Age Dependent Changes in Cartilage Matrix, Subchondral Bone Mass, and Estradiol Levels in Blood Serum, in Naturally Occurring Osteoarthritis in Guinea Pigs
Abstract
:1. Introduction
2. Results
2.1. Gross Morphology and Histopathology Changes in Cartilage



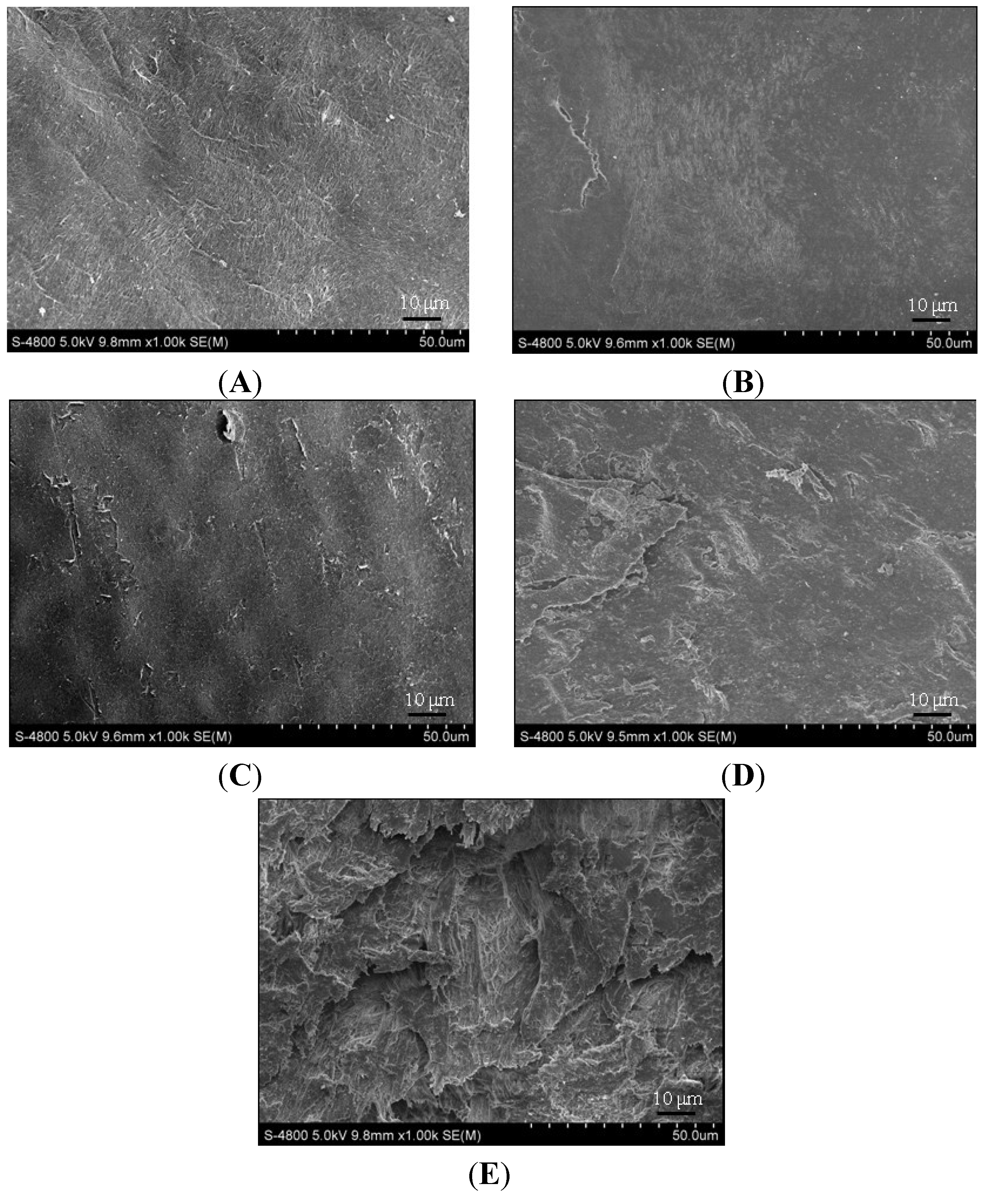
2.2. Electron Microscopy Findings


2.3. Serum Estradiol Levels

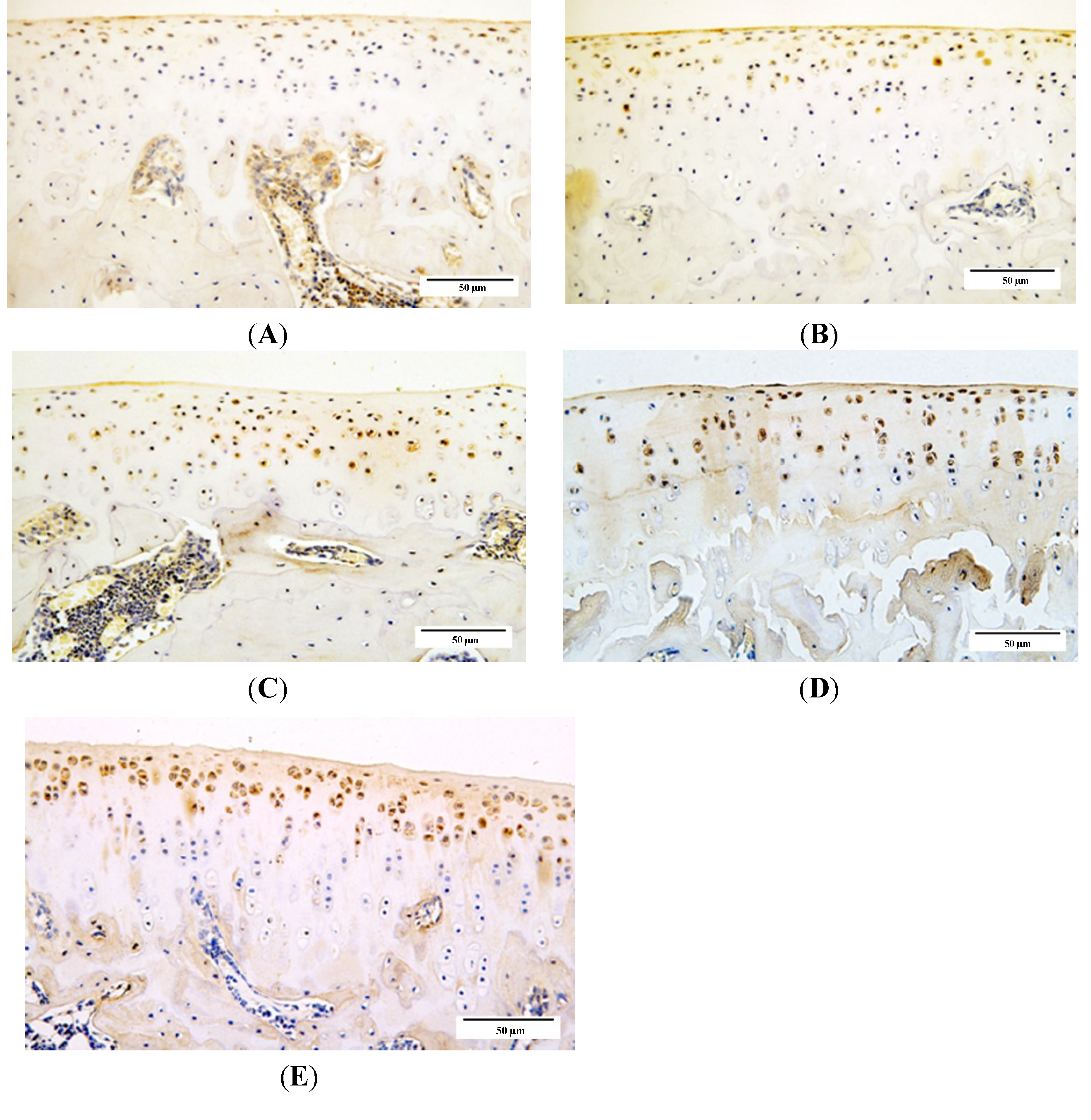
2.4. Qualitative Analysis of Matrix Metalloproteinase-3 (MMP-3) and Glycosaminoglycans (GAG) Expression


2.5. Subchondral Bone Mineral Density (BMD) Evaluation

3. Discussion
4. Methods
4.1. Animal Handling
4.2. Gross Observations and Specimen Processing
4.3. Preparation and Cartilage Histopathological Analysis
4.4. Electron Microscopy Analysis
4.5. ELISA Analysis
4.6. Immunohistochemistry for Glycosaminoglycans and Matrix Metalloproteinase-3
4.7. Subchondral BMD Analysis
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hardingham, T. Extracellular matrix and pathogenic mechanisms in osteoarthritis. Curr. Rheumatol. Rep. 2008, 10, 30–36. [Google Scholar] [CrossRef]
- Bendele, A.M. Animal models of osteoarthritis. J. Musculoskelet. Neuron. Interact. 2001, 1, 363–376. [Google Scholar]
- Arunakul, M.; Tochigi, Y.; Goetz, J.E.; Diestelmeier, B.W.; Heiner, A.D.; Rudert, J.; Fredericks, D.C.; Brown, T.D.; McKinley, T.O. Replication of chronic abnormal cartilage loading by medial meniscus destabilization for modeling osteoarthritis in the rabbit knee in vivo. J. Orthop. Res. 2013, 31, 1555–1560. [Google Scholar] [CrossRef]
- Mohan, G.; Perilli, E.; Parkinson, I.H.; Humphries, J.M.; Fazzalari, N.L.; Kuliwaba, J.S. Pre-emptive, early, and delayed alendronate treatment in a rat model of knee osteoarthritis: Effect on subchondral trabecular bone microarchitecture and cartilage degradation of the tibia, bone/cartilage turnover, and joint discomfort. Osteoarthr. Cartel. 2013, 21, 1595–1604. [Google Scholar] [CrossRef]
