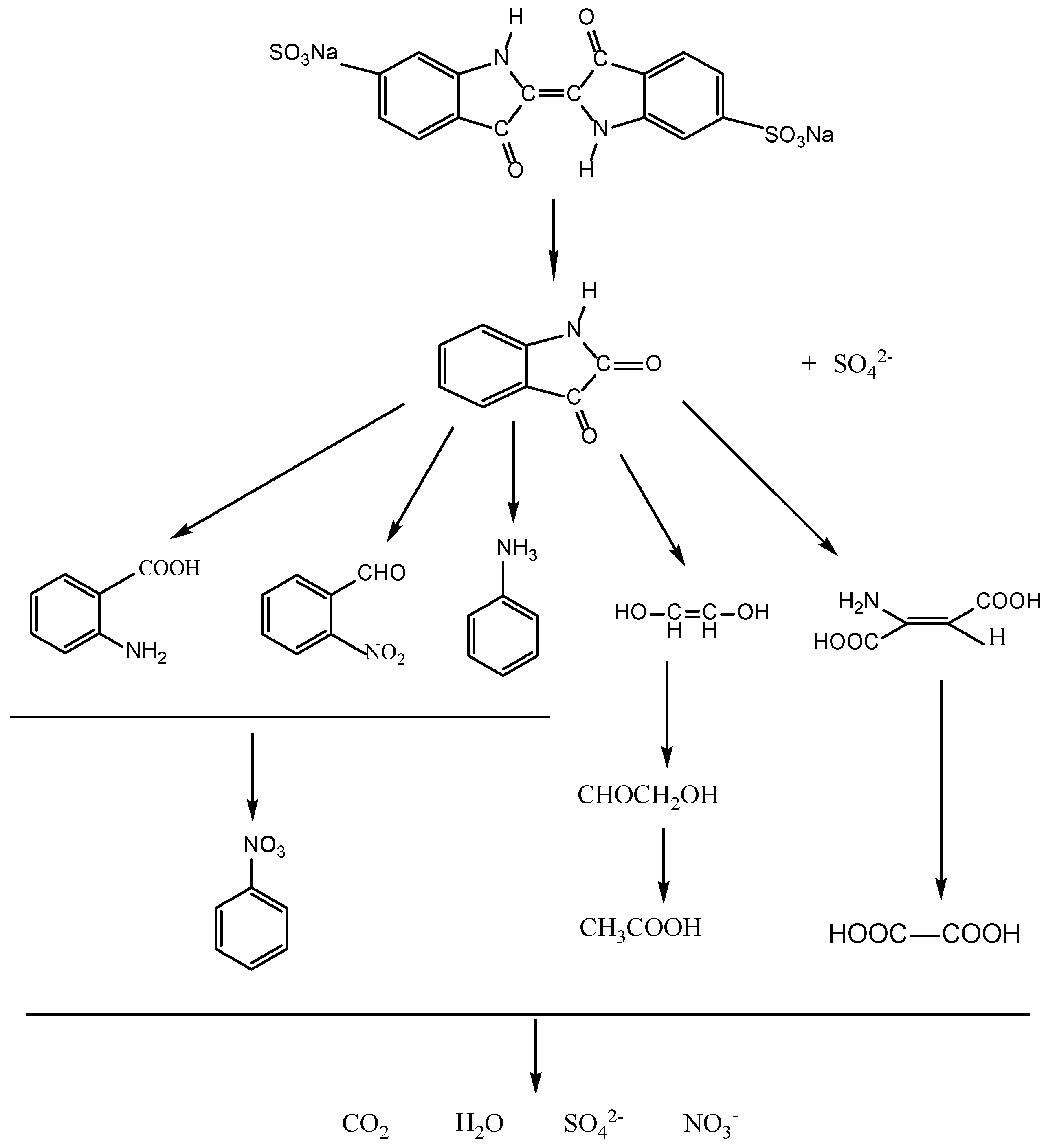
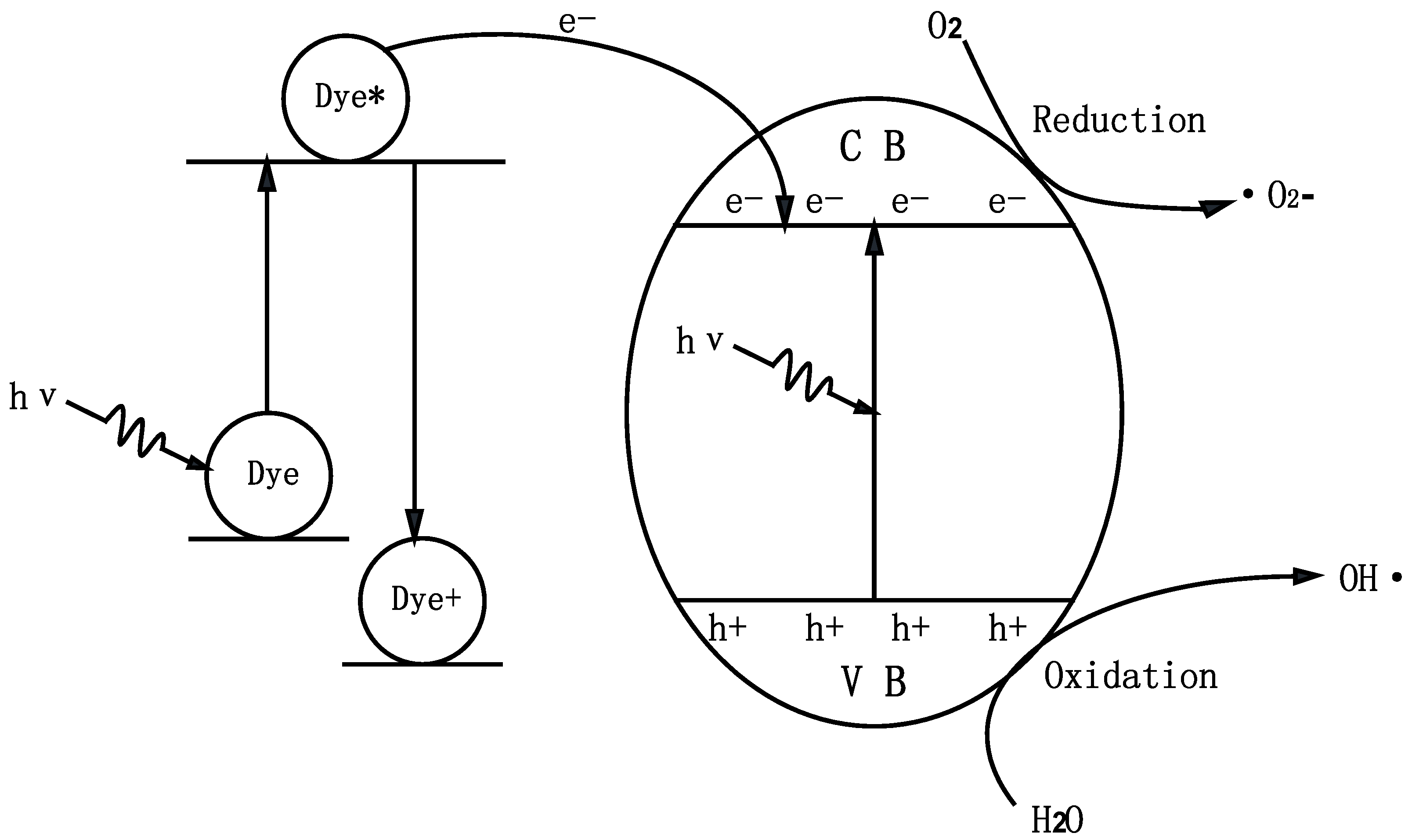
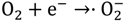
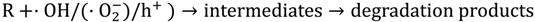
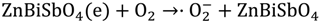
2.3. Phytotoxicity Properties
| Parameters | Sorghum vulgare | ||||||
|---|---|---|---|---|---|---|---|
| Control | Sample of different photocatalytic reaction time (min) | ||||||
| 0 | 40 | 80 | 120 | 160 | 220 | ||
| Germination (%) | 100 | 0 | 7.2 ± 0.6 | 21 ± 4.1 | 44 ± 6.5 | 68 ± 8.0 | 92 ± 3.5 |
| Plumule (cm) | 9.82 ± 1.23 | 0 | 1.15 ± 0.18 | 2.02 ± 0.36 | 4.16 ± 0.58 | 5.33 ± 0.79 | 8.54 ± 1.23 |
| Radicle (cm) | 6.79 ± 9.52 | 0 | 0.36 ± 0.12 | 1.04 ± 0.22 | 2.29 ± 0.29 | 3.22 ± 0.54 | 5.47 ± 0.89 |
| Parameters | Triticum aestivum | ||||||
|---|---|---|---|---|---|---|---|
| Control | Sample of different photocatalytic reaction time (min) | ||||||
| 0 | 40 | 80 | 120 | 160 | 220 | ||
| Germination (%) | 100 | 0 | 5.7 ± 1.3 | 16 ± 2.0 | 37 ± 6.4 | 63 ± 8.7 | 87 ± 2.5 |
| Plumule (cm) | 8.24 ± 1.12 | 0 | 1.02 ± 0.22 | 1.79 ± 0.29 | 3.84 ± 0.68 | 5.11 ± 0.82 | 8.32 ± 1.25 |
| Radicle (cm) | 6.13 ± 1.07 | 0 | 0.31 ± 0.05 | 0.96 ± 0.14 | 2.03 ± 0.57 | 3.02 ± 0.44 | 5.25 ± 0.78 |
3. Experimental Section
3.1. Synthesis of Nanocatalyst
3.2. Characterization of ZnBiSbO4
3.3. Photocatalytic Activity Experiments
3.4. Phytotoxicity Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siddique, M.; Farooq, R.; Khalid, A.; Farooq, A.; Mahmood, Q.; Farooq, U.; Raja, I.A.; Shaukat, S.F. Thermal-pressure-mediated hydrolysis of reactive blue 19 dye. J. Hazard. Mater. 2009, 172, 1007–1012. [Google Scholar] [CrossRef]
- Wang, S.B.; Li, H.T. Dye adsorption on unburned carbon: Kinetics and equilibrium. J. Hazard. Mater. 2005, 126, 71–77. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Shao, C.L.; Guo, Z.C.; Zhang, Z.Y.; Mu, J.B.; Zhang, P.; Cao, T.P.; Liu, Y.C. Highly efficient decomposition of organic dye by aqueous-solid phase transfer and in situ photocatalysis using hierarchical copper phthalocyanine hollow spheres. ACS Appl. Mater. Interfaces 2011, 3, 2573–2578. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Binding, D.; Steinbach, F. Homogeneous photocatalysis by organic dyes in liquid phase. Nature 1970, 227, 832–833. [Google Scholar] [CrossRef]
