Morphology, Composition, and Bioactivity of Strontium-Doped Brushite Coatings Deposited on Titanium Implants via Electrochemical Deposition
Abstract
:1. Introduction
2. Results
2.1. Phase Composition of Sr-Doped Brushite Coatings
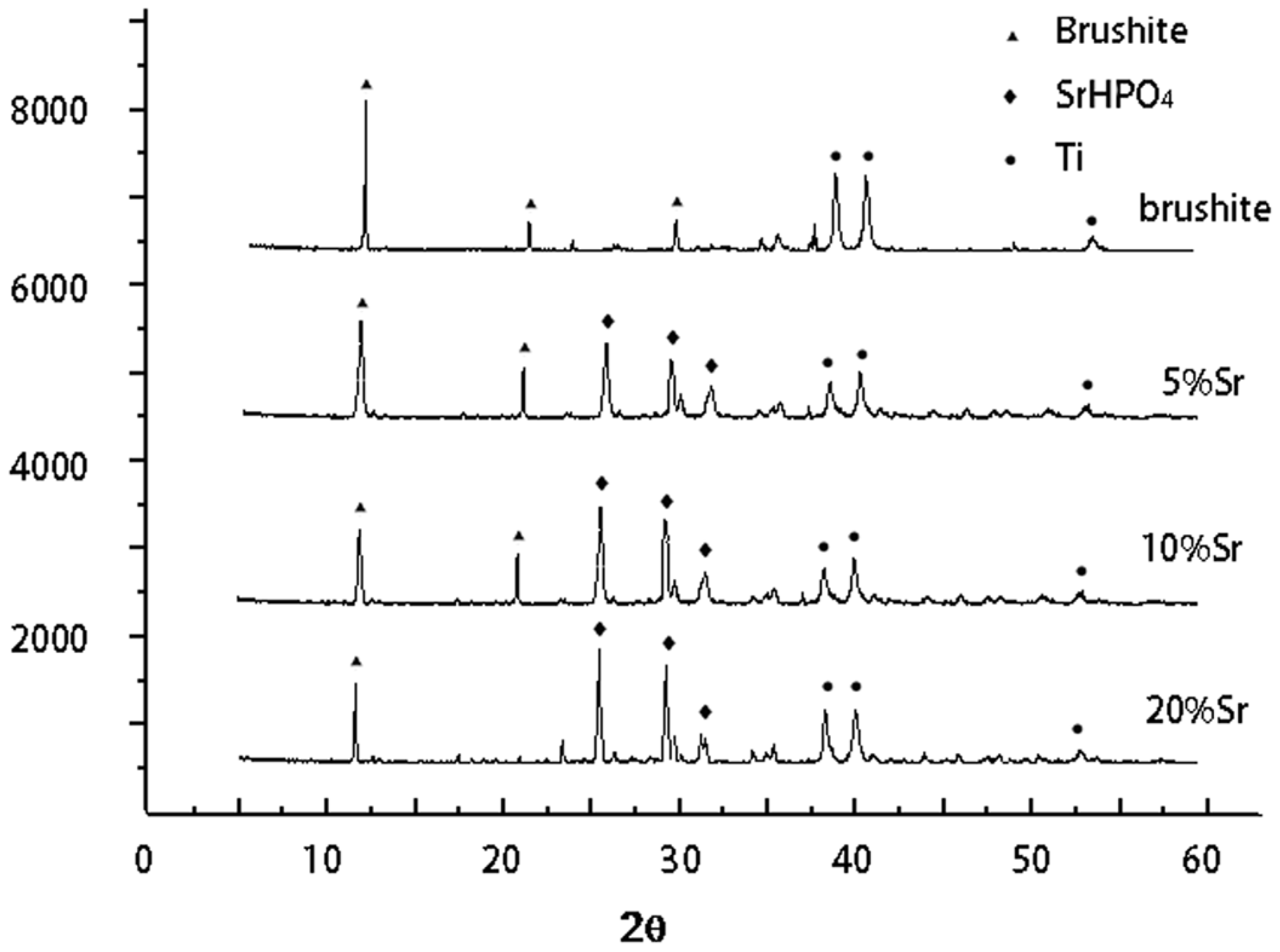
2.2. Morphology of Sr-Doped Brushite Coatings
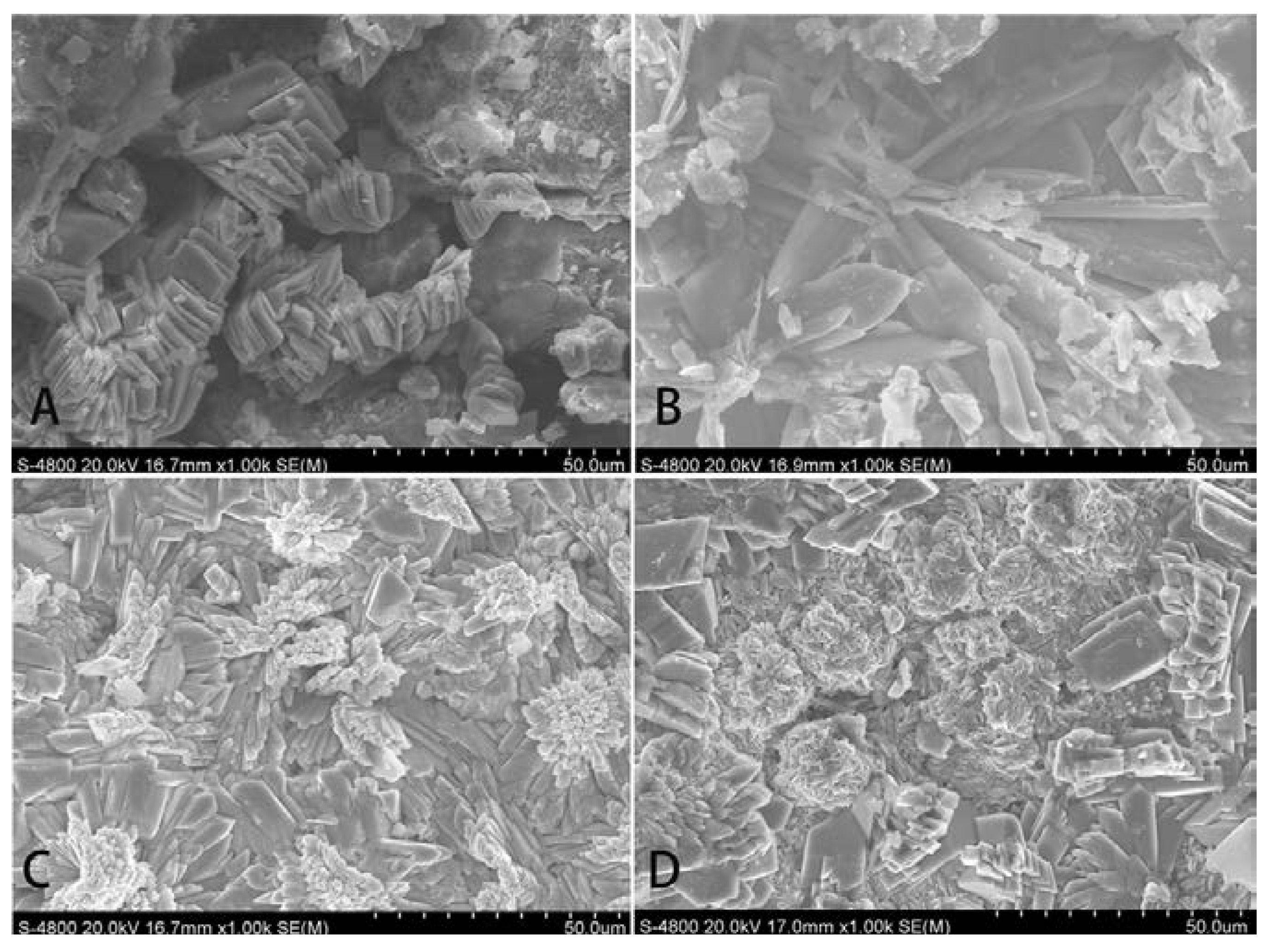
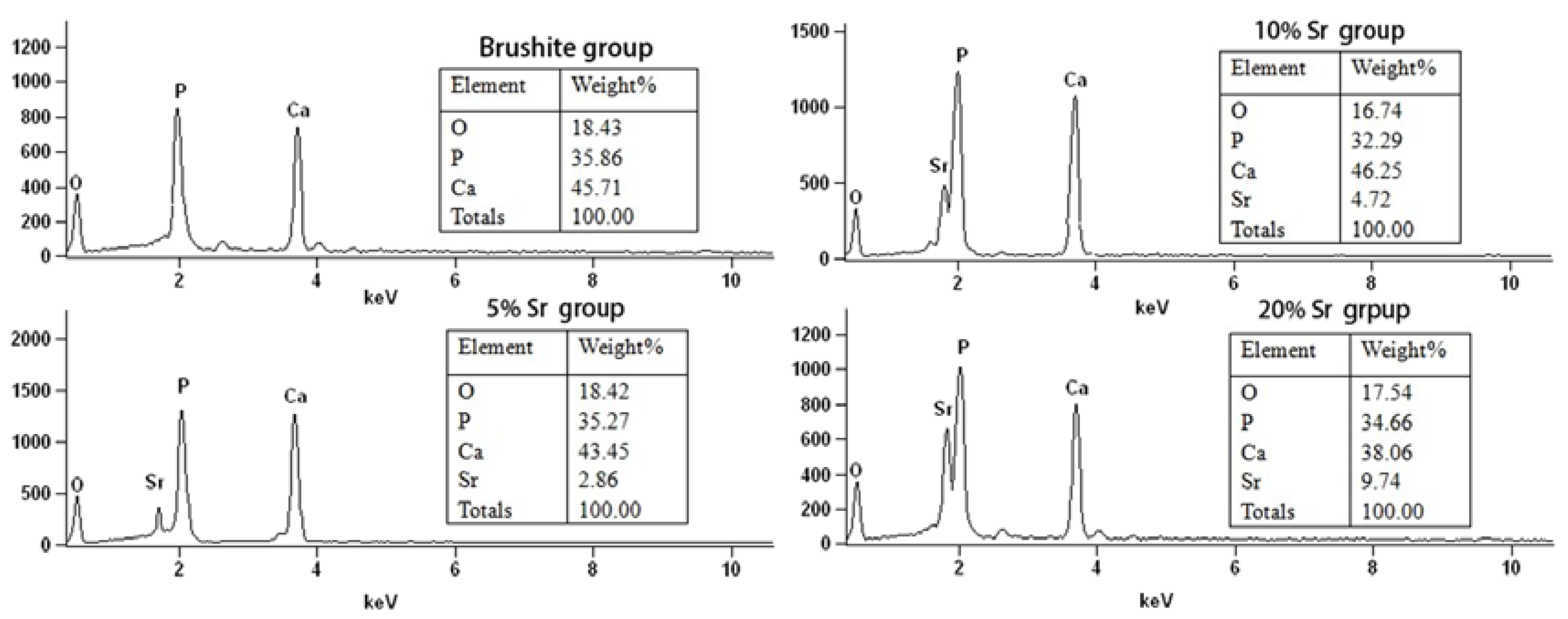
2.3. Elemental Analysis of Sr-Doped Brushite Coatings
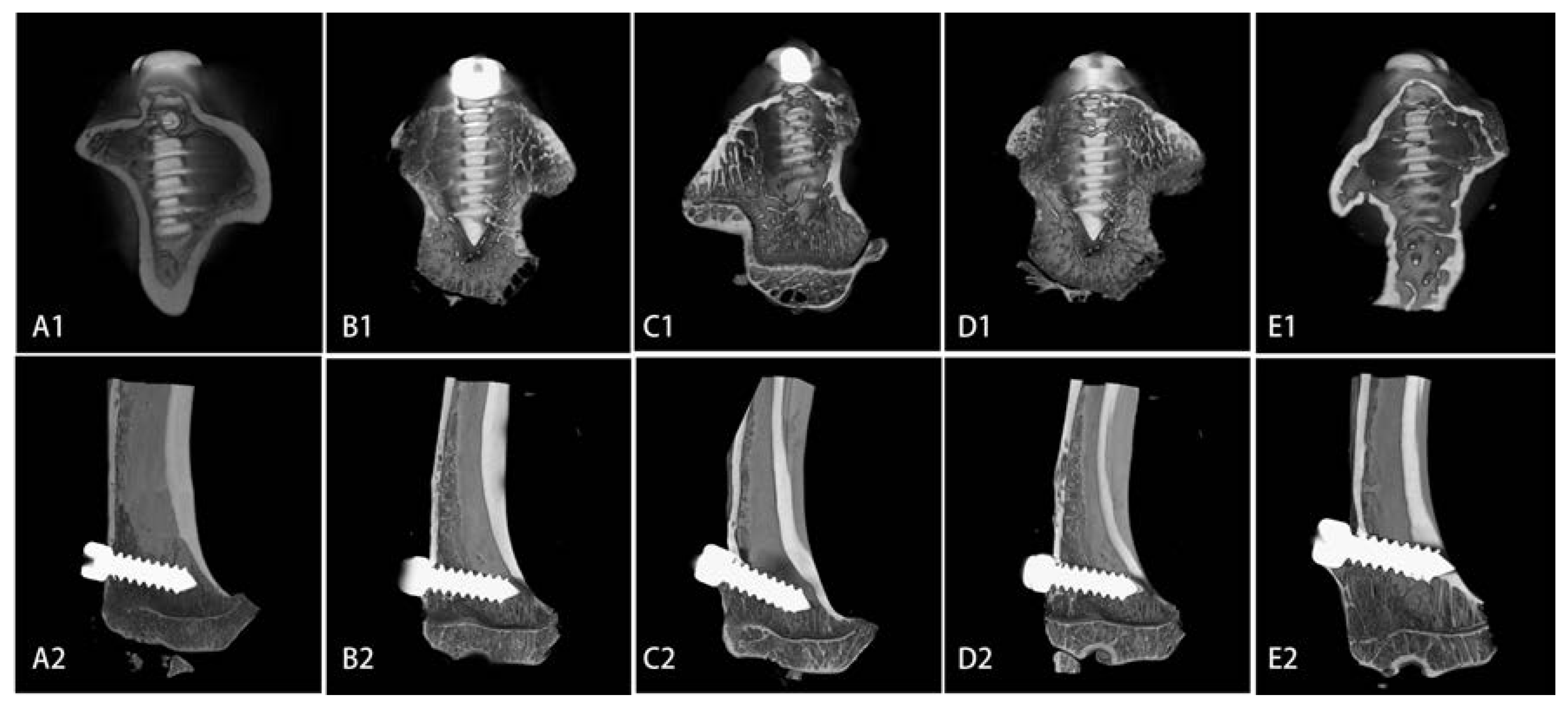
2.4. Microarchitecture of Bone Formed Around Sr-Doped Brushite-Coated Ti Implants
| Indices | Ti | Brushite | 5% Sr | 10% Sr | 20% Sr |
|---|---|---|---|---|---|
| IBCR | 55.76 ± 3.95 | 60.47 ± 3.97 | 61.99 ± 3.55 * | 67.43 ± 3.53 *Δ■ | 63.04 ± 4.65 * |
| BV/TV (%) | 52.77 ± 6.81 | 54.22 ± 5.72 | 57.12 ± 6.78 * | 65.43 ± 4.68 *Δ■ | 58.19 ± 5.62 |
| Conn.D | 103.70 ± 6.25 | 109.45 ± 8.90 | 114.57 ± 8.94 * | 137.42 ± 6.76 *Δ■ | 117.51 ± 7.01 *□ |
| Tb.Th | 0.1621 ± 0.0100 | 0.1796 ± 0.0111 *■ | 0.2077 ± 0.0226 * | 0.2089 ± 0.0188 * | 0.1719 ± 0.0124 ■□ |
| Tb.Sp | 0.2117 ± 0.0071 | 0.2206 ± 0.0053 * | 0.3241 ± 0.03 *Δ | 0.5694 ± 0.3293 *■ | 0.3133 ± 0.0119 *□ |
| Tb.N | 5.44 ± 0.28 | 7.63 ± 0.70 * | 7.98 ± 0.80 * | 8.96 ± 0.64 *Δ■ | 6.85 ± 0.62 *■□ |
2.5. Biomechanical Strength of Sr-Doped Brushite-Coated Implants
2.6. Cytotoxicity of Sr-Doped Brushite Coatings on Ti Implants
