Flavonoids and Wnt/β-Catenin Signaling: Potential Role in Colorectal Cancer Therapies
Abstract
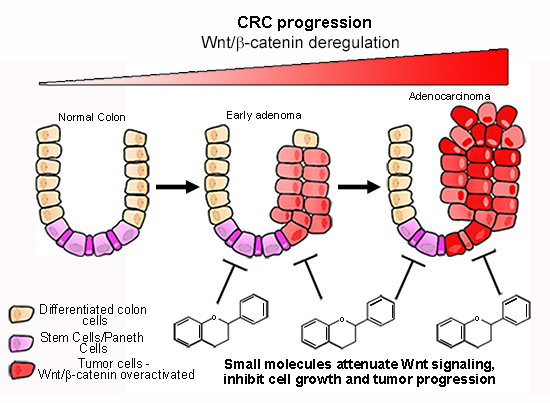
:1. Introduction
2. WNT/β-Catenin Signaling and Colorectal Cancer


3. Small Molecules as Wnt Inhibitors: Protein–Drug Interaction
| Protein | Flavonoids | Molecules | References |
|---|---|---|---|
| sFRP | ECGC | – | [55] |
| WIF-1 | ECGC | – | [56,57,5859] |
| Dsh | – | NSC668036; 3289-8625; FJ9; Pen-N3 | [17,47,48,49] |
| CK1a | Pyrvinium | [39] | |
| TNKS | Flavonone | XAV939; JW55; JW74; AZD2281; IWR-1; G007-LK; G244-LM | [40,41,42,43,44,60,61] |
| GSK-3β | Luteolin; Apigenin; Genistein | BIO | [62] |
| Destruction complex * | Kaempferol; Isorhamnetin; Baicalein | – | [63] |
| β-Catenin | Isoquercitrin | CCT031374; CCT036477; CCT070535 | [53,64] |
| β-Catenin/TCF | Quercetin | iCRT3; iCRT5; iCRT14; PKF118–310; PKF115–584; CGP049090; BC21; UU-T02 | [10,50,51,52,54,65] |
| CBP/β-catenin | – | ICG-001 | [45] |
| p300/β-catenin | – | Windorphen | [46] |
| Unknown | Silibinin; Wogonin | – | [9,36] |
4. Flavonoids and Wnt/β-Catenin Modulation in Colorectal Cancer
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yao, H.; Xu, W.; Shi, X.; Zhang, Z. Dietary flavonoids as cancer prevention agents. J. Environ. Sci. Health C 2011, 29, 1–31. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar]
- Amado, N.G.; Fonseca, B.F.; Cerqueira, D.M.; Neto, V.M.; Abreu, J.G. Flavonoids: Potential Wnt/β-catenin signaling modulators in cancer. Life Sci. 2011, 89, 545–554. [Google Scholar] [CrossRef]
- Gullett, N.P.; Ruhul Amin, A.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.-J.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat. Res. 2004, 555, 53–64. [Google Scholar] [CrossRef]
- Le Marchand, L. Cancer preventive effects of flavonoids—A review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: http://www.who.int (accessed on 19 June 2014).
