High Cytoplasmic Expression of Krüppel-like Factor 4 Is an Independent Prognostic Factor of Better Survival in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Patient Characteristics
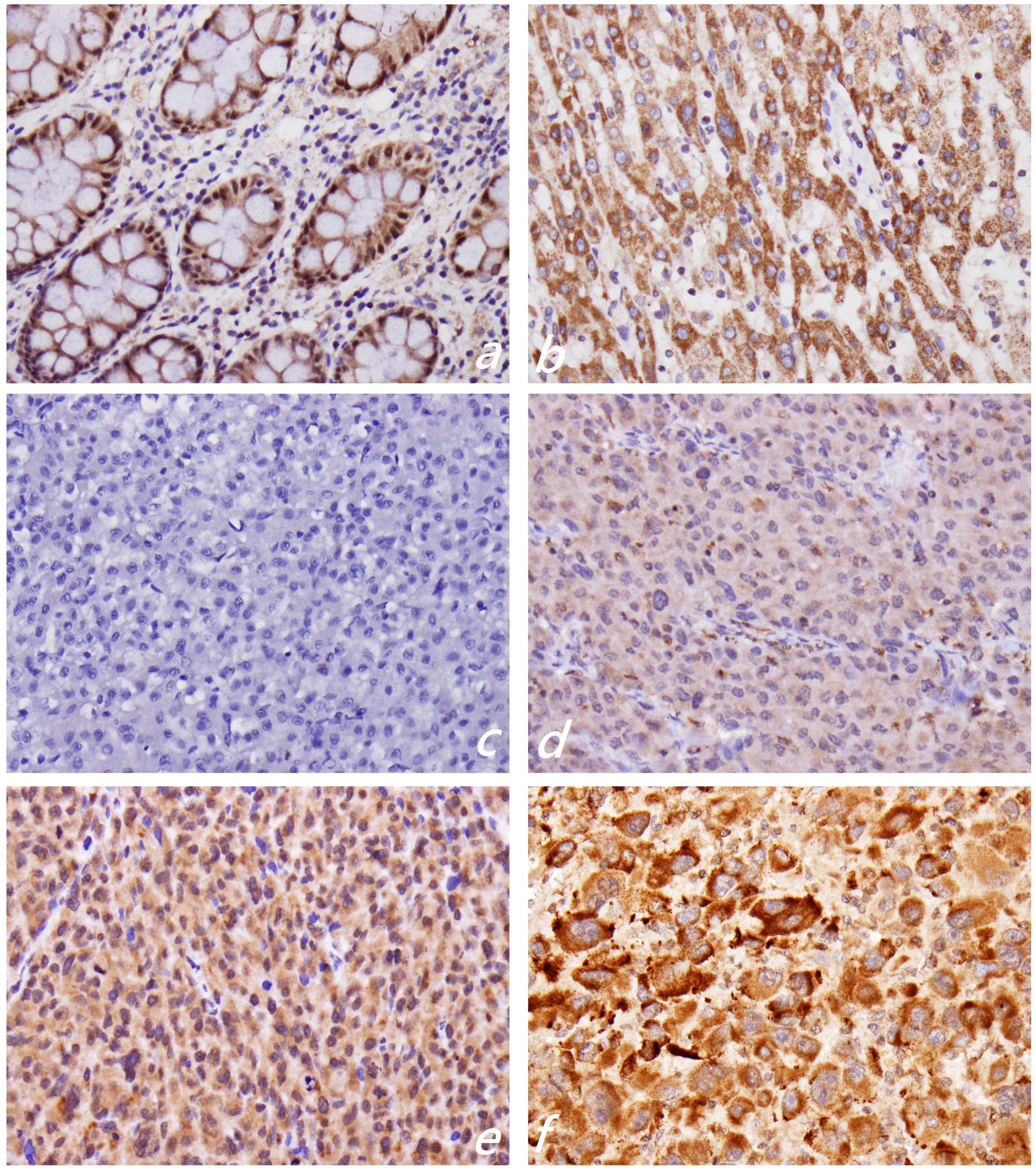
2.2. KLF4 Expression Is Associated with Tumor Differentiation in Hepatocellular Carcinoma
| Variables | Total, n (%) | KLF4, n (%) | p-Value | |
|---|---|---|---|---|
| KLF4 (−) | KLF4 (+) | |||
| Total number | 205 (100) | 160 (78.0) | 45 (22.0) | |
| Age | ||||
| <62 | 84 (41.0) | 60 (37.5) | 24 (53.3) | 0.056 |
| ≥62 | 121 (59.0) | 100 (62.5) | 21 (46.7) | |
| Gender | ||||
| Male | 151 (73.7) | 120 (75.0) | 31 (68.9) | 0.411 |
| Female | 54 (26.3) | 40 (25.0) | 14 (31.1) | |
| Differentiation | ||||
| G1 | 21 (10.2) | 11 (6.9) | 10 (22.2) | 0.001 * |
| G2 | 115 (56.1) | 99 (61.9) | 16 (35.6) | |
| G3 | 69 (33.7) | 50 (31.3) | 19 (42.2) | |
| T classification | ||||
| T1 | 103 (50.2) | 77 (48.1) | 26 (57.8) | 0.520 |
| T2 | 59 (28.8) | 48 (30.0) | 11 (24.4) | |
| T3–T4 | 43 (21.0) | 35 (21.9) | 8 (17.8) | |
| Stage | ||||
| I | 102 (49.8) | 76 (47.5) | 26 (57.8) | 0.366 |
| II | 57 (27.8) | 48 (30.0) | 9 (20.0) | |
| III−IV | 46 (22.4) | 36 (22.5) | 10 (22.2) | |
| Recurrence | ||||
| No | 82 (40.0) | 61 (38.1) | 21 (46.7) | 0.301 |
| Yes | 123 (60.0) | 99 (61.9) | 24 (53.3) | |
| Distant metastasis | ||||
| No | 198 (96.6) | 156 (97.5) | 42 (93.3) | 0.174 |
| Yes | 7 (3.4) | 4 (2.5) | 3 (6.7) | |
| Survival | ||||
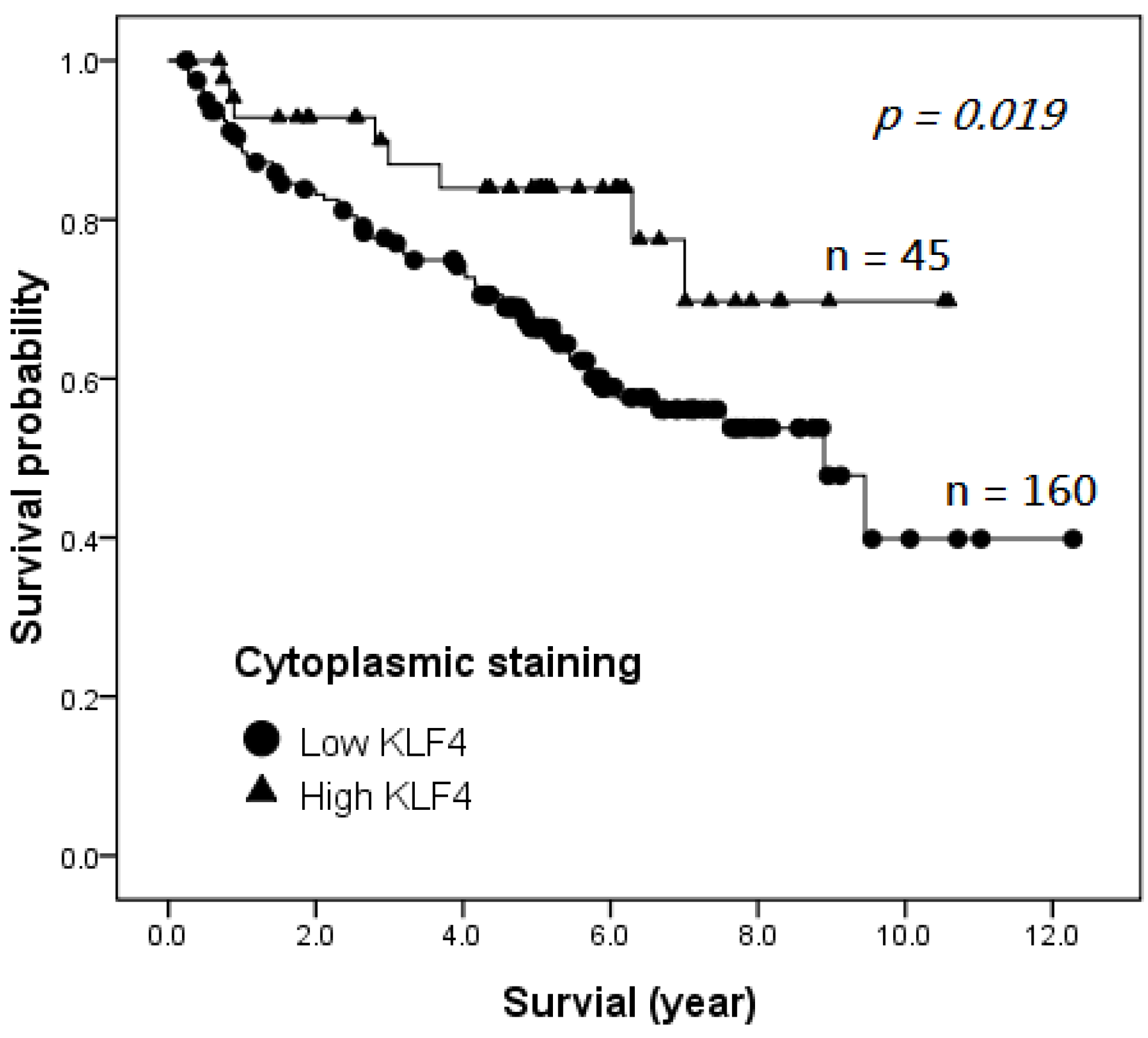
| ≤5 years | 126 (69.6) | 94 (74.6) | 32 (25.4) | 0.028 * |
| >5 years | 55 (30.4) | 49 (89.1) | 6 (10.9) | |
| Disease-specific survival | ||||
| Alive | 101 (59.4) | 74 (73.3) | 27 (26.7) | 0.017 * |
| Death | 69 (40.6) | 61 (88.4) | 8 (11.6) | |
2.3. Ki-67 Proliferative Index
2.4. Survival Analysis
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | |
| KLF4 expression | ||||||
| low | 1.0 | 0.22–0.95 | 0.036 * | 1.0 | 0.19–0.85 | 0.017 * |
| high | 0.455 | 0.398 | ||||
| Differentiation grade | ||||||
| G1–G2 | 1.0 | 1.30–2.09 | 0.000 * | 1.0 | 1.32–2.17 | 0.000 * |
| G3 | 1.649 | 1.686 | ||||
