Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review
Abstract
:1. Introduction
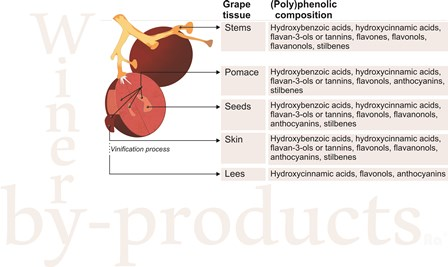
2. Main By-Products Derived from Winery Industry
2.1. Grape Pomace or Press Residues
2.2. Grape Stems
2.3. Grape Leaves
2.4. Wine Lees
3. Functional Compounds in By-Products from Organic Wineries
3.1. Phenolic Acids
3.1.1. Hydroxybenzoic Acids
3.1.2. Hydroxycinnamic Acids
3.2. Flavonoids
| Compound | Stem | Skin | Seed | Pomace | Leaves | Lees |
|---|---|---|---|---|---|---|
| Hydroxybenzoic acid | ||||||
| Gallic acid | 1.30–2.40 (µg·100 g−1·dw; HPLC–DAD) [92]; 0.07–33.00 (mg·g−1·dw, HPLC–DAD) [65,81,82] | – | 6.80–9.80 (mg·kg−1·fw, HPLC–FD) [84] | ≤3.97 (mg·g−1·dw, HPLC–UV) [17]; 0.05–0.19 (mg·g−1·dw, HPLC–DAD) [32] | – | – |
| p-Hydroxybenzoic acid | – | – | – | ≤6.59 (mg·g−1·dw, HPLC–UV) [17] | – | – |
| Protocatecuic acid | – | 1.50–2.40 (mg·kg−1·fw, HPLC–FD) [83] | 3.30–8.70 (mg·kg−1·fw, HPLC–FD) [84] | ≤98.65 (mg·g−1·dw, HPLC–UV) [17] | – | – |
| Syringic acid | ≤32.20 (mg·g−1·dw, HPLC–DAD) [81] | – | – | – | – | – |
| Tannic acid | – | – | – | ≤3.85 (mg·g−1·dw, HPLC–UV) [17] | – | – |
| Vanillic acid | – | – | – | ≤0.59 (mg·g−1·dw, HPLC–UV) [17] | – | – |
| Hydroxycinnamic acid | ||||||
| Caffeic acid | ≤0.60 (mg·g−1·dw, HPLC–DAD) [81]; 0.60–1.90 (µg·g−1·dw; HPLC–DAD) [92] | – | – | – | – | 663 (µg·g−1·dw, HPLC–DAD) [61] |
| Caftaric acid | – | – | – | – | ≤7.32 (mg·g−1·fw, HPLC–UV) [86] | |
| cis-Caftaric acid | – | 0.20–0.50 (mg·kg−1·fw, HPLC–FD) [83] | – | – | – | |
| trans-Caftaric acid | 0.04–16.10 (mg·g−1·dw, HPLC–DAD) [65,81]; ≤40.00 (mg·kg−1·fw; HPLC–DAD) [44] | 4.90–9.50 (mg·kg-1·fw, HPLC–FD) [83] | – | – | 1.40–3.28 (mg·g−1·dw, HPLC–DAD) [59] | |
| Chlorogenic acid | – | ≤0.23 (mg·g−1·dw, HPLC–DAD) [32] | 2.90–6.80 (mg·g−1·dw, HPLC–DAD) [33] | – | – | |
| p-Coumaric acid | 0.04–0.90 (mg·g−1·dw, HPLC–DAD) [81]; 0.90–2.20 (µg·g−1·dw, HPLC–DAD) [92] | – | – | – | – | 2449 (µg·g−1·dw, HPLC–DAD) [61] |
| trans-Coumaroyltartaric acid | – | – | – | – | 0.49–1.49 (mg·g−1·dw, HPLC–DAD) [59] | |
| Coutaric acid | ≤4.50 (mg·kg−1·fw; HPLC–DAD) [44] | – | – | – | – | |
| cis-Coutaric acid | – | 0.90–2.70 (mg·kg−1·fw, HPLC–FD) [83] | – | – | – | |
| trans-Coutaric acid | – | 3.20–10.00 (mg·kg−1·fw, HPLC–FD) [83] | – | – | – | |
| Ferulic acid | ≤2.50 (mg·g−1·dw, HPLC–DAD) [80] | – | – | – | – | |
| Compound | Stem | Skin | Seed | Pomace | Leaves | |
|---|---|---|---|---|---|---|
| Hydroxybenzoic acid | ||||||
| Gallic acid | 0.01–0.03 (mg·g−1·dw, HPLC–DAD) [92]; 1.05–22.60 (µg·g−1·dw; HPLC–DAD) [65,81] | ≤1.20 (mg·g−1·dw, HPLC–UV) [81] | ≤1.90 (mg·g−1·dw, HPLC–UV) [82]; 15.70–19.70 (mg·g−1·dw, HPLC–UV) [93] | <1.98 (mg·g−1·dw, HPLC–DAD) [94] | – | |
| Gallic acid hexose | – | – | ≤0.80 (mg·g−1·dw, HPLC–UV) [82] | – | – | |
| Gallic acid dihexose | – | ≤1.10 (mg·g−1·dw, HPLC–UV) [81] | ≤1.20 (mg·g−1·dw, HPLC–UV) [82] | – | – | |
| Protocatecuic acid | – | – | ≤6.00 (mg·kg−1·fw, HPLC–FD) [84]; <1.10 (mg·g−1·dw, HPLC–UV) [49] | – | ||