- Lepourcelet, M.; Chen, Y.-N.P.; France, D.S.; Wang, H.; Crews, P.; Petersen, F.; Bruseo, C.; Wood, A.W.; Shivdasani, R.A. Small-molecule antagonists of the oncogenic TCF/β-catenin protein complex. Cancer Cell 2004, 5, 91–102. [Google Scholar] [CrossRef]
- Banerjee, S.; Wang, Z.; Mohammad, M.; Sarkar, F.H.; Mohammad, R.M. Efficacy of selected natural products as therapeutic agents against cancer. J. Nat. Prod. 2008, 71, 492–496. [Google Scholar] [CrossRef]
- HemaIswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef]
- Patterson, R.E.; Neuhouser, M.L.; Hedderson, M.M.; Schwartz, S.M.; Standish, L.J.; Bowen, D.J.; Marshall, L.M. Types of alternative medicine used by patients with breast, colon, or prostate cancer: Predictors, motives, and costs. J. Altern. Complement. Med. 2002, 8, 477–485. [Google Scholar] [CrossRef]
- Meijerman, I.; Beijnen, J.H.; Schellens, J.H. Herb–drug interactions in oncology: Focus on mechanisms of induction. Oncologist 2006, 11, 742–752. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Shan, J.; Shi, D.-L.; Wang, J.; Zheng, J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry 2005, 44, 15495–15503. [Google Scholar] [CrossRef]
- Miller, J.R. The Wnts. Genome Biol. 2002, 3, 1–15. [Google Scholar]
- Veeman, M.T.; Axelrod, J.D.; Moon, R.T. A second canon: Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 2003, 5, 367–377. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Hoppler, S.P.; Moon, R.T. Wnt Signaling in Development and Disease: Molecular Mechanisms and Biological Functions; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Gordon, M.D.; Nusse, R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef]
- Vermeulen, L.; Felipe de Sousa, E.M.; van der Heijden, M.; Cameron, K.; de Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef]
- Astin, M.; Griffin, T.; Neal, R.D.; Rose, P.; Hamilton, W. The diagnostic value of symptoms for colorectal cancer in primary care: A systematic review. Br. J. Gen. Pract. 2011, 61, e231–e243. [Google Scholar]
- Ferlay, J.; Shin, H.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D. GLOBOCAN 2008 v1. 2, Cancer Incidence and Mortality Worldwide: IARC Cancer Base; No. 10; International Agency for Research on Cancer: Lyon, France, 1 May 2010. [Google Scholar]
- Duval, A.; Gayet, J.; Zhou, X.-P.; Iacopetta, B.; Thomas, G.; Hamelin, R. Frequent frameshift mutations of the TCF-4 gene in colorectal cancers with microsatellite instability. Cancer Res. 1999, 59, 4213–4215. [Google Scholar]
- Samowitz, W.S.; Powers, M.D.; Spirio, L.N.; Nollet, F.; van Roy, F.; Slattery, M.L. β-Catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res. 1999, 59, 1442–1444. [Google Scholar]
- Sparks, A.B.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998, 58, 1130–1134. [Google Scholar]
- Kitaeva, M.N.; Grogan, L.; Williams, J.P.; Dimond, E.; Nakahara, K.; Hausner, P.; DeNobile, J.W.; Soballe, P.W.; Kirsch, I.R. Mutations in β-catenin are uncommon in colorectal cancer occurring in occasional replication error-positive tumors. Cancer Res. 1997, 57, 4478–4481. [Google Scholar]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- Fodde, R. The APC gene in colorectal cancer. Eur. J. Cancer 2002, 38, 867–871. [Google Scholar] [CrossRef]
- Frayling, I.M.; Beck, N.E.; Ilyas, M.; Dove-Edwin, I.; Goodman, P.; Pack, K.; Bell, J.A.; Williams, C.B.; Hodgson, S.V.; Thomas, H.J.; et al. The APC variants I1307K and E1317Q are associated with colorectal tumors, but not always with a family history. Proc. Natl. Acad. Sci. USA 1998, 95, 10722–10727. [Google Scholar] [CrossRef]
- Schoen, R.E. The case for population-based screening for colorectal cancer. Nat. Rev. Cancer 2002, 2, 65–70. [Google Scholar] [CrossRef]
- Network, C.G.A. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- National Cancer Institute. Available online: http://www.cancer.gov (accessed on 19 June 2014).