- Luan, J.F.; Pan, B.C.; Paz, Y.; Li, Y.M.; Wu, X.S.; Zou, Z.G. Structural, photophysical and photocatalytic properties of new Bi2SbVO7 under visible light irradiation. Phys. Chem. Chem. Phys. 2009, 11, 6289–6298. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.C.; Wang, X.M.; Mai, B.X.; Sheng, G.Y.; Peng, P.; Fu, J.M. Preparation, characterization and photocatalytic activity of nano-sized ZnO/SnO2 coupled photocatalysts. Appl. Catal. B 2002, 39, 269–279. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.P.; Wu, Z.N. Pesticide removal from water suspension by UV/TiO2 process: A parametric study. Desalin. Water Treat. 2014, 52, 1956–1964. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 surfaces—Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Torimoto, T.; Ito, S.; Kuwabata, S.; Yoneyama, H. Effects of adsorbents used as supports for titanium dioxide loading on photocatalytic degradation of propyzamide. Environ. Sci. Technol. 1996, 30, 1275–1281. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K. Cu doped TiO2/GF for photocatalytic disinfection of escherichia coli in bioaerosols under visible light irradiation: Application and mechanism. Appl. Surf. Sci. 2014, 296, 15–23. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, A.B. Ultrasound assisted synthesis of doped TiO2 nano-particles: Characterization and comparison of effectiveness for photocatalytic oxidation of dyestuff effluent. Ultrason. Sonochem. 2013, 20, 277–286. [Google Scholar]
- Charanpahari, A.; Umare, S.S.; Gokhale, S.P.; Sudarsan, V.; Sreedhar, B.; Sasikala, R. Enhanced photocatalytic activity of multi-doped TiO2 for the degradation of methyl orange. Appl. Catal. A 2012, 443, 96–102. [Google Scholar]
- Karunakaran, C.; SakthiRaadha, S.; Gomathisankar, P. Hot-injection synthesis of bactericidal Sn-doped TiO2 nanospheres for visible-light photocatalysis. Mater. Express 2012, 2, 319–326. [Google Scholar] [CrossRef]
- Sano, T.; Mera, N.; Kanai, Y.; Nishimoto, C.; Tsutsui, S.; Hirakawa, T.; Negishi, N. Origin of visible-light activity of n-doped TiO2 photocatalyst: Behaviors of N and S atoms in a wet n-doping process. Appl. Catal. B 2012, 128, 77–83. [Google Scholar] [CrossRef]
- Neville, E.M.; Mattle, M.J.; Loughrey, D.; Rajesh, B.; Rahman, M.; MacElroy, J.M.D.; Sullivan, J.A.; Thampi, K.R. Carbon-doped TiO2 and carbon, tungsten-codoped TiO2 through sol-gel processes in the presence of melamine borate: Reflections through photocatalysis. J. Phys. Chem. C 2012, 116, 16511–16521. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C.H.; Ni, Y.R.; Su, M.X.; Xu, Z.Z. A new sight on hydrogenation of F and N–F doped {001} facets dominated anatase TiO2 for efficient visible light photocatalyst. Appl. Catal. B 2012, 127, 28–35. [Google Scholar] [CrossRef]