| Syringic acid | ≤0.10 (µg·g−1·dw, HPLC–DAD) [81] | – | – | – | – | |
| Hydroxycinnamic acid | ||||||
| Caffeic acid | ≤0.05 (µg·g−1·dw, HPLC–DAD) [81]; 1.00–1.50 (mg·g−1·dw, HPLC–DAD) [92] | – | – | – | – | |
| cis-Caftaric acid | – | ≤1.30 (mg·kg−1·fw, HPLC–FD) [84]; ≤2.50 (mg·g−1·dw, HPLC–UV) [82] | – | – | – | |
| trans-Caftaric acid | 0.05–12.20 (mg·g−1·dw, HPLC–DAD) [65,81] | 2.40–31.00 (mg·kg−1·fw, HPLC–FD) [84]; ≤4.00 (mg·g−1·dw, HPLC–UV) [82] | – | – | 18.81–83.36 (mg·g−1·dw, HPLC–DAD) [59] | |
| p-Coumaric acid | 0.01–0.08 (µg·g−1·dw, HPLC–DAD) [81]; 0.02–0.03 (mg·g−1·dw, HPLC–DAD) [92] | ≤2.0 (mg·g−1·dw, HPLC–UV) [82] | – | 0.03 (mg·g−1·dw, HPLC–DAD) [94] | – | |
| trans-Coumaroyltartaric acid | – | – | – | – | 2.12–10.71 (mg·g−1·dw, HPLC–DAD) [59] | |
| Coutaric acid-O-hexoside | – | 2.27 (mg·g−1·dw, HPLC–UV) [82] | – | – | – | |
| cis-Coutaric acid | – | 1.60–5.90 (mg·kg−1·fw , HPLC–FD) [84]; ≤6.20 (mg·g−1·dw, HPLC–UV) [82] | – | – | – | |
| trans-Coutaric acid | – | 1.90–18.00 (mg·kg−1·fw, HPLC–FD) [84]; ≤0.30 (mg·g−1·dw, HPLC–UV) [82] | – | – | – | |
| trans-Fertaric acid | – | ≤2.60 (mg·kg−1·fw, HPLC–FD) [84]; ≤1.70 (mg·g−1·dw, HPLC–UV) [82] | – | – | – | |
| trans-Ferulic acid | – | – | – | 0.04 (mg·g−1·dw, HPLC–DAD) [94] | – | |
3.2.1. Flavonols
| Compound | Stem | Skin | Seed | Pomace | Leaves | Lees |
|---|---|---|---|---|---|---|
| Flavan-3-ols and tannins | ||||||
| Catechin Z | 0.71–85.80 (mg·g−1·dw, HPLC–DAD Y) [65,81,102] 1.24–2.58 (µg·g−1·dw; HPLC–DAD) [92] ≤60.00 (mg·kg−1·fw, HPLC–DAD) [44] 0.12–1.27 (mg·g−1·dw, HPLC–UV) [103] | 8.50–25.0 (mg·kg−1·fw, HPLC–FD) [84] ≤13.20 (mg·g−1·dw, HPLC–DAD) [32] 0.01–10.00 (mg·g−1·dw, HPLC–FD) [104] | 0.08–4.50 (mg·g−1·fw, HPLC–FD) [85,105] 0.27–1.17 (mg·g−1·dw, HPLC–DAD) [33] | 1.12–1.50 (mg·g−1·dw, HPLC–DAD) [97] | 71.00 (mg·kg−1·fw, HPLC–UV) [86] | – |
| Epicatechin | ≤1.00–13.30 (mg·g−1·dw, HPLC–DAD) [44,61,78,106] ≤0.11 (mg·g−1·dw, HPLC–UV) [103] | 6.20–13.00 (mg·kg−1·fw, HPLC–FD) [84] ≤1.10 (mg·g−1·dw, HPLC–FD) [104] | 0.06–0.21 (mg·g−1·fw, HPLC–FD) [85,105] ≤0.47 (mg·g−1·dw, HPLC–DAD) [33] | ≤2.01 (mg·g−1·dw, HPLC–UV) [17] | 15.00 (mg·kg−1·fw, HPLC–UV) [86] | – |
| Epicatechin gallate | 0.06–7.80 (mg·g−1·dw, HPLC–DAD) [65,81] 15.50–19.80 (mg·kg−1·fw, HPLC–FD) [44] | – | 13.00–70.00 (mg·kg−1·fw, HPLC–FD) [85] | – | 15.00 (mg·kg−1·fw, HPLC–UV) [86] | – |
| Epigallocatechin | 1.50–5.40 (mg·kg−1·fw, HPLC–DAD) [44] | – | – | – | – | – |
| Procyanidin B1 | 6.20–13.73 (mg·g−1·dw, HPLC–DAD) [102] 0.25–1.96 (mg·g−1·dw, HPLC–UV) [103] | 8.40–22.00 (mg·kg−1·fw, HPLC–FD) [82] | 74.00–170.00 (mg·kg−1·fw, HPLC–FD) [85] | – | – | – |
| Procyanidin B2 | 0.11–5.10 (mg·g−1·dw, HPLC–DAD) [65,81] ≤0.09 (mg·g−1·dw, HPLC–UV) [103] | 0.70–2.20 (mg·kg−1·fw, HPLC–FD) [82] | 21.00–41.00 (mg·kg−1·fw, HPLC–FD) [85] | – | – | – |
| Procyanidin B3 | 0.14–20.50 (mg·g−1·dw, HPLC–DAD) [65,81] 0.04–0.23 (mg·g−1·dw, HPLC–UV) [103] | 16.00–39.00 (mg·kg−1·fw, HPLC–FD) [84] | 43.00–64.00 (mg·kg−1·fw , HPLC–FD) [85] | – | – | – |