| T classification | ||||||
| T1–T2 | 1.0 | 1.48–2.43 | 0.000 * | 1.0 | 1.49–2.50 | 0.000 * |
| T3–T4 | 1.897 | 1.928 | ||||
| Distant metastasis | ||||||
| no | 1.0 | 2.89–15.99 | 0.000 * | 1.0 | 1.56–9.60 | 0.003 * |
| yes | 6.793 | 3.871 | ||||
| Recurrence | ||||||
| no | 1 | 1.72–5.97 | 0.000 * | 1 | 1.35–4.84 | 0.004 * |
| yes | 3.20 | 2.556 | ||||
3. Experimental Section
3.1. Ethics Statement
3.2. Patients
3.3. Tissue Microarray
3.4. Immunohistochemistry of KLF4
3.5. Ki-67 Proliferative Index
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Mittal, S.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma: Consider the population. J. Clin. Gastroenterol. 2013, 47, S2–S6. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef]
- Wong, C.M.; Ng, I.O. Molecular pathogenesis of hepatocellular carcinoma. Liver Int. 2008, 28, 160–174. [Google Scholar] [CrossRef]
- Zucman-Rossi, J. Molecular classification of hepatocellular carcinoma. Dig. Liver Dis. 2010, 42 (Suppl. 3), S235–S241. [Google Scholar] [CrossRef]
- Zender, L.; Villanueva, A.; Tovar, V.; Sia, D.; Chiang, D.Y.; Llovet, J.M. Cancer gene discovery in hepatocellular carcinoma. J. Hepatol. 2010, 52, 921–929. [Google Scholar] [CrossRef]
- Wei, D.; Kanai, M.; Huang, S.; Xie, K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis 2006, 27, 23–31. [Google Scholar]
- McConnell, B.B.; Yang, V.W. Mammalian Kruppel-like factors in health and diseases. Physiol. Rev. 2010, 90, 1337–1381. [Google Scholar] [CrossRef]
- Hu, R.; Zuo, Y.; Zuo, L.; Liu, C.; Zhang, S.; Wu, Q.; Zhou, Q.; Gui, S.; Wei, W.; Wang, Y. KLF4 expression correlates with the degree of differentiation in colorectal cancer. Gut Liver 2011, 5, 154–159. [Google Scholar] [CrossRef]
- Yang, W.T.; Zheng, P.S. Krüppel-like factor 4 functions as a tumor suppressor in cervical carcinoma. Cancer 2012, 118, 3691–702. [Google Scholar] [CrossRef]
- Yoon, O.; Roh, J. Downregulation of KLF4 and the Bcl-2/Bax ratio in advanced epithelial ovarian cancer. Oncol. Lett. 2012, 4, 1033–1036. [Google Scholar]