- Ma, X.G.; Wu, Y.; Lu, Y.H.; Xu, J.; Wang, Y.J.; Zhu, Y.F. Effect of compensated codoping on the photoelectrochemical properties of anatase TiO2 photocatalyst. J. Phys. Chem. C 2011, 115, 16963–16969. [Google Scholar] [CrossRef]
- Zhang, M.L.; Li, L.F.; Meng, X.D. A simple new way to prepare Cu-doped nano-TiO2 with visible light photocatalytic activity. Adv. Mater. Res. 2011, 197–198, 1028–1031. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Rawal, S.B.; Bera, S.; Lee, W.I. Visible-light photocatalytic properties of W18O19/TiO2 and WO3/TiO2 heterocomposites. Catal. Lett. 2012, 142, 1482–1488. [Google Scholar] [CrossRef]
- Su, K.; Ai, Z.H.; Zhang, L.Z. Efficient visible light-driven photocatalytic degradation of pentachlorophenol with Bi2O3/TiO2-xBx. J. Phys. Chem. C 2012, 116, 17118–17123. [Google Scholar]
- Wu, D.W.; Li, S.; Zhang, Q.L.; Chen, Y.Q.; Gong, M.C. Composite photocatalyst TiO2/BiNbO4: Preparation and degradation performance for gas phase benzene. Chin. J. Inorg. Chem. 2012, 28, 1383–1388. [Google Scholar]
- Fei, X.N.; Jia, G.Z.; Xu, X.J.; Hao, Y.C.; Wang, D.; Guo, J. Study on preparation and sunlight photocatalytic activity of porous coupled ZnO/TiO2 photocatalyst. Optoelectron. Adv. Mater. 2012, 6, 709–712. [Google Scholar]
- Lin, B.Z.; Li, X.L.; Xu, B.H.; Chen, Y.L.; Gao, B.F.; Fan, X.R. Improved photocatalytic activity of anatase TiO2-pillared HTaWO6 for degradation of methylene blue. Microporous Mesoporous Mater. 2012, 155, 16–23. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.Y.; Mao, J.; Li, K.; Zan, L. Graphitic carbon nitride (g-C3N4)-Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys. Chem. Chem. Phys. 2012, 14, 16745–16752. [Google Scholar] [CrossRef]
- Sun, M.; Chen, G.D.; Zhang, Y.K.; Wei, Q.; Ma, Z.M.; Du, B. Efficient degradation of azo dyes over Sb2S3/TiO2 heterojunction under visible light irradiation. Ind. Eng. Chem. Res. 2012, 51, 2897–2903. [Google Scholar] [CrossRef]
- Qian, S.S.; Wang, C.S.; Liu, W.J.; Zhu, Y.H.; Yao, W.J.; Lu, X.H. An enhanced CdS/TiO2 photocatalyst with high stability and activity: Effect of mesoporous substrate and bifunctional linking molecule. J. Mater. Chem. 2011, 21, 4945–4952. [Google Scholar] [CrossRef]