| Procyanidin B4 | – | – | 33.00–80.00 (mg·kg−1·fw, HPLC–FD) [85] | – | – | – |
| Procyanidin trimmer C1 | – | ≤0.04 (mg·g−1·dw, HPLC–FD) [104] | 0.20–0.30 (mg·g−1·dw, HPLC–FD) [105] | – | – | – |
| Flavones | ||||||
| Luteolin | 0.02–0.04 (µg·g−1·dw; HPLC–DAD) [92] | – | – | – | – | – |
| Flavonols | ||||||
| Engeletin | Traces (HPLC–DAD) [44] | – | – | – | – | – |
| Isorhamnetin | – | – | – | ≤ 0.20 (mg·g−1·dw, HPLC–DAD) [97] | – | – |
| Isrhm-3-O-Glc | – | 11.00–48.00 (mg·kg−1·fw, HPLC–FD) [84] | – | 0.06 (mg·g−1·dw, HPLC–DAD) [96] ≤0.10 (mg·g−1·dw, HPLC–DAD) [97] | – | – |
| Isrhm-3-O-Gluc | – | – | – | ≤ 0.90(mg·g−1·dw, HPLC–DAD) [97] | – | – |
| Kaempferol | ≤1.80 (mg·g−1·dw, HPLC–DAD) [81] | – | – | <0.01 (mg·g−1·dw, HPLC–DAD) [98] ≤0.01 (mg·g−1·dw, HPLC–DAD) [97] | – | – |
| K-3-O-Glc | Traces (HPLC–DAD) [44] | 8.00–14.00 (mg·kg−1·fw, HPLC–FD) [84] | – | 0.01 (mg·g−1·dw, HPLC–DAD) [98] ≤0.20 (mg·g−1·dw, HPLC–DAD) [97] | ≤30.0 (mg·kg−1·fw, HPLC–UV) [86] 2.03–5.51 (mg·g−1·dw, HPLC–DAD) [59] | – |
| K-3-O-Gluc | Traces (HPLC–DAD) [44] | – | – | – | – | – |
| Laricitin | – | – | – | ≤ 0.20 (mg·g−1·dw, HPLC–DAD)[97] | – | – |
| Myricetin | – | – | – | – | – | 4292 (µg·g−1·dw, HPLC–DAD) [61] |
| Myr-3-O-Glc | Traces (HPLC–DAD) [44] | 13.00–26.00 (mg·kg−1·fw, HPLC–FD) [84] | – | 0.49–1.49(mg·g−1·dw, HPLC–DAD) [59] | – | |
| Myr-3-O-Gluc | Traces (HPLC–DAD) [44] | 5.80–10.00 (mg·kg−1·fw, HPLC–FD) [84] | – | – | – | – |
| Quercetin | 0.60–8.20 (µg·g−1·dw, HPLC–DAD) [81] | – | – | 15.30 (mg·kg−1·dw, HPLC–DAD)[97] 0.50–2.80 (mg·100 g−1·dw, HPLC–DAD) [97] | 47.00 (mg·kg−1·fw, HPLC–UV) [86] | 13,656 (µg·g−1·dw, HPLC–DAD) [61] |
| Q-3-O-Gal | 6.60–15.00 (mg·g−1·dw, HPLC–DAD) [81] | – | – | – | – | – |
| Q-3-O-Glc | 0.28–7.30 (mg·g−1·dw, HPLC–DAD) [81,102] 18.0 (mg·kg−1·fw, HPLC–DAD) [44] | 31.00–55.00 (mg·kg−1·fw, HPLC–FD) [84] | – | 0.18 (mg·g−1·dw, HPLC–DAD) [98] ≤0.90–5.11 (mg·g−1·dw, HPLC–DAD) [97,99] | 142.00 (mg·kg−1·fw, HPLC–UV) [86] | – |
| Q-3-O-GlcXyl | – | 9.00–18.00 (mg·kg−1·fw, HPLC–FD) [84] | – | – | – | – |
| Q-3-O-Gluc | 0.20–126.80 mg·g−1·dw, HPLC–DAD) [44,102] | 29.00–59.00 (mg·kg−1·fw, HPLC–FD) [84] | – | ≤1.30–130.00 (mg·g−1·dw, HPLC–DAD) [97,98] ≤105.40 (mg·g−1·dw, HPLC–DAD) [99] | – | 8900 (µg·g−1·dw, HPLC–DAD) [61] |
| Q-3-O-Rha | 0.30–2.80 (mg·g−1·dw, HPLC–DAD) [81] | – | – | – | – | – |
| Q-3-O-Rut | 1.90–41.80 (mg·g−1·dw, HPLC–DAD) [81] | 0.01–0.57 (mg·g−1·dw, HPLC–DAD) [32,99] | – | 0.12–0.50 (mg·g−1·dw, HPLC–DAD) [33,97] | – | – |
| Flavanonols | ||||||
| Astilbin | 35.00(HPLC–DAD; mg·kg−1·fw) [44] | – | – | – | – | – |
| Compound | Stem | Skin | Seed | Pomace | Leaves | |
|---|---|---|---|---|---|---|
| Flavan-3-ols and tannins | ||||||