| Groups | 24 h | 48 h | 72 h |
|---|---|---|---|
| Ti | 0.3126 ± 0.0113 | 0.4956 ± 0.0082 | 0.5725 ± 0.0170 |
| brushite | 0.4133 ± 0.0183 * | 0.5153 ± 0.0109 * | 0.6155 ± 0.0125 * |
| 5% Sr | 0.4782 ± 0.0188 *Δ | 0.5873 ± 0.0099 *Δ | 0.6890 ± 0.0128 *Δ |
| 10% Sr | 0.5119 ± 0.0402 *Δ | 0.6193 ± 0.0315 *Δ■ | 0.7561 ± 0.0582 *Δ■ |
| 20% Sr | 0.4222 ± 0.0136 * | 0.5278 ± 0.0139 * | 0.6180 ± 0.0270 * |
3. Discussion
4. Materials and Methods
4.1. Preparation of Sr-Doped Brushite Coating by Electrochemical Deposition
4.2. Morphology and Characterization of Sr-Doped Brushite Coatings
4.3. Preparation of Ti Implants Coated with Sr-Doped Brushite
4.4. Implantation of Brushite-Coated Ti Implants
4.5. Micro-Computed Tomography (Micro-CT) Examination
4.6. Biomechanical Testing of Brushite-Coated Ti
4.7. MTT Assay for Cytotoxicity of Brushite-Coated Ti
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Szmukler-Moncler, S.; Perrin, D.; Ahossi, V.; Pointaire, P. Evaluation of BONIT®, a fully Resorbable CaP Coating Obtained by Electrochemical Deposition, after 6 Weeks of Healing: A Pilot study in the Pig Maxilla. Key Eng. Mater. 2001, 192–195, 395–398. [Google Scholar] [CrossRef]