- Markowitz, S.D.; Bertagnolli, M.M. Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Voronkov, A.; Krauss, S. Wnt/β-catenin signaling and small molecule inhibitors. Curr. Pharm. Des. 2013, 19, 634–664. [Google Scholar] [CrossRef]
- Thorne, C.A.; Hanson, A.J.; Schneider, J.; Tahinci, E.; Orton, D.; Cselenyi, C.S.; Jernigan, K.K.; Meyers, K.C.; Hang, B.I.; Waterson, A.G.; et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat. Chem. Biol. 2010, 6, 829–836. [Google Scholar] [CrossRef]
- Huang, S.M.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Waaler, J.; Machon, O.; Tumova, L.; Dinh, H.; Korinek, V.; Wilson, S.R.; Paulsen, J.E.; Pedersen, N.M.; Eide, T.J.; Machonova, O.; et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012, 72, 2822–2832. [Google Scholar] [CrossRef]
- Lau, T.; Chan, E.; Callow, M.; Waaler, J.; Boggs, J.; Blake, R.A.; Magnuson, S.; Sambrone, A.; Schutten, M.; Firestein, R.; et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013, 73, 3132–3144. [Google Scholar] [CrossRef]
- Narwal, M.; Venkannagari, H.; Lehtio, L. Structural basis of selective inhibition of human tankyrases. J. Med. Chem. 2012, 55, 1360–1367. [Google Scholar] [CrossRef]
- Shultz, M.D.; Kirby, C.A.; Stams, T.; Chin, D.N.; Blank, J.; Charlat, O.; Cheng, H.; Cheung, A.; Cong, F.; Feng, Y.; et al. [1,2,4](triazol-3-ylsulfanylmethyl)-3-phenyl-[1,2,4]oxadiazoles: Antagonists of the Wnt pathway that inhibit tankyrases 1 and 2 via novel adenosine pocket binding. J. Med. Chem. 2012, 55, 1127–1136. [Google Scholar] [CrossRef]
- Emami, K.H.; Nguyen, C.; Ma, H.; Kim, D.H.; Jeong, K.W.; Eguchi, M.; Moon, R.T.; Teo, J.L.; Kim, H.Y.; Moon, S.H.; et al. A small molecule inhibitor of β-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. USA 2004, 101, 12682–12687. [Google Scholar] [CrossRef]
- Hao, J.; Ao, A.; Zhou, L.; Murphy, C.K.; Frist, A.Y.; Keel, J.J.; Thorne, C.A.; Kim, K.; Lee, E.; Hong, C.C. Selective small molecule targeting β-catenin function discovered by in vivo chemical genetic screen. Cell Rep. 2013, 4, 898–904. [Google Scholar] [CrossRef]
- Fujii, N.; You, L.; Xu, Z.; Uematsu, K.; Shan, J.; He, B.; Mikami, I.; Edmondson, L.R.; Neale, G.; Zheng, J.; et al. An antagonist of dishevelled protein–protein interaction suppresses β-catenin-dependent tumor cell growth. Cancer Res. 2007, 67, 573–579. [Google Scholar] [CrossRef]
- Grandy, D.; Shan, J.; Zhang, X.; Rao, S.; Akunuru, S.; Li, H.; Zhang, Y.; Alpatov, I.; Zhang, X.A.; Lang, R.A.; et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J. Biol. Chem. 2009, 284, 16256–16263. [Google Scholar] [CrossRef]
- Zhang, Y.; Appleton, B.A.; Wiesmann, C.; Lau, T.; Costa, M.; Hannoush, R.N.; Sidhu, S.S. Inhibition of Wnt signaling by Dishevelled PDZ peptides. Nat. Chem. Biol. 2009, 5, 217–219. [Google Scholar] [CrossRef]
- Tian, W.; Han, X.; Yan, M.; Xu, Y.; Duggineni, S.; Lin, N.; Luo, G.; Li, Y.M.; Han, X.; Huang, Z.; et al. Structure-based discovery of a novel inhibitor targeting the β-catenin/TCF4 interaction. Biochemistry 2012, 51, 724–731. [Google Scholar] [CrossRef]
- Gonsalves, F.C.; Klein, K.; Carson, B.B.; Katz, S.; Ekas, L.A.; Evans, S.; Nagourney, R.; Cardozo, T.; Brown, A.M.; DasGupta, R. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 5954–5963. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Wang, S.; Hao, X.; Li, L. A diterpenoid derivative 15-oxospiramilactone inhibits Wnt/β-catenin signaling and colon cancer cell tumorigenesis. Cell Res. 2011, 21, 730–740. [Google Scholar] [CrossRef]