| Catechin Z | 46.50–98.30 (µg·g−1·dw, HPLC–DAD Y) [84] 0.13–2.89 (mg·g−1·dw, HPLC–DAD) [92,93] 3.85–18.58 (µg·g−1·dw; HPLC–DAD) [65] 9.30–133.90 (mg·g−1·dw, HPLC–UV) [103] | ≤23.00 (mg·kg−1·fw, HPLC–FD) [84] 11.40 (mg·g−1·dw, HPLC–UV) [82] 0.72–0.84 (mg·g−1·dw, HPLC–DAD) [93] | 120.00–500.00 (mg·kg−1·fw, HPLC–FD) [84] 106.50 (mg·g−1·dw, HPLC–UV) [82] 83.1–98.3 (mg·g−1·dw, HPLC–UV) [107] 0.45–0.53 (mg·g−1·dw, HPLC–DAD) [93] | <0.30 (mg·g−1·dw, HPLC–DAD) [108,109] | – | |
| Dimer gallate | – | – | 38.1–46.8 (mg·g-1·dw, HPLC–UV) [107] | – | – | |
| Epicatechin | ≤4.00–0.58 (µg·g−1·dw, HPLC–DAD) [65,81] 0.04–1.13 (mg·g−1·dw, HPLC–DAD) [93,110] 0.50–5.80 (mg·g−1·dw, HPLC–UV) [103] | ≤8.30 (mg·kg−1·fw, HPLC–FD) [84] 2.70 (mg·g−1·dw, HPLC–UV) [82] 0.77–0.85 (mg·g−1·dw, HPLC–DAD) [93] | 110.00–310.00 (mg·kg−1·fw, HPLC–FD) [84] 77.50 (mg·g−1·dw, HPLC–UV) [82] 54.2–55.8 (mg·g−1·dw, HPLC–UV) [107] 0.79–0.89 (mg·g−1·dw, HPLC–DAD) [107] | <0.07 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Epicatechin-epicatechingallate I | – | – | 26.80 (mg·g−1·dw, HPLC–UV) [82] | – | – | |
| Epicatechin-epicatechingallate II | – | – | 23.70 (mg·g−1·dw, HPLC–UV) [82] | – | – | |
| Epicatechin-epicatechingallate III | – | – | 21.40 (mg·g−1·dw, HPLC–UV) [82] | – | – | |
| Epicatechin gallate | 0.34–15.70 (µg·g−1·dw, HPLC–DAD) [44,65,81] | – | 13.00–67.00 (mg·kg−1·fw, HPLC–FD) [84] 76.50 (mg·g−1·dw, HPLC–UV) [82] 53.8–56.8 (mg·g−1·dw, HPLC–UV) [107] | <0.05 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Epicatechin-3-hexose | – | – | 1.50 (mg·g−1·dw, HPLC–UV) [82] | – | – | |
| Epigallocatechin | 0.80–0.90 (mg·kg−1·fw, HPLC–DAD) [44] | 2.10 (mg·g−1·dw, HPLC–UV) [82] | – | – | – | |
| Epigallocatechin gallate | – | – | 0.30–0.50 (mg·g−1·dw, HPLC–UV) [107] | – | – | |
| Epigallocatechin-epicatechin | – | – | 3.60 (mg·g−1·dw, HPLC–UV) [82] | – | – | |
| Procyanidin dimmer B1 | 13.30–187.70 (mg·g−1·dw, HPLC–UV) [103] 0.07–0.11 (mg·g−1·dw, HPLC–DAD) [93] | ≤48.00 (mg·kg−1·fw, HPLC–FD) [84] 0.36–0.48 (mg·g−1·dw, HPLC–DAD) [93] | 200.00–620.00 (mg·kg−1·fw, HPLC–FD) [82] 3.10 (mg·g−1·dw, HPLC–UV) [82] 0.10–0.14 (mg·g−1·dw, HPLC–DAD) [107] | <0.22 (mg·g−1·dw, HPLC–DAD) [93,108] | – | |
| Procyanidin dimmer B2 | 0.02–8.50 (mg·g−1·dw, HPLC–DAD) [65,81,93] 1.10–4.80 (mg·g−1·dw, HPLC–UV) [103] | 8.70 (mg·g−1·dw, HPLC–UV) [82] 0.59–0.65 (mg·g−1·dw, HPLC–DAD) [93] | 15.00–33.00 (mg·kg−1·fw, HPLC–FD) [84] 64.50 (mg·g−1·dw, HPLC–UV) [82] 0.79–0.91 (mg·g−1·dw, HPLC–DAD) [107] | <0.06 (mg·g−1·dw, HPLC–DAD) [93,108] | – | |
| Procyanidin dimmer B3 | 0.02–31.60 (mg·g−1·dw, HPLC–DAD) [65,81,93] 4.50–22.20 mg·g−1·dw (HPLC–UV) [111] | ≤37.00(mg·kg−1·fw, HPLC–FD) [84] 0.31–0.35 (mg·g−1·dw, HPLC–DAD) [93] | 39.00–56.00 (mg·kg−1·fw, HPLC–FD) [84] 44.60 (mg·g−1·dw, HPLC–UV) [82] 0.11–0.15 (mg·g−1·dw, HPLC–DAD) [107] | <0.06 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Procyanidin dimmer B4 | 0.03–0.05 (mg·g−1·dw, HPLC–DAD) [93] | 8.00 (mg·g−1·dw, HPLC–UV) [82] 0.26–0.30 (mg·g−1·dw, HPLC–DAD) [93] | 40.00–95.00 (mg·kg−1·fw, HPLC–FD) [84] 58.40 (mg·g−1·dw, HPLC–UV) [82] 0.21–0.31 (mg·g−1·dw, HPLC–DAD) [107] | <0.07 (mg g−1 dw, HPLC–DAD) [108] | – | |