- Malchiodi, L.; Ghensi, P.; Cucchi, A.; Trisi, P.; Szmukler-Moncler, S.; Corrocher, G.; Gerosa, R. Early bone formation around immediately loaded FBR-coated implants after 8, 10, and 12 weeks: A human histologic evaluation of three retrieved implants. Minerva Stomatol. 2011, 60, 205–216. [Google Scholar]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res. 2008, 85, 279–299. [Google Scholar] [CrossRef]
- Ince, A.; Schutze, N.; Hendrich, C.; Thull, R.; Eulert, J.; Lohr, J.F. In vitro investigation of orthopedic titanium-coated and brushite-coated surfaces using human osteoblasts in the presence of gentamycin. J. Arthroplast. 2008, 23, 762–771. [Google Scholar] [CrossRef]
- Schwarz, M.L.; Kowarsch, M.; Rose, S.; Becker, K.; Lenz, T.; Jani, L. Effect of surface roughness, porosity, and a resorbable calcium phosphate coating on osseointegration of titanium in a minipig model. J. Biomed. Mater. Res. 2009, 89, 667–678. [Google Scholar]
- Brennan, T.C.; Rybchyn, M.S.; Green, W.; Atwa, S.; Conigrave, A.D.; Mason, R.S. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br. J. Pharmacol. 2009, 157, 1291–1300. [Google Scholar] [CrossRef]
- Fonseca, J.E. Rebalancing bone turnover in favour of formation with strontium ranelate: Implications for bone strength. Rheumatology 2008, 47, iv17–iv19. [Google Scholar] [CrossRef]
- Eliaz, N.; Eliyahu, M. Electrochemical processes of nucleation and growth of hydroxyapatite on titanium supported by real-time electrochemical atomic force microscopy. J. Biomed. Mater. Res. 2007, 80, 621–634. [Google Scholar] [CrossRef]
