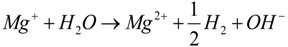The Influence of MgH2 on the Assessment of Electrochemical Data to Predict the Degradation Rate of Mg and Mg Alloys
Abstract
:1. Introduction
2. Literature Survey
- (1)
- NDE is attributed to the breakdown of a partially protective film on the magnesium surface during anodic dissolution. The formation of this layer is based on the creation of the Mg(OH)2 layer, which covers the surface and which changes with time to the MgO layer, liberating a molecule of water, according to Equation (2).Mg2+ + 2OH− ⇒ Mg(OH)2 ⇒ MgO + H2O
- (2)
- Mg reacts in two steps, creating a monovalent Mg+ ion, which, in a second step, oxidizes to Mg2+, where some site steps are discussed for the creation of hydrogen as the final product; see Equations (3) and (4).
- (3)
- The first layer created on the surface of Mg is a MgH2 layer, which, in a following reaction step with (OH)− ions, liberates hydrogen and creates Mg(OH)2; see Equations (5) and (6).Mg + 2e− + 2H2O → MgH2 + 2OH−MgH2 + 2H2O → Mg(OH)2 + 2H2
3. Results
3.1. Open Circuit Potential (OCP) Measurements
| Material | EOCP (V vs. SCE) up to Break | EOCP (V vs. SCE) after 15 min |
|---|---|---|
| Al pure | – | −0.709 |
| Fe pure | – | −0.597 |
| Zn pure | – | −1.025 |
| Mg pure | −1.664 | −0.744 |
| WE43 | −1.601 | −0.44 |

3.2. Polarization Using a Scan Rate of 100 mV/s

3.3. Comparison of Scan Rates at 100 and 10 mV/s




| Material Scan Rate Cathodic Threshold Potential | icorrfw (A/cm²) | CR fw (mm/year) | Ecorrfw (Vvs. SCE) | icorrbw (A/cm²) | CR bw (mm/year) | Ecorrbw (V vs. SCE) | Rp (Ω cm²) |
|---|---|---|---|---|---|---|---|
| WE43 10 mV/s ET = −1 V vs. OCP | 3.92 × 10−5 | 5.68 × 10−1 | −1.51214 | 6.67 × 10−5 | 9.66 × 10−1 | −1.5432 | 276 |
| SD | 7.71 × 10−6 | 1.12 × 10−1 | 0.01187468 | 6.47 × 10−5 | 9.37 × 10−1 | 0.05866018 | |
| WE43 100 mV/s ET = −0.5 V vs. OCP | 2.75 × 10−5 | 3.98 × 10−1 | −1.51482 | 2.48 × 10−5 | 3.59 × 10−1 | −1.5146 | 393 |
| SD | 1.07 × 10−6 | 1.55 × 10−2 | 0.00584739 | 1.52 × 10−6 | 2.20 × 10−2 | 0.00335857 | |
| WE43 100 mV/s ET = −1 V vs. OCP | 3.03 × 10−5 | 4.38 × 10−1 | −1.54254 | 2.91 × 10−5 | 4.22 × 10−1 | −1.5400 | |
| SD | 4.77 × 10−6 | 6.92 × 10−2 | 0.00609286 | 2.95 × 10−6 | 4.28 × 10−2 | 0.00758571 | |
| Mg 10 mV/s ET = −1 V vs. OCP | 1.48 × 10−4 | 2.14 | −1.4752 | 2.08 × 10−4 | 3.01 | −1.6444 | 59 |
| SD | 9.34 × 10−5 | 1.35 | 0.0671203 | 8.39 × 10−6 | 1.22 × 10−1 | 0.01472678 | |
| Mg 100 mV/s ET = −0.5 V vs. OCP | 1.68 × 10−4 | 2.44 | −1.61504 | 1.72 × 10−4 | 2.49 | −1.6475 | 68 |
| SD | 1.24 × 10−5 | 1.80 × 10−1 | 0.01142817 | 3.56 × 10−6 | 5.16 × 10−2 | 0.00302476 | |
| Mg 100 mV/s ET = −1 V vs. OCP | 5.40 × 10−5 | 7.82 × 10−1 | −1.5261 | 5.00 × 10−5 | 7.24 × 10−1 | −1.5457 | |
| SD | 2.51 × 10−6 | 3.64 × 10−2 | 0.00435603 | 2.28 × 10−6 | 3.31 × 10−2 | 0.00314754 |
4. Discussion
| Electrolyte | pH | OCP vs. NHE | Reference |
|---|---|---|---|