| Procyanidin B1-O-gallate | 0.04 (mg·g−1·dw, HPLC–DAD) [93] | 0.31–0.35 (mg·g−1·dw, HPLC–DAD) [93] | 0.66–0.82 (mg·g−1·dw, HPLC–DAD) [107] | – | – | |
| Procyanidin B2-O-gallate | 0.02 (mg·g−1·dw, HPLC–DAD) [93] | 0.27–0.30 (mg·g−1·dw, HPLC–DAD) [93] | 0.66–0.79 (mg·g−1·dw, HPLC–DAD) [107] | <0.14 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Procyanidin trimmer C1 | 0.11–0.19 (mg·g−1·dw, HPLC–DAD) [93] | 0.35 (mg·g−1·dw, HPLC–DAD) [93] | 12.70–31.40 (mg·g−1·dw, HPLC–UV) [82] 0.51–0.61 (mg·g−1·dw, HPLC–DAD) [107] | <0.03 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Procyanidin trimmer C2 | – | – | 18.50 (mg·g−1·dw, HPLC–UV) [82] | <0.04 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Procyanidin trimmer C3 | – | – | 13.50 (mg·g−1·dw, HPLC–UV) [82] | – | – | |
| Galloyled-procyanidin | – | – | – | <0.07 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Gallocatechin-catechin dimer | – | – | – | <0.03 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Procyanidin tetramer | – | – | 1.80–2.20 (mg·g−1·dw, HPLC–UV) [107] | <0.06 (mg·g−1·dw, HPLC–DAD) [108] | ||
| Flavones | ||||||
| Luteolin | 0.01–0.07(µg·g−1·dw, HPLC–DAD) [16] | – | – | – | – | |
| Flavonols | ||||||
| Isrhm-3-O-Glc | – | ≤2.30 (mg·kg-1·fw, HPLC–FD) [84] | – | <0.01 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Kaempferol | 0.04–0.20 (µg·g−1·dw, HPLC–DAD) [81] | 3.2 (mg·g−1·dw, HPLC–UV) [82] | – | – | – | |
| K-3-O-Glc | – | 2.00–26.00 (mg·kg−1·fw, HPLC–FD) [84] 8.40 (mg·g−1·dw, HPLC–UV) [82] 0.10 (mg·g−1·dw, HPLC–UV) [107] | – | <0.02 (mg·g−1·dw, HPLC–DAD) [108] | 34.79–62.04 (mg·g−1·dw, HPLC–DAD) [59] | |
| K-3-O-Gluc | 3.20 (mg·g−1·dw, HPLC–UV) [82] | – | – | <0.01 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Myr-3-O-Glc | – | – | – | – | 1.11–8.50 (mg·g−1·dw, HPLC–DAD) [59] | |
| Quercetin | 0.30–2.20 (µg·g−1·dw, HPLC–DAD) [81] | 0.30 (mg·g−1·dw, HPLC–UV) [107] | – | – | – | |
| Q-3-O-Gal | 13.50–19.20 (µg·g−1·dw, HPLC–DAD) [81] | – | 0.20 (mg·g−1·dw, HPLC–UV) [107] | <0.02 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Q-3-O-Glc | 4.50–7.20 (µg·g−1·dw, HPLC–DAD) [81] | 8.90–66.00 (mg·kg−1·fw, HPLC–FD) [84] 12.40 (mg·g−1·dw, HPLC–UV) [82] | – | <0.11 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Q-3-O-Gluc | – | 12.00–67.00 (mg·kg−1·fw, HPLC–FD) [84] 1.00 (mg·g−1·dw, HPLC–UV) [82] | – | <0.09 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Q-3-O-pentoside | – | 0.20 (mg·g−1·dw, HPLC–UV) [82] | – | – | – | |
| Q-3-O-Rha | 0.30–1.90 (µg·g−1·dw, HPLC–DAD) [81] | – | – | – | – | |
| Q-3-O-Rut | – | 0.40 (mg·g−1·dw, HPLC–UV) [7] | – | <0.02 (mg·g−1·dw, HPLC–DAD) [108] | – | |
| Flavanonols | ||||||
| Astilbin | – | 5.60 (mg·g−1·dw, HPLC–UV) [80] | – | – | – | |
3.2.2. Flavanols
| Compound | Skin | Pomace | Lees |
|---|---|---|---|
| Cy-3-O-Glc Z | ≤0.04 (mg·g−1·dw, HPLC–DAD Y) [99] ≤1.00 (mg·g−1·dw, HPLC–FD) [104] | <0.01 (mg·g−1·dw, HPLC–UV–DAD) [97,99] 0.01–1.79 (mg·g−1·dw, HPLC–DAD) [99] | – |
| Cy-3-O-(6'-O-acetyl)-Glc | – | <0.01 (mg·g−1·dw, HPLC–UV–DAD) [99] | – |