- Ewan, K.; Pajak, B.; Stubbs, M.; Todd, H.; Barbeau, O.; Quevedo, C.; Botfield, H.; Young, R.; Ruddle, R.; Samuel, L.; et al. A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res. 2010, 70, 5963–5973. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, M.; Burton, S.D.; Katsakhyan, L.N.; Ji, H. Targeting the Tcf4 G13ANDE17 binding site to selectively disrupt β-catenin/T-cell factor protein-protein interactions. ACS Chem. Biol. 2014, 9, 193–201. [Google Scholar] [CrossRef]
- Godeke, J.; Maier, S.; Eichenmuller, M.; Muller-Hocker, J.; von Schweinitz, D.; Kappler, R. Epigallocatechin-3-gallate inhibits hepatoblastoma growth by reactivating the Wnt inhibitor SFRP1. Nutr. Cancer 2013, 65, 1200–1207. [Google Scholar] [CrossRef]
- Mazieres, J.; He, B.; You, L.; Xu, Z.; Lee, A.Y.; Mikami, I.; Reguart, N.; Rosell, R.; McCormick, F.; Jablons, D.M. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004, 64, 4717–4720. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Yang, T.-M.; Leu, S.-W.; Li, J.-M.; Hung, M.-S.; Lin, C.-H.; Lin, Y.-C.; Huang, T.-J.; Tsai, Y.-H.; Yang, C.-T. WIF-1 promoter region hypermethylation as an adjuvant diagnostic marker for non-small cell lung cancer-related malignant pleural effusions. J. Cancer Res. Clin. Oncol. 2009, 135, 919–924. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Z.; Hung, M.-S.; Lin, Y.-C.; Wang, T.; Gong, M.; Zhi, X.; Jablon, D.M.; You, L. Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells. Anticancer Res. 2009, 29, 2025–2030. [Google Scholar]
- Narwal, M.; Koivunen, J.; Haikarainen, T.; Obaji, E.; Legala, O.E.; Venkannagari, H.; Joensuu, P.; Pihlajaniemi, T.; Lehtio, L. Discovery of tankyrase inhibiting flavones with increased potency and isoenzyme selectivity. J. Med. Chem. 2013, 56, 7880–7889. [Google Scholar] [CrossRef]
- Menear, K.A.; Adcock, C.; Boulter, R.; Cockcroft, X.-L.; Copsey, L.; Cranston, A.; Dillon, K.J.; Drzewiecki, J.; Garman, S.; Gomez, S. 4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2 H-phthalazin-1-one: A novel bioavailable inhibitor of poly (ADP-ribose) polymerase-1. J. Med. Chem. 2008, 51, 6581–6591. [Google Scholar] [CrossRef]
- Johnson, J.L.; Rupasinghe, S.G.; Stefani, F.; Schuler, M.A.; Gonzalez de Mejia, E. Citrus flavonoids luteolin, apigenin, and quercetin inhibit glycogen synthase kinase-3β enzymatic activity by lowering the interaction energy within the binding cavity. J. Med. Food 2011, 14, 325–333. [Google Scholar] [CrossRef]
- Park, S.; Choi, J. Inhibition of β-catenin/Tcf signaling by flavonoids. J. Cell. Biochem. 2010, 110, 1376–1385. [Google Scholar] [CrossRef]
- Amado, N.G.; Cerqueira, D.M.; Menezes, F.S.; da Silva, J.F.; Neto, V.M.; Abreu, J.G. Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization. Anticancer Drugs 2009, 20, 543–552. [Google Scholar] [CrossRef]
- Amado, N.G.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Simas, A.B.; Kuster, R.M.; Mendes, F.A.; Abreu, J.G. Effects of natural compounds on xenopus embryogenesis: A potential read out for functional drug discovery targeting Wnt/β-catenin signaling. Curr. Top. Med. Chem. 2012, 12, 2103–2113. [Google Scholar] [CrossRef]
- Mabry, T.J.; Ulubelen, A. Chemistry and utilization of phenylpropanoids including flavonoids, coumarins, and lignans. J. Agric. Food Chem. 1980, 28, 188–196. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Johnson, I.T. Phytochemicals and cancer. Proc. Nutr. Soc. 2007, 66, 207–215. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Manson, M.M. Cancer prevention—The potential for diet to modulate molecular signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar] [CrossRef]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Potential targets for colorectal cancer prevention. Int. J. Mol. Sci. 2013, 14, 17279–17303. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Park, C.H.; Chang, J.Y.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 328, 227–234. [Google Scholar] [CrossRef]