- Cropek, D.; Kemme, P.A.; Makarova, O.V.; Chen, L.X.; Rajh, T. Selective photocatalytic decomposition of nitrobenzene using surface modified TiO2 nanoparticles. J. Phys. Chem. C 2008, 112, 8311–8318. [Google Scholar]
- Lu, W.S.; Xiao, G.C.; Li, D.Z.; Fu, X.Z.; Wang, X.X. Synthesis and characterization of visible light-driven photocatalyst Pt/InVO4/TiO2. Chin. J. Inorg. Chem. 2005, 21, 1495–1499. [Google Scholar]
- Tatsuma, T.; Saitoh, S.; Ngaotrakanwiwat, P.; Ohko, Y.; Fujishima, A. Energy storage of TiO2–WO3 photocatalysis systems in the gas phase. Langmuir 2002, 18, 7777–7779. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z.; Fan, X.X.; Zou, Z.G. Selective synthesis and visible-light photocatalytic activities of BiVO4 with different crystalline phases. Mater. Chem. Phys. 2007, 103, 162–167. [Google Scholar] [CrossRef]
- Zhou, J.K.; Zou, Z.G.; Ray, A.K.; Zhao, X.S. Preparation and characterization of polycrystalline bismuth titanate Bi12TiO20 and its photocatalytic properties under visible light irradiation. Ind. Eng. Chem. Res. 2007, 46, 745–749. [Google Scholar] [CrossRef]
- Zhang, G.K.; Zou, X.; Gong, J.; He, F.S.; Zhang, H.; Zhang, Q.; Liu, Y.; Yang, X.; Hu, B. Preparation and photocatalytic property of potassium niobate K6Nb10.8O30. J. Alloy. Compd. 2006, 425, 76–80. [Google Scholar] [CrossRef]
- Peng, F.; Zhou, C.M.; Wang, H.J.; Yu, H.; Liang, J.H.; Yang, J.A. The role of RuO2 in the electrocatalytic oxidation of methanol for direct methanol fuel cell. Catal. Commun. 2009, 10, 533–537. [Google Scholar] [CrossRef]
- Li, X.K.; Kako, T.; Ye, J.H. 2-Propanol photodegradation over lead niobates under visible light irradiation. Appl. Catal. A 2007, 326, 1–7. [Google Scholar]
- Hou, L.R.; Yuan, C.Z.; Peng, Y. Preparation and photocatalytic property of sunlight-driven photocatalyst Bi38ZnO58. J. Mol. Catal. A 2006, 252, 132–135. [Google Scholar] [CrossRef]
- Xie, L.J.; Ma, J.F.; Xu, G.J. Preparation of a novel Bi2MoO6 flake-like nanophotocatalyst by molten salt method and evaluation for photocatalytic decomposition of rhodamine B. Mater. Chem. Phys. 2008, 110, 197–200. [Google Scholar] [CrossRef]
- Tang, J.W.; Zou, Z.G.; Yin, J.; Ye, J. Photocatalytic degradation of methylene blue on Caln2O4 under visible light irradiation. Chem. Phys. Lett. 2003, 382, 175–179. [Google Scholar] [CrossRef]
