MRC2 Expression Correlates with TGFβ1 and Survival in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mannose Receptor C Type 2 (MRC2) Was Over-Expressed in Hepatocellular Carcinoma (HCC) Tissues and Associated with Poor Prognosis after Liver Resection

| Clinicopathological Parameters | No. of Higher MRC2 Expression in HCC Tissues | r-Value | p-Value | |
|---|---|---|---|---|
| Gender | Female (n = 39) | 28 | −0.16 | 0.062 |
| Male (n = 57) | 34 | |||
| Age | >50 (n = 49) | 43 | 0.344 | 0.121 |
| <50 (n = 47) | 19 | |||
| HBV Infection | Yes (n = 76) | 59 | 0.312 | 0.075 |
| No (n = 20) | 3 | |||
| Liver Cirrhosis | Yes (n = 89) | 61 | 0.12 | 0.063 |
| No (n = 7) | 1 | |||
| High Serum AFP Level | >400 ng/mL | 38 | 0.322 | 0.130 |
| <400 ng/mL | 24 | |||
| Tumor Size | >5 cm | 40 | 0.271 | 0.091 |
| <5 cm | 22 | |||
| Intrahepatic Metastases | Yes (n = 18) | 16 | 0.485 | 0.005 |
| No (n = 78) | 46 | |||
| Portal Vein Invasion | Yes (n = 9) | 9 | 0.214 | 0.002 |
| No (n = 87) | 53 | |||
| Portal Vein Invasion | Yes (n = 9) | 9 | 0.214 | 0.002 |
| No (n = 87) | 53 | |||
| Edmonson–Steiner Classification | I and II (n = 34) | 24 | 0.332 | 0.130 |
| III and IV (n = 62) | 38 | |||
| Advanced TNM Stage | I and II (n = 52) | 26 | 0.151 | 0.032 |
| III and IV (n = 44) | 36 | |||
| Vasculature Invasion * | Yes (n = 12) | 7 | 0.070 | 0.071 |
| No (n = 84) | 55 | |||
| Clinicopathologic Parameters | Unvariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| RR (95% CI) | p-Value | RR (95% CI) | p-Value | |
| Intrahepatic Metastases | 3.120 (2.132–6.324) | 0.001 | 2.975 (1.587–4.525) | 0.003 |
| Higher MRC2 Expression in HCC Tissue | 1.856 (1.323–2.241) | 0.012 | 2.035 (1.785–2.995) | 0.015 |
| Higher TGFβ1 Expression in HCC Tissue | 1.697 (1.022–1.975) | 0.030 | 1.882 (1.211–2.214) | 0.022 |
2.2. Transforming Growth Factor (TGFβ1) Was Up-Regulated in HCC Tissues and Associated with MRC2 Positively
2.3. TGFβ1 Treatment Up-Regulated MRC2 Expression and Enhanced Cell Mobility and Invasion of HCC Cells


2.4. Knockdown of MRC2 Abolished the Effect of TGFβ1 on Cell Mobility and Invasion

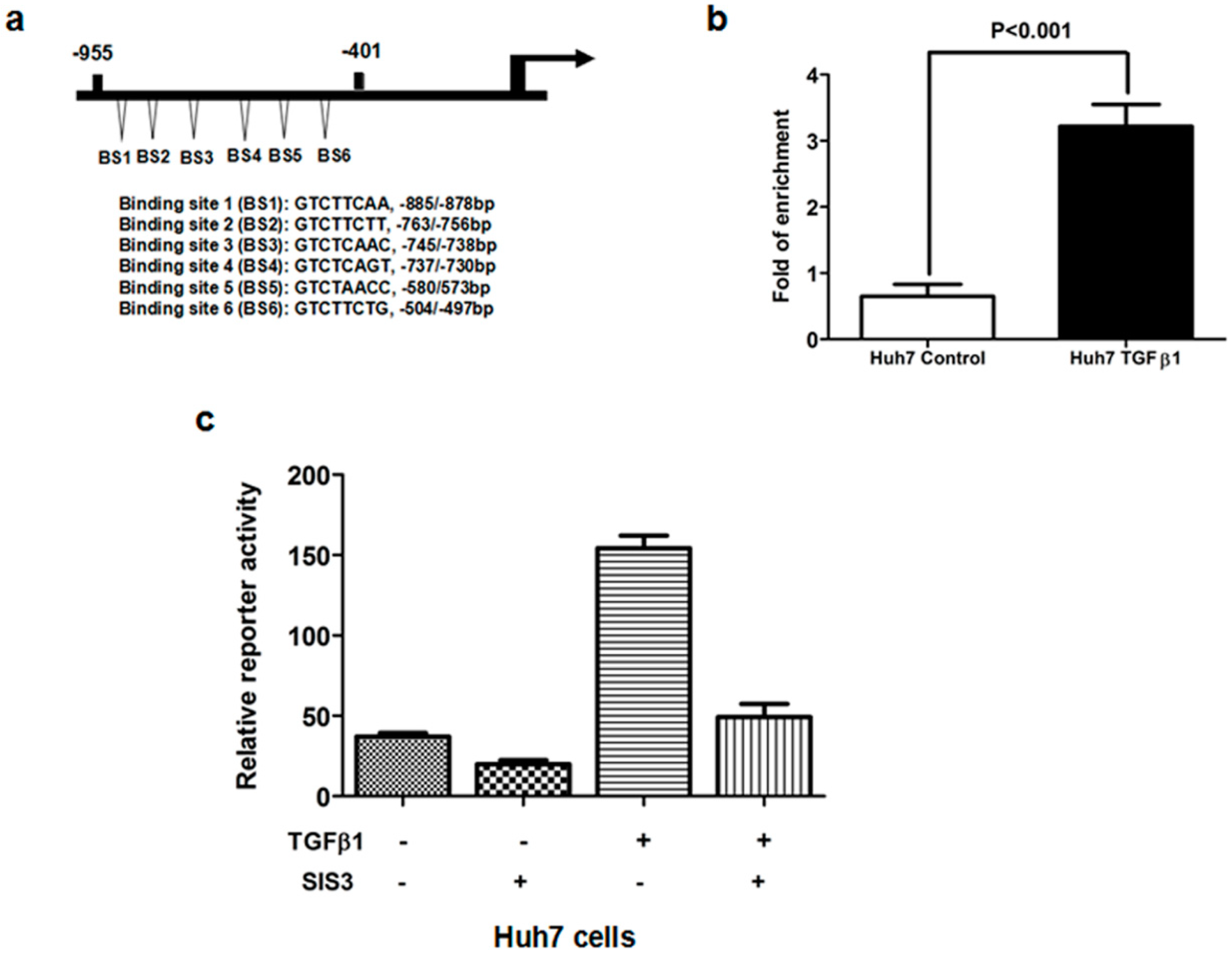
2.5. TGFβ1/Smad3 Pathway Regulated MRC2 Expression in HCC Directly
3. Experimental Section
3.1. Patients and Specimens
3.2. Immunohistochemistry Staining

3.3. Cell Culture and TGFβ1 Treatment
3.4. RNAi Transfections
3.5. Western Immunoblotting
3.6. Quantitative Reverse-Transcription-Polymerase Chain Reaction (RT-PCR)
3.7. Scratch Wound Healing Migration Assay
3.8. Invasion Assay
3.9. Chromatin Immunoprecipitation (ChIP) Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roberts, L.R.; Gores, G.J. Hepatocellular carcinoma: Molecular pathways and new therapeutic targets. Semin. Liver Dis. 2005, 25, 212–225. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.I.; Devesa, S.S. Cancer burden in the year 2000. The global picture. Eur. J. Cancer 2001, 37, S4–S66. [Google Scholar] [CrossRef]