| 0.05 M NaOH, 0.186 M H3BO3, 0.0002 M NaCl | 8.9 | −1.48 | p. 18 in Perrault 26 |
| 0.1 M NaCl | 10.6 | −1.44 | p. 18 in Perrault 26 |
| 0.2 N MgCl2 8.33 × 10−3 N HCl | 1.85 | −1.72 | p. 19 in Perrault 26 |
| 1 N NaCl | 7 | −1.72 | p. 20 in Perrault 26 |
| 1 N Na2SO4 | 7 | −1.75 | p. 20 in Perrault 26 |
| 1 N HCl | 1 | −1.49 | p. 20 in Perrault 26 |
| 3% NaCl | −1.39 → −1.44 | Volovitch et al. [29] | |
| Simulated body fluid (SBF) | 7 | −1.51 | Gao et al. [30] |
| 5% NaCl | 7 | −1.34 → −1.51 | Chong et al. [31] |
| 3.5% NaCl | 7 | −1.36 → −1.62 | Shin et al. [32] |
| 0.1 N NaCl | 7 | −1.66 | Zhao et al. [33] |
| Mg-Alloy | Electrolyte | pH | OCP vs. SCE | Reference |
|---|---|---|---|---|
| Mg–1Al | 3.5% NaCl | 7 | −1.62 | Lee et al. [23] |
| Mg–3Al | 3.5% NaCl | 7 | −1.62 | Lee et al. [23] |
| Mg–6Al | 3.5% NaCl | 7 | −1.64 | Lee et al. [23] |
| Mg–9Al | 3.5% NaCl | 7 | −1.65 | Lee et al. [23] |
| Mg–12Al | 3.5% NaCl | 7 | −1.63 | Lee et al. [23] |
| Mg–12Al–1Zn | 3.5% NaCl | 7 | −1.58 → −1.64 | Lee et al. [23] |
| AZ91 | Simulated body fluid (SBF) | 7 | −1.4 3 → −1.48 | Kannan et al. [34] |
| AZ91 | 5% NaCl | 7 | −1.55 | Chong et al. [31] |
| AZ80 | 5% NaCl | 7 | −1.55 | Chong et al. [31] |
| AZ65 | 5% NaCl | 7 | −1.58 | Chong et al. [31] |
- (1)
- Initial step: As Perrault [26] described, first, MgH2 film will be created on the Mg or Mg alloy surface, according to Equations (13) and (14). These reactions cause an increase of pH in the vicinity of the surface, because the protons from the water equilibrium are consumed during the reaction.Mg ⇒ Mg2+ + 2e−Mg2+ + 4e− + 2H+ ⇒ MgH2
- (2)
- Next step: Under stationary conditions as a consequence of the increase of OH− ions at the surface, a chemically degradation of the MgH2 layer takes place with the liberation of 1 mol of hydrogen at the end; see Equations (15) and (16). The reaction pathway in Equation (6) would explain that Mg(OH)2 or MgO under vacuum conditions can be detected [18].MgH2 + 2OH− ⇒ Mg(OH)2 + 2H+ + 4e−MgH2 + 2OH− ⇒ Mg(OH)2 + H2 + 2e−
- (3)
- OCP: At the OCP, measured in the range between −1.4 to −1.7 V (vs. SCE), reactions according to Equations (11) and (12) take place.
- (4)
- Polarization: During the polarization, the cathodic and the anodic arm of the I–E curve can be discussed separately. The cathodic arm follows Equations (11) and (12). In the cathodic arm of the forward scan, the formation of the MgH2 film takes place. This film induces in the anodic arm of the forward scan a so-called passivation effect, because of the temporary stability of the MgH2 and delayed H2 formation following Equation (15) or (16). That is the reason that H2 formation takes place in the anodic arm of the forward scan. The Mg oxidation becomes dominant; no passivation occurs, and the NDE can be explained considering that a part of the surface will be changed chemically according to Equation (6). Further discussion has to be separated for the different scan rates of 10 and 100 mV/s.