| Del-3-O-Glc | 0.02–0.19 (mg·g−1·dw, HPLC–DAD) [99] ≤4.2 (mg·g−1·dw, HPLC–FD) [104] | ≤1.20 (mg·g−1·dw, HPLC–UV–DAD) [97,99] 0.03–4.24 (mg·g−1·dw, HPLC–DAD) [99] | – |
| Del-3-O-(6'-O-p-coumaryl)-Glc | – | ≤2.2 (mg·g−1·dw, HPLC–UV–DAD) [97] | – |
| Mv-3-O-Glc | 12.10–16.50 (mg·g−1·dw, HPLC–DAD) [99] | 0.06–10.40 (mg·g−1·dw, HPLC–UV–DAD) [97,99] 0.095–14.61 (mg·g−1·dw, HPLC–DAD) [99] | 0.091 (mg·g−1·dw, HPLC–DAD) [61] |
| Mv-3-O-(6'-O-acetyl)-Glc | – | ≤2.0 (mg·g−1·dw, HPLC–UV–DAD) [97,99] | – |
| Mv-3-O-(6'-O-caffeoyl)-Glc | 29.0–59.0 (mg·kg−1·fw, HPLC–FD) [104] | ≤0.3 (mg·g−1·dw, HPLC–UV–DAD) [97,99] | – |
| Mv-3-O-(6'-p-coumaroyl)-Glc | – | ≤27.10 (mg·g−1·dw, HPLC–UV–DAD) [97,99] ≤0.10 (mg·g−1·dw, HPLC–DAD) [99] | 11.7 (mg·g−1·dw, HPLC–DAD) [61] |
| Pn-3-O-Glc | 1.90–7.10 (mg·g−1·dw, HPLC–DAD) [99] 0.30–11.50 (mg·g−1·dw, HPLC–FD) [104] | 0.02 (mg·g−1·dw, HPLC–UV–DAD) [99] 0.10–1.50 (mg·g−1·dw, HPLC–UV–DAD) [97] 0.03–3.47 (mg·g−1·dw, HPLC–DAD) [99] | – |
| Pn-3-O-Gal | 0.02–0.10 (mg·g−1·dw, HPLC–DAD) [99] | – | – |
| Pn-3-O-(6''-O-p-coumaryl)-Glc | – | ≤1.20 (mg·g−1·dw, HPLC–UV–DAD) [97,99] | – |
| Compound | Stem | Skin | Seed | Pomace | Leaf |
|---|---|---|---|---|---|
| trans-Res | ≤0.09–124.10 (mg·g−1·dw, HPLC–DAD Y) [65,81,92,102] | – | ≤0.01 (mg·g−1·dw, HPLC–DAD) [33] | ≤0.06 (mg·g−1·dw, HPLC–DAD) [33] | – |
| trans-Res-3-O-Glc Z | – | – | – | – | ≤96.00 (mg·kg−1·fw, HPLC–UV) [86] |
| cis-Res-3-O-Glc | – | – | – | – | ≤129.00 (mg·kg−1·fw, HPLC–UV) [86] |
| ε-Viniferin | 0.20–49.10 (mg·g−1·dw, HPLC–DAD) [65,81,102] | – | – | – | – |
| Compound | Stem | Skin | Seed | Pomace | Leaf |
|---|---|---|---|---|---|
| trans-Piceid | – | ≤6.90 (mg·g−1·dw, HPLC–UV) [82] | – | – | – |
| trans-Res Z | ≤0.02 (mg·g−1·dw, HPLC–DAD Y) [65,81,92] | ≤1.40 (mg·g−1·dw, HPLC–UV) [82] | – | – | – |
| ε-Viniferin | 12.10–50.10 (mg·g−1·dw, HPLC–DAD) [81] 1.67–4.99 (µg·g−1·dw; HPLC–DAD) [65] | – | – | – | – |
3.2.3. Flavones
3.2.4. Anthocyanins
3.3. Stilbenes
4. Biological Activities and Potential Health Benefits of Winery Wastes Polyphenols
5. Current Extractive Techniques for Bioactive Phytochemicals of Industrial By-Products: Limitations and Possibilities to Improve
| Extractive Procedure | Vinification Residue | Effective Solvent | Target Compound/Assay | Ref. |
|---|---|---|---|---|
| Solvent extraction | Grape pomace | EtOH/Water (6:4, v/v) | Total phenolic compounds | [175] |
| EtOH/Water (7:3, v/v) | Total flavonoid compounds | [175] | ||
| Ethyl acetate/Water (1:1, v/v) | Anthocyanins (aqueous phase) and phenolics (organic phase) | [100] | ||
| Ethyl acetate/water (9:1, v/v) | Total phenolic compounds | [176] | ||
| MeOH/water (7:3, v/v) and EtOH/water (7:3, v/v) | Total phenolic compounds | [177] | ||
| Grape seeds | MeOH/Water/Acetone (3:3.5:3.5, v/v/v) | Total phenolic compounds, total flavonoids, quercetin-3-rutinoside, myricetin | [175] | |
| MeOH/Water (7:3, v/v) | Catechin, epicatechin | [175] | ||