- Hotary, K.B.; Allen, E.D.; Brooks, P.C.; Datta, N.S.; Long, M.W.; Weiss, S.J. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 2003, 114, 33–45. [Google Scholar] [CrossRef]
- Curino, A.C.; Engelholm, L.H.; Yamada, S.S.; Holmbeck, K.; Lund, L.R.; Molinolo, A.A.; Behrendt, N.; Nielsen, B.S.; Bugge, T.H. Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J. Cell Biol. 2005, 169, 977–985. [Google Scholar]
- Prud’homme, G.J. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Investig. 2007, 87, 1077–1091. [Google Scholar] [CrossRef]
- Silberstein, G.B.; Daniel, C.W. Reversible inhibition of mammary gland growth by transforming growth factor-β. Science 1987, 237, 291–293. [Google Scholar]
- Forrester, E.; Chytil, A.; Bierie, B.; Aakre, M.; Gorska, A.E.; Sharif-Afshar, A.R.; Muller, W.J.; Moses, H.L. Effect of conditional knockout of the type II TGF-β receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005, 65, 2296–2302. [Google Scholar] [CrossRef]
- Tian, F.; Byfield, S.D.; Parks, W.T.; Stuelten, C.H.; Nemani, D.; Zhang, Y.E.; Roberts, A.B. Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res. 2004, 64, 4523–4530. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Wang, F.; Gu, Q.; Xu, X. Snail involves in the transforming growth factor β-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS One 2011, 6, e23322. [Google Scholar]
- Maitah, M.Y.; Ali, S.; Ahmad, A.; Gadgeel, S.; Sarkar, F.H. Up-regulation of sonic hedgehog contributes to TGF-β1-induced epithelial to mesenchymal transition in NSCLC cells. PLoS One 2011, 6, e16068. [Google Scholar]
- Zheng, X.; Vittar, N.B.; Gai, X.; Fernandez-Barrena, M.G.; Moser, C.D.; Hu, C.; Almada, L.L.; McCleary-Wheeler, A.L.; Elsawa, S.F.; Vrabel, A.M.; et al. The transcription factor GLI1 mediates TGFβ1 driven EMT in hepatocellular carcinoma via a SNAI1-dependent mechanism. PLoS One 2012, 7, e49581. [Google Scholar] [CrossRef]
- Wakefield, L.M.; Roberts, A.B. TGF-β signaling: Positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002, 12, 22–29. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef]
- Pardali, K.; Moustakas, A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta 2007, 1775, 21–62. [Google Scholar]
- Engelholm, L.H.; Ingvarsen, S.; Jurgensen, H.J.; Hillig, T.; Madsen, D.H.; Nielsen, B.S.; Behrendt, N. The collagen receptor uPARAP/Endo180. Front Biosci. 2009, 14, 2103–2114. [Google Scholar]
- Behrendt, N. The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): Membrane proteins engaged in matrix turnover during tissue remodeling. Biol. Chem. 2004, 385, 103–136. [Google Scholar] [CrossRef]
- Palmieri, C.; Caley, M.P.; Purshouse, K.; Fonseca, A.V.; Rodriguez-Teja, M.; Kogianni, G.; Woodley, L.; Odendaal, J.; Elliott, K.; Waxman, J.; et al. Endo180 modulation by bisphosphonates and diagnostic accuracy in metastatic breast cancer. Br. J. Cancer 2013, 108, 163–169. [Google Scholar] [CrossRef]