- Lin, S.; LeGeros, R.Z.; LeGeros, J.P. Adherent octacalciumphosphate coating on titanium alloy using modulated electrochemical deposition method. J. Biomed. Mater. Res. 2003, 66, 819–828. [Google Scholar]
- Choudhary, S.; Halbout, P.; Alander, C.; Raisz, L.; Pilbeam, C. Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: Involvement of prostaglandins. J. Bone Miner. Res. 2007, 22, 1002–1010. [Google Scholar] [CrossRef]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Kwik, D.; Zreiqat, H. The effect of strontium incorporation into CaSiO3 ceramics on their physical and biological properties. Biomaterials 2007, 28, 3171–3181. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-kappaB activation. Mol. Cell. Biochem. 2012, 359, 399–407. [Google Scholar] [CrossRef]
- Ni, G.X.; Shu, B.; Huang, G.; Lu, W.W.; Pan, H.B. The effect of strontium incorporation into hydroxyapatites on their physical and biological properties. J. Biomed. Mater. Res. 2011, 100B, 562–568. [Google Scholar]
- Caverzasio, J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 2008, 42, 1131–1136. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef]
- Caverzasio, J.; Thouverey, C. Activation of FGF receptors is a new mechanism by which strontium ranelate induces osteoblastic cell growth. Cell. Physiol. Biochem. 2011, 27, 243–250. [Google Scholar] [CrossRef]