| Grape skins | MeOH/Water/Acetone (3:3.5:3.5, v/v/v) | Total phenolic compounds, total flavonoids, myricetin | [175] | |
| Grape skins | MeOH/Ethyl acetate (1:1, v/v) | Resveratrol | [121] | |
| MeOH/Water (7:3, v/v) | Quercetin and quercetin-3-rutinoside | [175] | ||
| Grape peduncules | MeOH/Water/Acetone (3:3.5:3.5, v/v/v) | Quercetin | [175] | |
| MeOH/Water (7:3, v/v) | Quercetin-3-rutinoside, myricetin, catechin | [175] | ||
| MeOH/Water (6:4, v/v) | Total phenolic compounds, total flavonoids | [175] | ||
| Grape stems | EtOH/Water (6:6, v/v) | Flavones | [52] | |
| EtOH/Water (6:4, v/v) | Flavonols | [52] | ||
| EtOH/Water (4.5:5.5, v/v) | Proanthocyanidins | [52] | ||
| Grape pomace/stems | EtOH/Water (9:1, v/v) | Total phenolic compounds | [176] | |
| Solvent extraction and supercritical fluid extraction | Grape pomace | Ethyl acetate and SFE CO2 | Total phenolic compounds | [49] |
| Grape seeds | EtOH and SFE CO2 | Total phenolic compounds | [182] | |
| Microwave assisted exraction | Grape seeds | 70 W, MeOH | Quercetin, Catechin | [158] |
| Grape skins | 500 W, MeOH:water (6:4, v/v) | Anthocyanins | [183] | |
| Ultrasound assisted extraction | Grape skins | 35 KHz, MeOH/HCl (99:1, v/v) | Anthocyanins, flavan-3-ols, and flavonols | [167] |
| 35 KHz, EtOH/Water (1:1, v/v) | Anthocyanins, total phenolic compounds | [46] | ||
| High pressure and temperature extraction | Grape seeds | Reactor (350 °C/200 bar) | Gallic acid, hydroxytyrosol, vanillic acid, syringic acid, and trans-resveratrol | [184] |
| Aqueous β-cyclodextrins | Grape pomace | 2.5% (w/v) aqueous β-CD solutions | Individuals flavonols, flavan-3-ols, stilbenes, and ortho-diohenols | [109] |
6. Conclusions
7. Future Prospects
Abbreviations
| BHT | Buthylated hydroxytoluene |
| BHA | Buthylated hydroxyanisole |
| Cy | Cyanidin |
| Del | Delphinidin |
| dw | dry weight |
| fw | fresh weight |
| GAE | Galic acid equivalents |
| Gal | Galactoside |
| Glc | Glucoside |
| Gluc | Glucuronide |
| HDL | High-density lipoprotein |
| HPLC–DAD | High Performance Liquid Chromatography–Diode Array Detector |
| HPLC–FD | High Performance Liquid Chromatography–Fluorescence Detector |
| HPLC–UV | High Performance Liquid Chromatography–Ultraviolet Detector |
| HPTE | High pressure and temperature extraction |
| Isrhm | Isorhamntin |
| LDL | Low-density lipoprotein |
| K | Kaempferol |
| MAE | Microwaves assisted extractions |
| Mv | Malvidin |
| Myr | Myricetin |
| Pn | Peonidin |
| Q | Quercetin |
| Res | Resveratrol |
| Rha | Rhamnoside |
| Rut | Rutinoside |
| ROS | Reactive Oxygen Species |
| SFE | Supercritical fluids extraction |
| SLE | Solvent extraction |
| TBHQ | tert-buthylhydroxyquinone |
| UAE | Ultrasound assisted extractions |
| Xyl | Xyloside |
Acknowledgments
Conflicts of Interest
References
- FAOSTAT, Food and Agriculture Organization of the United Nations. Available online: http://faostat3.fao.org/faostat-gateway/go/to/download/Q/QC/E (accessed on 2 March 2014).