- Mapp, P.I.; Sagar, D.R.; Ashraf, S.; Burston, J.J.; Suri, S.; Chapman, V.; Walsh, D.A. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthr. Cartel. 2013, 21, 1336–1345. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, L.; Fu, X.; Wang, W.; Xu, H.; Song, H.; Zhang, Y. The potential protective effects of calcitonin involved in coordinating chondrocyte response, extracellular matrix, and subchondral trabecular bone in experimental osteoarthritis. Connect. Tissue Res. 2013, 54, 139–146. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartel. 2009, 17, 971–979. [Google Scholar] [CrossRef]
- Bendele, A.M.; Hulman, J.F. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis Rheumatol. 1991, 34, 1180–1184. [Google Scholar] [CrossRef]
- Wei, L.; de Bri, E.; Lundberg, A.; Svensson, O. Mechanical load and primary guinea pig osteoarthrosis. Acta Orthop. Scand. 1998, 69, 351–357. [Google Scholar] [CrossRef]
- Huebner, J.L.; Hanes, M.A.; Beekman, B.; TeKoppele, J.M.; Kraus, V.B. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthr. Cartil. 2002, 10, 758–767. [Google Scholar] [CrossRef]
- Wang, T.; Wen, C.Y.; Yan, C.H.; Lu, W.W.; Chiu, K.Y. Spatial and temporal changes of subchondral bone proceed to microscopic articular cartilage degeneration in guinea pigs with spontaneous osteoarthritis. Osteoarthr. Cartil. 2013, 21, 574–581. [Google Scholar] [CrossRef]
- Johnson, K.; Svensson, C.I.; Etten, D.V.; Ghosh, S.S.; Murphy, A.N.; Powell, H.C.; Terkeltaub, R. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis Rheumatol. 2004, 50, 1216–1225. [Google Scholar] [CrossRef]
- Gurkan, I.; Ranganathan, A.; Yang, X.; Horton, W.E., Jr.; Todman, M.; Huckle, J.; Pleshko, N.; Spencer, R.G. Modification of osteoarthritis in the guinea pig with pulsed low-intensity ultrasound treatment. Osteoarthr. Cartel. 2010, 18, 724–733. [Google Scholar] [CrossRef]
- Martin-Millan, M.; Castaneda, S. Estrogens, osteoarthritis and inflammation. Jt. Bone Spine 2013, 80, 368–373. [Google Scholar] [CrossRef]
- Sniekers, Y.H.; van Osch, G.J.; Ederveen, A.G.; Inzunza, J.; Gustafsson, J.A.; van Leeuwen, J.P.; Weinans, H. Development of osteoarthritic features in estrogen receptor knockout mice. Osteoarthr. Cartel. 2009, 17, 1356–1361. [Google Scholar] [CrossRef]