4.1. Mixed Potential Effect during Polarization with a Scan Rate of 10 mV/s

4.2. Passive-Like Behavior during Polarization with a Scan Rate of 100 mV/s
4.3. Development of New Mg Alloys

5. Materials and Methods
6. Conclusions

Author Contributions
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Atrens, A.; Dietzel, W. The negative difference effect and unipositive Mg+. Adv. Eng. Mater. 2007, 9, 292–297. [Google Scholar]
- Mueller, W.D.; Nascimento, M.L.; Zeddies, M.; Córsico, M.; Gassa, L.M.; de Mele, M.F. Magnesium and its alloys as degradable biomaterials.Corrosion studies using potentiodynamic and EIS electrochemical techniques. Mater. Res. 2007, 10, 5–10. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Liu, L.J.; Schlesinger, M. Corrosion of magnesium and its alloys. Corros. Sci. 2009, 51, 1733–1737. [Google Scholar] [CrossRef]
- Weber, C.R.; Knörnschild, G.; Dick, L.F. The negative-difference effect during the localized corrosion of magnesium and of the AZ91HP alloy. J. Braz. Chem. Soc. 2003, 14, 584–593. [Google Scholar] [CrossRef]
- Virtanen, S. Biodegradable Mg and Mg alloys: Corrosion and biocompatibility. Mater. Sci. Eng. B 2011, 176, 1600–1608. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Stjohn, D.; Nairn, J.; Li, Y. The electrochemical corrosion of pure magnesium in 1 N NaCl. Corros. Sci. 1997, 39, 855–875. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Stjohn, D.; Wu, X.; Nairn, J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corros. Sci. 1997, 39, 1981–2004. [Google Scholar] [CrossRef]
- Mueller, W.D.; de Mele, M.F.; Nascimento, M.L.; Zeddies, M. Degradation of magnesium and its alloys: Dependence on the composition of the synthetic biological media. J. Biomed. Mater. Res. A 2008, 90, 487–495. [Google Scholar]
- Kaese, V.; Niemeyer, M.; Tai, P.; Röttger, J. Enhancing corrosion resistance of magnesium based alloys by alloying Part 1: Dynamic alkalinization of the boundary layer—Ternary alloys. Mater. Corros. 1999, 50, 191–198. [Google Scholar] [CrossRef]
- Xue, D.C.; Yun, Y.; Schulz, M.J.; Shanov, V. Corrosion protection of biodegradable magnesium implants using anodization. Mater. Sci. Eng. C 2011, 31, 215–223. [Google Scholar] [CrossRef]
- Mensah-Darkwa, K.; Gupta, R.K.; Kumar, D. Mechanical and corrosion properties of magnesium–hydroxyapatite (Mg–HA) composite thin films. J. Mater. Sci. Technol. 2013, 29, 788–794. [Google Scholar] [CrossRef]
- Pereda, M.D.; Alonso, C.; Gamero, M.; del Valle, J.A.; de Mele, M.F. Comparative study of fluoride conversion coatings formed on biodegradable powder metallurgy Mg: The effect of chlorides at physiological level. Mater. Sci. Eng. C 2011, 31, 858–865. [Google Scholar] [CrossRef] [Green Version]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Kirkland, N.T.; Stace, E.; Woodfield, T.; Staiger, M.P.; Dias, G.J. Magnesium alloys: Predicting in vivo corrosion with in vitro immersion testing. J. Biomed. Mater. Res. B 2012, 100, 1134–1141. [Google Scholar]
- Bender, S.; Goellner, J.; Heyn, A.; Schmigalla, S. A new theory for the negative difference effect in magnesium corrosion. Mater. Corros. 2012, 63, 707–712. [Google Scholar]
- Santamaria, M.; di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Seyeux, A.; Liu, M.; Schmutz, P.; Song, G.; Atrens, A.; Marcus, P. ToF-SIMS depth profile of the surface film on pure magnesium formed by immersion in pure water and the identification of magnesium hydride. Corros. Sci. 2009, 51, 1883–1886. [Google Scholar] [CrossRef]
- Abidin, N.I.; Atrens, A.D.; Martin, D.; Atrens, A. Corrosion of high purity Mg, Mg2Zn0.2Mn, ZE41 and AZ91 in Hank’s solution at 37 °C. Corros. Sci. 2011, 53, 3542–3556. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R; Han, E.H. Corrosion characterization of Mg-8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [Google Scholar] [CrossRef]
- Dietzel, W.; Pfuff, M.; Winzer, N. Testing and mesoscale modelling of hydrogen assisted cracking of magnesium. Eng. Fract. Mech. 2010, 77, 257–263. [Google Scholar] [CrossRef]