- Zhu, L.L.; Zaidi, S.; Peng, Y.; Zhou, H.; Moonga, B.S.; Blesius, A.; Dupin-Roger, I.; Zaidi, M.; Sun, L. Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochem. Biophys. Res. Commun. 2007, 355, 307–311. [Google Scholar] [CrossRef]
- Baier, M.; Staudt, P.; Klein, R.; Sommer, U.; Wenz, R.; Grafe, I.; Meeder, P.J.; Nawroth, P.P.; Kasperk, C. Strontium enhances osseointegration of calcium phosphate cement: A histomorphometric pilot study in ovariectomized rats. J. Orthop. Surg. Res. 2013, 8, 8–16. [Google Scholar] [CrossRef]
- Pan, H.B.; Zhao, X.L.; Zhang, X.; Zhang, K.B.; Li, L.C.; Li, Z.Y.; Lam, W.M.; Lu, W.W.; Wang, D.P.; Huang, W.H.; et al. Strontium borate glass: Potential biomaterial for bone regeneration. J. R. Soc. Interface 2010, 7, 1025–1031. [Google Scholar] [CrossRef]
- Bagi, C.M.; Hanson, N.; Andresen, C.; Pero, R.; Lariviere, R.; Turner, C.H.; Laib, A. The use of micro-CT to evaluate cortical bone geometry and strength in nude rats: Correlation with mechanical testing, pQCT and DXA. Bone 2006, 38, 136–144. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, Y.J.; Jeong, S.H.; Kwon, T.G. Density of the alveolar and basal bones of the maxilla and the mandible. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 30–37. [Google Scholar]