- Chu, X.F.; Zheng, C.M. Sulfide-sensing characteristics of MFe2O4 (M = Zn, Cd, Mg and Cu) thick film prepared by co-precipitation method. Sens. Actuators B 2003, 96, 504–508. [Google Scholar] [CrossRef]
- Chen, C.H.; Liang, Y.H.; Zhang, W.D. ZnFe2O4/MWCNTs composite with enhanced photocatalytic activity under visible-light irradiation. J. Alloy. Compd. 2010, 501, 168–172. [Google Scholar] [CrossRef]
- Qiu, J.X.; Wang, C.Y.; Gu, M.Y. Photocatalytic properties and optical absorption of zinc ferrite nanometer films. Mater. Sci. Eng. B 2004, 112, 1–4. [Google Scholar] [CrossRef]
- Du, H.Y.; Luan, J.F. Synthesis, characterization and photocatalytic activity of new photocatalyst CdBiYO4. Solid State Sci. 2012, 14, 1295–1305. [Google Scholar] [CrossRef]
- Yang, S.X.; Zhu, W.P.; Jiang, Z.P.; Chen, Z.X.; Wang, J.B. The surface properties and the activities in catalytic wet air oxidation over CeO2-TiO2 catalysts. Appl. Surf. Sci. 2006, 252, 8499–8505. [Google Scholar] [CrossRef]
- Matuana, L.M.; Balatinecz, J.J.; Sodhi, R.N.S.; Park, C.B. Surface characterization of esterified cellulosic fibers by XPS and FTIR spectroscopy. Wood Sci. Technol. 2001, 35, 191–201. [Google Scholar] [CrossRef]
- Izumi, F. Rietan: A software package for the rietveld analysis and simulation of X-ray and neutron diffraction patterns. J. Crystallogr. 1985, 27, 23. [Google Scholar]
- Culea, E.; Pop, L.; Simon, S. Spectroscopic and magnetic behaviour of xGd2O3 (1−x) (Bi2O3 PbO) glasses. Mater. Sci. Eng. B 2004, 112, 59–63. [Google Scholar] [CrossRef]
- Soltani, M.; Haddad, S.; Ouennes, K.; Boulegroun, A.; Poulain, M.; Aslan, M.H.; Oral, A.Y. In structural investigations of Sb2O3–M2O (M = Li, Na or K) glasses by the mean of thermal and elastics characterization. AIP Conf. Proc. 2012, 1476, 87–90. [Google Scholar]
- Pillai, S.C.; Kelly, J.M.; McCormack, D.E.; O’Brien, P.; Ramesh, R. The effect of processing conditions on varistors prepared from nanocrystalline ZnO. J. Mater. Chem. 2003, 13, 2586–2590. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Luan, J.F.; Wang, S.; Ma, K.; Li, Y.M.; Pan, B.C. Structural property and catalytic activity of new In2YbSbO7 and Gd2YbSbO7 nanocatalysts under visible light irradiation. J. Phys. Chem. C 2010, 114, 9398–9407. [Google Scholar]
- Yang, Y.J.; Luan, J.F. Synthesis, property characterization and photocatalytic activity of the novel composite polymer polyaniline/Bi2SnTiO7. Molecules 2012, 17, 2752–2772. [Google Scholar] [CrossRef]