- Lee, C.D.; Kang, C.S.; Shin, K.S. Effects of chunk breakage and surface protective film on negative difference effect of magnesium alloys. Met. Mater. Int. 2001, 7, 385–391. [Google Scholar] [CrossRef]
- Thomaz, T.R.; Weber, C.R.; Pelegrini, T., Jr.; Dick, L.F.; Knörnschild, G. The negative difference effect of magnesium and of the AZ91 alloy in chloride and stannate-containing solutions. Corros. Sci. 2010, 52, 2235–2243. [Google Scholar] [CrossRef]
- Nam, N.D.; Mathesch, M.; Forsyth, M.; Jo, D.S. Effect of manganese additions on the corrosion behavior of an extruded Mg–5Al based alloy. J. Alloy. Compd. 2012, 542, 199–206. [Google Scholar] [CrossRef]
- Perrault, G.G. Magnesium. In Encyclopedia of Electrochemistry of Elements; Bard, A.J, Ed.; Dekker: New York, NY, USA, 1978; p. 266. [Google Scholar]
- Pourbaix, M.; de Zoubov, N.; van Muylder, J. Atlas d'Equilibres Electrochimiques; Gauthier-Villars & Cie: Paris, France, 1963. [Google Scholar]
- San-Martin, A.; Manchester, F.D. The H–Mg (hydrogen–magnesium) system. Bull. Alloy Phase Diagr. 1987, 8, 431–437. [Google Scholar]
- Volovitch, P.; Serdechnova, M.; Ogle, K. Aqueous corrosion of Mg–Al binary alloys: Roles of Al and Mg. Corrosion 2012, 68, 557–570. [Google Scholar] [CrossRef]
- Gao, J.H.; Shi, X.Y.; Yang, B.; Hou, S.S.; Meng, E.C.; Guan, F.X.; Guan, S.K. Fabrication and characterization of bioactive composite coatings on Mg–Zn–Ca alloy by MAO/sol–gel. J. Mater. Sci. Mater. Med. 2011, 22, 1681–1687. [Google Scholar]
- Chong, K.Z.; Shih, T.S. Conversion coating treatment for magnesium alloys by a permanganate-phosphate solution. Mater. Chem. Phys. 2003, 80, 191–200. [Google Scholar] [CrossRef]
- Shin, K.S.; Bian, M.Z.; Nam, N.D. Effects of crystallographic orientation on corrosion behavior of magnesium single crystals. JOM 2012, 64, 664–670. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, L.L.; Xu, J. A comparison of corrosion behaviour in saline environment: Rare earth metals (Y, Nd, Gd, Dy) for alloying of biodegradable magnesium alloys. J. Mater. Sci. Technol. 2013, 29, 781–787. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.; Witte, F.; Blawert, C.; Dietzel, W. Influence of circumferential notch and fatigue crack on the mechanical integrity of biodegradable magnesium-based alloy in simulated body fluid. J. Biomed. Mater. Res. B 2010, 96, 303–309. [Google Scholar]
- Swiatowska, J.; Volovitch, P.; Ogle, K. The anodic dissolution of Mg in NaCl and Na2SO4 electrolytes by atomic emission spectroelectrochemistry. Corros. Sci. 2010, 52, 2372–2378. [Google Scholar] [CrossRef]
- Ezaki, H.; Morinaga, M.; Watanabe, S. Hydrogen overpotential for transition metals and alloys and its interpretation using an electronic model. Electrochim. Acta 1993, 38, 557–564. [Google Scholar]
- Tie, D.; Feyerabend, F.; Müller, W.D.; Schade, R.; Liefeith, K.; Kainer, K.U.; Willumeit, R. Antibacterial biodegradable Mg–Ag alloys. Eur. Cell. Mater. 2013, 25, 284–298. [Google Scholar]
- Zhang, E.; Yin, D.; Xu, L.; Yang, L.; Yang, K. Microstructure, mechanical and corrosion properties and biocompatibility of Mg–Zn–Mn alloys for biomedical application. Mater. Sci. Eng. C 2009, 29(3), 987–993. [Google Scholar] [CrossRef]
- Drazic, D.M.; Popic, J.P. Corrosion rates and negative difference effects for Al and some Al alloys. J. Appl. Electrochem. 1999, 29, 43–50. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mueller, W.-D.; Hornberger, H. The Influence of MgH2 on the Assessment of Electrochemical Data to Predict the Degradation Rate of Mg and Mg Alloys. Int. J. Mol. Sci. 2014, 15, 11456-11472. https://doi.org/10.3390/ijms150711456
Mueller W-D, Hornberger H. The Influence of MgH2 on the Assessment of Electrochemical Data to Predict the Degradation Rate of Mg and Mg Alloys. International Journal of Molecular Sciences. 2014; 15(7):11456-11472. https://doi.org/10.3390/ijms150711456
Chicago/Turabian StyleMueller, Wolf-Dieter, and Helga Hornberger. 2014. "The Influence of MgH2 on the Assessment of Electrochemical Data to Predict the Degradation Rate of Mg and Mg Alloys" International Journal of Molecular Sciences 15, no. 7: 11456-11472. https://doi.org/10.3390/ijms150711456