- Le Nihouannen, D.; Hacking, S.A.; Gbureck, U.; Komarova, S.V.; Barralet, J.E. The use of RANKL-coated brushite cement to stimulate bone remodelling. Biomaterials 2008, 29, 3253–3259. [Google Scholar] [CrossRef]
- Qi, M.; Hu, J.; Li, J.; Li, J.; Dong, W.; Feng, X.; Yu, J. Effect of zoledronate acid treatment on osseointegration and fixation of implants in autologous iliac bone grafts in ovariectomized rabbits. Bone 2012, 50, 119–127. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, Y.; Li, H.; Xu, J.; Li, X.; Qi, M.; Hu, M. Morphology, Composition, and Bioactivity of Strontium-Doped Brushite Coatings Deposited on Titanium Implants via Electrochemical Deposition. Int. J. Mol. Sci. 2014, 15, 9952-9962. https://doi.org/10.3390/ijms15069952
Liang Y, Li H, Xu J, Li X, Qi M, Hu M. Morphology, Composition, and Bioactivity of Strontium-Doped Brushite Coatings Deposited on Titanium Implants via Electrochemical Deposition. International Journal of Molecular Sciences. 2014; 15(6):9952-9962. https://doi.org/10.3390/ijms15069952
Chicago/Turabian StyleLiang, Yongqiang, Haoyan Li, Jiang Xu, Xin Li, Mengchun Qi, and Min Hu. 2014. "Morphology, Composition, and Bioactivity of Strontium-Doped Brushite Coatings Deposited on Titanium Implants via Electrochemical Deposition" International Journal of Molecular Sciences 15, no. 6: 9952-9962. https://doi.org/10.3390/ijms15069952