- Zammarchi, F.; Morelli, M.; Menicagli, M.; di Cristofano, C.; Zavaglia, K.; Paolucci, A.; Campani, D.; Aretini, P.; Boggi, U.; Mosca, F.; et al. KLF4 is a novel candidate tumor suppressor gene in pancreatic ductal carcinoma. Am. J. Pathol. 2011, 178, 361–372. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Luo, W.; Jiang, Q.; Mai, C.; Chen, Y.; Zhen, Y.; Yu, X.; Long, X.; Fang, W. Loss of cytoplasmic KLF4 expression is correlated with the progression and poor prognosis of nasopharyngeal carcinoma. Histopathology 2013, 63, 362–370. [Google Scholar]
- Hu, W.; Hofstetter, W.L.; Li, H.; Zhou, Y.; He, Y.; Pataer, A.; Wang, L.; Xie, K.; Swisher, S.G.; Fang, B. Putative tumor-suppressive function of Kruppel-like factor 4 in primary lung carcinoma. Clin. Cancer Res. 2009, 15, 5688–5695. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Liu, X.; Shi, M.; Chen, G.; Zhang, B.; Li, Z.; Song, L. Correlation of KLF4 and SPARC expression with the clinical characteristics of non-small cell lung cancer. Chin. J. Lung Cancer 2012, 15, 720–724. [Google Scholar]
- Ohnishi, S.; Ohnami, S.; Laub, F.; Aoki, K.; Suzuki, K.; Kanai, Y.; Haga, K.; Asaka, M.; Ramirez, F.; Yoshida, T. Downregulation and growth inhibitory effect of epithelial-type Krüppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem. Biophys. Res. Commun. 2003, 308, 251–256. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Wang, Z.W.; Zha, L.; Huang, Z. Altered expression of Krüppel-like factor 4 and β-catenin in human gastric cancer. Oncol. Lett. 2012, 3, 1017–1022. [Google Scholar]
- Li, H.; Wang, J.; Xiao, W.; Xia, D.; Lang, B.; Yu, G.; Guo, X.; Guan, W.; Wang, Z.; Hu, Z.; et al. Epigenetic alterations of Kruppel-like factor 4 and its tumor suppressor function in renal cell carcinoma. Carcinogenesis 2013, 34, 2262–2270. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wu, C.Y.; Chang, C.C.; Ma, C.J.; Li, M.C.; Chen, C.M. Nuclear Krüppel-like factor 4 expression is associated with human skin squamous cell carcinoma progression and metastasis. Cancer Biol. Ther. 2008, 7, 777–782. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Jia, Z.; Mishra, L.; Guo, K.; Li, Z.; Le, X.; Wei, D.; Huang, S.; Xie, K. Dysregulated Krüppel-like factor 4 and Vitamin D receptor signaling contribute to progression of hepatocellular carcinoma. Gastroenterology 2012, 143, 799–810. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.W.; Jin, J.J.; Zhou, Y.; Ren, Z.G.; Qiu, S.J.; Zhang, B.H. The clinical and prognostic implications of pluripotent stem cell gene expression in hepatocellular carcinoma. Oncol. Lett. 2013, 5, 1155–1162. [Google Scholar]
- Pandya, A.Y.; Talley, L.I.; Frost, A.R.; Fitzgerald, T.J.; Trivedi, V.; Chakravarthy, M.; Chhieng, D.C.; Grizzle, W.E.; Engler, J.A.; Krontiras, H.; et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin. Cancer Res. 2004, 10, 2709–2719. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, B.; Zhang, X.H.; Nie, C.J.; Li, Y.H.; Wen, J.K. Localization and function of KLF4 in cytoplasm of vascular smooth muscle cell. Biochem. Biophys. Res. Commun. 2013, 436, 162–168. [Google Scholar] [CrossRef]
- Le Magnen, C.; Bubendorf, L.; Ruiz, C.; Zlobec, I.; Bachmann, A.; Heberer, M.; Spagnoli, G.C.; Wyler, S.; Mengus, C. KLF4 transcription factor is expressed in the cytoplasm of prostate cancer cells. Eur. J. Cancer 2013, 49, 955–963. [Google Scholar]
- D’Errico, A.; Grigioni, W.F.; Fiorentino, M.; Baccarini, P.; Grazi, G.L.; Mancini, A.M. Overexpression of p53 protein and Ki-67 proliferative index in hepatocellular carcinoma: An immuno-histochemical study on 109 Italian patients. Pathol. Int. 1994, 44, 682–687. [Google Scholar]
- Nakanishi, K.; Sakamoto, M.; Yamasaki, S.; Todo, S.; Hirohashi, S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer 2005, 103, 307–312. [Google Scholar]
- Tuominen, V.J.; Ruotoistenmäki, S.; Viitanen, A.; Jumppanen, M.; Isola, J. ImmunoRatio: A publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010, 12, R56. [Google Scholar] [CrossRef]
- Han, D.H.; Choi, G.H.; Kim, K.S.; Choi, J.S.; Park, Y.N.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Han, K.H. Prognostic significance of the worst grade in hepatocellular carcinoma with heterogeneous histologic grades of differentiation. J. Gastroenterol. Hepatol. 2013, 28, 1384–1390. [Google Scholar] [CrossRef]