- Ou, Y.; Tan, C.; An, H.; Jiang, D.; Quan, Z.; Tang, K.; Luo, X. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med. Sci. Monit. 2012, 18, BR247–BR252. [Google Scholar]
- Bendele, A.M.; Hulman, J.F. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheumatol. 1988, 31, 561–565. [Google Scholar] [CrossRef]
- Jimenez, P.A.; Glasson, S.S.; Trubetskoy, O.V.; Haimes, H.B. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: Histologic, radiologic, and biochemical changes. Lab. Anim. Sci. 1997, 47, 598–601. [Google Scholar]
- Zamli, Z.; Adams, M.A.; Tarlton, J.F.; Sharif, M. Increased chondrocyte apoptosis is associated with progression of osteoarthritis in spontaneous Guinea pig models of the disease. Int. J. Mol. Sci. 2013, 14, 17729–17743. [Google Scholar] [CrossRef]
- Cawston, T.E.; Wilson, A.J. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract. Res. Clin. Rheumatol. 2006, 20, 983–1002. [Google Scholar] [CrossRef]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Aigner, T.; Zien, A.; Gehrsitz, A.; Gebhard, P.M.; McKenna, L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheumatol. 2001, 44, 2777–2789. [Google Scholar] [CrossRef]
- Mankin, H.J.; Lippiello, L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J. Bone. Jt. Surg. Am. 1970, 52, 424–434. [Google Scholar]
- Thompson, R.C., Jr.; Oegema, T.R., Jr. Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J. Bone Jt. surg. Am. 1979, 61, 407–416. [Google Scholar]
- Rizkalla, G.; Reiner, A.; Bogoch, E.; Poole, A.R. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J. Clin. Investig. 1992, 90, 2268–2277. [Google Scholar] [CrossRef]
- Venkatesan, N.; Barre, L.; Bourhim, M.; Magdalou, J.; Mainard, D.; Netter, P.; Fournel-Gigleux, S.; Ouzzine, M. Xylosyltransferase-I regulates glycosaminoglycan synthesis during the pathogenic process of human osteoarthritis. PLoS One 2012, 7, e34020. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Castañeda, S.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis associated with estrogen deficiency. Arthritis Res. Ther. 2009, 11, 241. [Google Scholar] [CrossRef]