- Wienke, D.; Davies, G.C.; Johnson, D.A.; Sturge, J.; Lambros, M.B.; Savage, K.; Elsheikh, S.E.; Green, A.R.; Ellis, I.O.; Robertson, D.; et al. The collagen receptor Endo180 (CD280) is expressed on basal-like breast tumor cells and promotes tumor growth in vivo. Cancer Res. 2007, 67, 10230–10240. [Google Scholar] [CrossRef]
- Kogianni, G.; Walker, M.M.; Waxman, J.; Sturge, J. Endo180 expression with cofunctional partners MT1-MMP and uPAR-uPA is correlated with prostate cancer progression. Eur. J. Cancer 2009, 45, 685–693. [Google Scholar] [CrossRef]
- Sulek, J.; Wagenaar-Miller, R.A.; Shireman, J.; Molinolo, A.; Madsen, D.H.; Engelholm, L.H.; Behrendt, N.; Bugge, T.H. Increased expression of the collagen internalization receptor uPARAP/Endo180 in the stroma of head and neck cancer. J. Histochem. Cytochem. 2007, 55, 347–353. [Google Scholar]
- Zheng, X.; Yao, Y.; Xu, Q.; Tu, K.; Liu, Q. Evaluation of glioma-associated oncogene 1 expression and its correlation with the expression of sonic hedgehog, E-cadherin and S100a4 in human hepatocellular carcinoma. Mol. Med. Rep. 2010, 3, 965–970. [Google Scholar]
- Zheng, X.; Gai, X.; Ding, F.; Lu, Z.; Tu, K.; Yao, Y.; Liu, Q. Histone acetyltransferase PCAF up-regulated cell apoptosis in hepatocellular carcinoma via acetylating histone H4 and inactivating AKT signaling. Mol. Cancer 2013, 12, 96. [Google Scholar] [CrossRef]
- Wienke, D.; MacFadyen, J.R.; Isacke, C.M. Identification and characterization of the endocytic transmembrane glycoprotein Endo180 as a novel collagen receptor. Mol. Biol. Cell 2003, 14, 3592–3604. [Google Scholar] [CrossRef]
- Huijbers, I.J.; Iravani, M.; Popov, S.; Robertson, D.; Al-Sarraj, S.; Jones, C.; Isacke, C.M. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One 2010, 5, e9808. [Google Scholar]
- Caley, M.P.; Kogianni, G.; Adamarek, A.; Gronau, J.H.; Rodriguez-Teja, M.; Fonseca, A.V.; Mauri, F.; Sandison, A.; Rhim, J.S.; Palmieri, C.; et al. TGFβ1-Endo180-dependent collagen deposition is dysregulated at the tumour-stromal interface in bone metastasis. J. Pathol. 2012, 226, 775–783. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gai, X.; Tu, K.; Lu, Z.; Zheng, X. MRC2 Expression Correlates with TGFβ1 and Survival in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2014, 15, 15011-15025. https://doi.org/10.3390/ijms150915011
Gai X, Tu K, Lu Z, Zheng X. MRC2 Expression Correlates with TGFβ1 and Survival in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2014; 15(9):15011-15025. https://doi.org/10.3390/ijms150915011
Chicago/Turabian StyleGai, Xiaohong, Kangsheng Tu, Zhongtang Lu, and Xin Zheng. 2014. "MRC2 Expression Correlates with TGFβ1 and Survival in Hepatocellular Carcinoma" International Journal of Molecular Sciences 15, no. 9: 15011-15025. https://doi.org/10.3390/ijms150915011