- Qin, L.X.; Tang, Z.Y. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J. Gastroenterol. 2002, 8, 193–199. [Google Scholar]
- Chen, C.J.; Hsu, L.S.; Lin, S.H.; Chen, M.K.; Wang, H.K.; Hsu, J.D.; Lee, H.; Yeh, K.T. Loss of nuclear expression of Krüppel-like factor 4 is associated with poor prognosis in patients with oral cancer. Hum. Pathol. 2012, 43, 1119–1125. [Google Scholar] [CrossRef]
- Tai, S.K.; Yang, M.H.; Chang, S.Y.; Chang, Y.C.; Li, W.Y.; Tsai, T.L.; Wang, Y.F.; Chu, P.Y.; Hsieh, S.L. Persistent Krüppel-like factor 4 expression predicts progression and poor prognosis of head and neck squamous cell carcinoma. Cancer Sci. 2011, 102, 895–902. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Yu, F.; Yu, T.; Liu, C.; Huang, S.; Wang, T.C.; Ai, W. Deficiency of the Kruppel-like factor KLF4 correlates with increased cell proliferation and enhanced skin tumorigenesis. Carcinogenesis 2012, 33, 1239–1246. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hsu, H.-T.; Wu, P.-R.; Chen, C.-J.; Hsu, L.-S.; Yeh, C.-M.; Hsing, M.-T.; Chiang, Y.-S.; Lai, M.-T.; Yeh, K.-T. High Cytoplasmic Expression of Krüppel-like Factor 4 Is an Independent Prognostic Factor of Better Survival in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2014, 15, 9894-9906. https://doi.org/10.3390/ijms15069894
Hsu H-T, Wu P-R, Chen C-J, Hsu L-S, Yeh C-M, Hsing M-T, Chiang Y-S, Lai M-T, Yeh K-T. High Cytoplasmic Expression of Krüppel-like Factor 4 Is an Independent Prognostic Factor of Better Survival in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2014; 15(6):9894-9906. https://doi.org/10.3390/ijms15069894
Chicago/Turabian StyleHsu, Hui-Ting, Pei-Ru Wu, Chih-Jung Chen, Li-Sung Hsu, Chung-Min Yeh, Ming-Tai Hsing, Yi-Shan Chiang, Ming-Tsung Lai, and Kun-Tu Yeh. 2014. "High Cytoplasmic Expression of Krüppel-like Factor 4 Is an Independent Prognostic Factor of Better Survival in Hepatocellular Carcinoma" International Journal of Molecular Sciences 15, no. 6: 9894-9906. https://doi.org/10.3390/ijms15069894