- Turner, A.S.; Athanasiou, K.A.; Zhu, C.F.; Alvis, M.R.; Bryant, H.U. Biochemical effects of estrogen on articular cartilage in ovariectomized sheep. Osteoarthr. Cartel. 1997, 5, 63–69. [Google Scholar] [CrossRef]
- Ham, K.D.; Oegema, T.R.; Loeser, R.F.; Carlson, C.S. Effects of long-term estrogen replacement therapy on articular cartilage IGFBP-2, IGFBP-3, collagen and proteoglycan levels in ovariectomized cynomolgus monkeys. Osteoarthr. Cartel. 2004, 12, 160–168. [Google Scholar] [CrossRef]
- Hoegh-Andersen, P.; Tanko, L.B.; Andersen, T.L.; Lundberg, C.V.; Mo, J.A.; Heegaard, A.M.; Delaisse, J.M.; Christgau, S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis Res. Ther. 2004, 6, R169–R180. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Chen, K.; Lan, Y.; Zhang, N.; Jiang, R.; Hu, J. Alendronate protects against articular cartilage erosion by inhibiting subchondral bone loss in ovariectomized rats. Bone 2013, 53, 340–349. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Bay-Jensen, A.C.; Srinivasan, B.; Tabassi, N.C.; Garnero, P.; Delaisse, J.M.; Khosla, S.; Kassem, M. Estrogen inhibits Dlk1/FA1 production: A potential mechanism for estrogen effects on bone turnover. J. Bone Miner. Res. 2011, 26, 2548–2551. [Google Scholar] [CrossRef]
- Claassen, H.; Schunke, M.; Kurz, B. Estradiol protects cultured articular chondrocytes from oxygen-radical-induced damage. Cell Tissue Res. 2005, 319, 439–445. [Google Scholar] [CrossRef]
- Castañeda, S.; Largo, R.; Calvo, E.; Bellido, M.; Gómez-Vaquero, C.; Herrero-Beaumont, G. Effects of estrogen deficiency and low bone mineral density on healthy knee cartilage in rabbits. J. Orthop. Res. 2010, 28, 812–818. [Google Scholar]
- Herrero-Beaumont, G.; Herrero-Beaumont, G.; Roman-Blas, J.A.; Largo, R.; Berenbaum, F.; Castañeda, S. Bone mineral density and joint cartilage: Four clinical settings of a complex relationship in osteoarthritis. Ann. Rheum. Dis. 2011, 70, 1523–1525. [Google Scholar] [CrossRef]