- Luan, J.F.; Wang, S.; Hu, Z.T.; Zhang, L.Y. Synthesis techniques, properties and applications of polymer nanocomposites. Curr. Org. Synth. 2012, 9, 114–136. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Vautier, M.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of dyes in water: Case study of indigo and of indigo carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- Shang, X.L.; Li, B.; Li, C.H.; Wang, X.; Zhang, T.Y.; Jiang, S. Preparation and enhanced visible light catalytic activity of TiO2 sensitized with Benzimidazolone Yellow H3G. Dyes Pigment. 2013, 98, 358–366. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Highly efficient, solar active, and reusable photocatalyst: Zr-loaded Ag–ZnO for Reactive Red 120 dye degradation with synergistic effect and dye-sensitized mechanism. Langmuir 2013, 29, 939–949. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Ding, H.M.; Sun, H.; Shan, Y.K. Preparation and characterization of mesoporous SBA-15 supported dye-sensitized TiO2 photocatalyst. J. Photochem. Photobiol. A 2005, 169, 101–107. [Google Scholar] [CrossRef]
- Girotto, E.M.; Gazotti, W.A.; Tormena, C.F.; de Paoli, M.A. Photoelectronic and transport properties of polypyrrole doped with a dianionic dye. Electrochim. Acta 2002, 47, 1351–1357. [Google Scholar]
- Marugan, J.; Hufschmidt, D.; Sagawe, G.; Selzer, V.; Bahnemann, D. Optical density and photonic efficiency of silica-supported TiO2 photocatalysts. Water Res. 2006, 40, 833–839. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luan, J.; Chen, M.; Hu, W. Synthesis, Characterization and Photocatalytic Activity of New Photocatalyst ZnBiSbO4 under Visible Light Irradiation. Int. J. Mol. Sci. 2014, 15, 9459-9480. https://doi.org/10.3390/ijms15069459
Luan J, Chen M, Hu W. Synthesis, Characterization and Photocatalytic Activity of New Photocatalyst ZnBiSbO4 under Visible Light Irradiation. International Journal of Molecular Sciences. 2014; 15(6):9459-9480. https://doi.org/10.3390/ijms15069459
Chicago/Turabian StyleLuan, Jingfei, Mengjing Chen, and Wenhua Hu. 2014. "Synthesis, Characterization and Photocatalytic Activity of New Photocatalyst ZnBiSbO4 under Visible Light Irradiation" International Journal of Molecular Sciences 15, no. 6: 9459-9480. https://doi.org/10.3390/ijms15069459