- Pahlke, G.; Ngiewih, Y.; Kern, M.; Jakobs, S.; Marko, D.; Eisenbrand, G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J. Agric. Food Chem. 2006, 54, 7075–7082. [Google Scholar] [CrossRef]
- Kawahara, T.; Kawaguchi-Ihara, N.; Okuhashi, Y.; Itoh, M.; Nara, N.; Tohda, S. Cyclopamine and quercetin suppress the growth of leukemia and lymphoma cells. Anticancer Res. 2009, 29, 4629–4632. [Google Scholar]
- Román-Gómez, J.; Cordeu, L.; Agirre, X.; Jiménez-Velasco, A.; San José-Eneriz, E.; Garate, L.; Calasanz, M.J.; Heiniger, A.; Torres, A.; Prosper, F. Epigenetic regulation of Wnt-signaling pathway in acute lymphoblastic leukemia. Blood 2007, 109, 3462–3469. [Google Scholar] [CrossRef]
- Hsieh, A.; Kim, H.-S.; Lim, S.-O.; Yu, D.-Y.; Jung, G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/β-catenin signaling. Cancer Lett. 2011, 300, 162–172. [Google Scholar] [CrossRef]
- Okuhashi, Y.; ITOH, M.; Nara, N.; Tohda, S. Effects of combination of notch inhibitor plus hedgehog inhibitor or Wnt inhibitor on growth of leukemia cells. Anticancer Res. 2011, 31, 893–896. [Google Scholar]
- Kim, J.; Zhang, X.; Rieger-Christ, K.M.; Summerhayes, I.C.; Wazer, D.E.; Paulson, K.E.; Yee, A.S. Suppression of Wnt signaling by the green tea compound (–)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells requirement of the transcriptional repressor HBP1. J. Biol. Chem. 2006, 281, 10865–10875. [Google Scholar]
- Ju, J.; Hong, J.; Zhou, J.-N.; Pan, Z.; Bose, M.; Liao, J.; Yang, G.-Y.; Liu, Y.Y.; Hou, Z.; Lin, Y. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005, 65, 10623–10631. [Google Scholar] [CrossRef]
- Bose, M.; Hao, X.; Ju, J.; Husain, A.; Park, S.; Lambert, J.D.; Yang, C.S. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (–)-epigallocatechin-3-gallate and fish oil. J. Agric. Food Chem. 2007, 55, 7695–7700. [Google Scholar] [CrossRef]
- Kaur, M.; Velmurugan, B.; Tyagi, A.; Agarwal, C.; Singh, R.P.; Agarwal, R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of β-catenin-dependent signaling. Neoplasia 2010, 12, 415–424. [Google Scholar]
- He, L.; Lu, N.; Dai, Q.; Zhao, Y.; Zhao, L.; Wang, H.; Li, Z.; You, Q.; Guo, Q. Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells. Toxicology 2013, 312, 36–47. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amado, N.G.; Predes, D.; Moreno, M.M.; Carvalho, I.O.; Mendes, F.A.; Abreu, J.G. Flavonoids and Wnt/β-Catenin Signaling: Potential Role in Colorectal Cancer Therapies. Int. J. Mol. Sci. 2014, 15, 12094-12106. https://doi.org/10.3390/ijms150712094
Amado NG, Predes D, Moreno MM, Carvalho IO, Mendes FA, Abreu JG. Flavonoids and Wnt/β-Catenin Signaling: Potential Role in Colorectal Cancer Therapies. International Journal of Molecular Sciences. 2014; 15(7):12094-12106. https://doi.org/10.3390/ijms150712094
Chicago/Turabian StyleAmado, Nathália G., Danilo Predes, Marcela M. Moreno, Igor O. Carvalho, Fábio A. Mendes, and José G. Abreu. 2014. "Flavonoids and Wnt/β-Catenin Signaling: Potential Role in Colorectal Cancer Therapies" International Journal of Molecular Sciences 15, no. 7: 12094-12106. https://doi.org/10.3390/ijms150712094