- Bruyere, O.; Dardenne, C.; Lejeune, E.; Zegels, B.; Pahaut, A.; Richy, F.; Seidel, L.; Ethgen, O.; Henrotin, Y.; Reginster, J.Y. Subchondral tibial bone mineral density predicts future joint space narrowing at the medial femoro-tibial compartment in patients with knee osteoarthritis. Bone 2003, 32, 541–545. [Google Scholar] [CrossRef]
- Cao, Y.; Stannus, O.P.; Aitken, D.; Cicuttini, F.; Antony, B.; Jones, G.; Ding, C. Cross-sectional and longitudinal associations between systemic, subchondral bone mineral density and knee cartilage thickness in older adults with or without radiographic osteoarthritis. Ann. Rheum. Dis. 2014. [Google Scholar] [CrossRef]
- Santangelo, K.S.; Pieczarka, E.M.; Nuovo, G.J.; Weisbrode, S.E.; Bertone, A.L. Temporal expression and tissue distribution of interleukin-1β in two strains of guinea pigs with varying propensity for spontaneous knee osteoarthritis. Osteoarthr. Cartil. 2011, 19, 439–448. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yan, J.-Y.; Tian, F.-M.; Wang, W.-Y.; Cheng, Y.; Xu, H.-F.; Song, H.-P.; Zhang, Y.-Z.; Zhang, L. Age Dependent Changes in Cartilage Matrix, Subchondral Bone Mass, and Estradiol Levels in Blood Serum, in Naturally Occurring Osteoarthritis in Guinea Pigs. Int. J. Mol. Sci. 2014, 15, 13578-13595. https://doi.org/10.3390/ijms150813578
Yan J-Y, Tian F-M, Wang W-Y, Cheng Y, Xu H-F, Song H-P, Zhang Y-Z, Zhang L. Age Dependent Changes in Cartilage Matrix, Subchondral Bone Mass, and Estradiol Levels in Blood Serum, in Naturally Occurring Osteoarthritis in Guinea Pigs. International Journal of Molecular Sciences. 2014; 15(8):13578-13595. https://doi.org/10.3390/ijms150813578
Chicago/Turabian StyleYan, Jin-Yin, Fa-Ming Tian, Wen-Ya Wang, Ying Cheng, Hua-Fang Xu, Hui-Ping Song, Ying-Ze Zhang, and Liu Zhang. 2014. "Age Dependent Changes in Cartilage Matrix, Subchondral Bone Mass, and Estradiol Levels in Blood Serum, in Naturally Occurring Osteoarthritis in Guinea Pigs" International Journal of Molecular Sciences 15, no. 8: 13578-13595. https://doi.org/10.3390/ijms150813578