- Poudel, P.R.; Tamura, H.; Kataoka, I.; Mochioka, R. Phenolic compounds and antioxidant activities of skins and seeds of five wild grapes and two hybrids native to Japan. J. Food Comp. Anal. 2008, 21, 622–625. [Google Scholar]
- Musee, N.; Lorenzen, L.; Aldrich, C. Cellar waste minimization in the wine industry: A systems approach. J. Clean. Prod. 2007, 15, 417–431. [Google Scholar] [CrossRef]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and chemical variability of grape pomaces from French vineyard. Ind. Crops Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Brito, A.G.; Peixoto, J.; Oliveria, J.M.; Oliveria, J.A.; Costa, C.; Nogueira, R.; Rodrigues, A. Brewery and winery wastewater treatment: Some focal points of design and operation. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer Science + Business Media Llc.: New York, NY, USA, 2007; pp. 109–131. [Google Scholar]
- Saura-Calixto, F. Antioxidant dietary fiber product: A new concept and a potential food ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar]
- Shrikhande, A.J. Wine by-products with health benefits. Food Res. Int. 2000, 33, 469–474. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Fernandez, A.; Pinto, M.G.V.; Butz, P.; Franz, C.M.A.P.; Schuele, E.; Tauscher, B. Characterization of phenolic content, in vitro biological activity, and pesticide loads of extracts from white grape skins from organic and conventional cultivars. Food Chem. Toxicol. 2010, 48, 3471–3476. [Google Scholar] [CrossRef] [PubMed]
- Kataliníc, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Terpinc, P.; Boban, M. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Wine as a biological fluid: history, production, and role in disease prevention. J. Clin. Lab. Anal. 1997, 11, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Trousdale, E. White wine phenolics—Varietal and processing differences as shown by HPLC. Am. J. Enol. Vitic. 1983, 34, 27–34. [Google Scholar]
- Ju, Z.Y.; Howard, L.R. Effects of solvent a temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.Y.; Howard, L.R. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2005, 70, 270–276. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Delgado-Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Pérez-Juan, P.M.; Luque de Castro, M.D. Comparison of accelerated methods for the extraction of phenolic compounds from different vine-shoot cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar]
- Oliveira, D.A.; Salvador, A.A.A.S.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. Food Sci. Technol. 2013, 53, 522–529. [Google Scholar]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Potential uses and applications of treated wine waste. Int. J. Food Sci. Technol. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- Harsha, P.S.C.S.; Gardana, C.; Simonetti, P.; Spigno, G.; Lavelli, V. Characterization of phenolics, in vitro reducing capacity and anti-glycation activity of red grape skins recovered from winemaking by-products. Bioresour. Technol. 2013, 140, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other Agri-food solid waste extracts. J. Food Comp. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Torres, J.L.; Varela, B.; García, M.T.; Carilla, J.; Matito, C.; Centelles, J.J.; Cascante, M.; Sort, X.; Bobet, R. Valorization of grape (Vitis vinifera) by-products. Antioxidant and biological properties of polyphenolic fractions differing in procyanidin composition and flavonol content. J. Agric. Food Chem. 2002, 50, 7548–7555. [Google Scholar]
- Laufenberg, G.; Kunz, B.; Nystroem, M. Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef] [PubMed]
- Panouillé, M.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F. Recovery and reuse of trimmings and pulps from fruit and vegetable processing. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Woodhead Publishing: Abington Hall, UK, 2007; pp. 417–447. [Google Scholar]
- Boussetta, N.; Lanoisellé, J.L.; Bedel-Cloutour, C.; Vorobiev, E. Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: Effect of sulphur dioxide and thermal treatments. J. Food. Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from tem grape varieties and their byproducts: Grape pomace and stems. LWT–Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar]
- Brahim, M.; Gambier, F.; Brosse, N. Optimization of polyphenols extraction from grape residues in water medium. Ind. Crop Prod. 2014, 52, 18–22. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Sánchez, M.; Franco, D.; Sineiro, J.; Magariños, B.; Nuñez, M.J. Antioxidant power, bacteriostatic activity, and characterization of white grape pomace extracts by HPLC–ESI–MS. Eur. Food Res. Technol. 2009, 230, 291–301. [Google Scholar]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Intern. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Shi, J.; Mittal, G.S.; Kakuda, Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep. Purif. Technol. 2006, 48, 176–181. [Google Scholar] [CrossRef]
- Stamatina, K.; Garcia-Viguera, C.; Bridle, P.; Bakker, J. Survey of solvents for the extraction of grape seed phenolics. Phyto. Anal. 1995, 6, 265–267. [Google Scholar] [CrossRef]
- de Campos, L.M.A.S.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R.S. Free radical scavenging of grape pomace extracts from Cabernet Sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar]
- González-Manzano, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Extraction offlavan-3-ols from grape seed and skin into wine using simulated maceration. Anal. Chim. Acta 2004, 513, 283–289. [Google Scholar]
- Ariga, T. The antioxidative function, preventive action on disease and utilization of proanthocyanidins. Biofactors 2004, 21, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Bucić-Kojić, A.; Sovová, H.; Planinić, M.; Tomas, S. Temperature-dependent kinetics of grape seed phenolic compounds extraction: Experiment and model. Food Chem. 2013, 136, 1136–1140. [Google Scholar]
- Furiga, A.; Lonvaud-Funel, A.; Badet, C. In vitro study of antioxidant capacity and antibacterial activity on oral anaerobes of a grape seed extract. Food Chem. 2009, 113, 1037–1040. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M.C. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatgr. A 1998, 823, 31–37. [Google Scholar]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Llobera, A.; Canellas, J. Dietary fiiet content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Souquet, J.M.; Labarbe, B.; Guernevé, C.L.; Cheynier, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Llobera, A.; Canellas, J. Antioxidant activity and dietary fiiet of Prensal Blanc white grape (Vitis vinifera) by-products. Int. J. Food Sci. Technol. 2008, 43, 1953–1959. [Google Scholar] [CrossRef]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Technol. 2004, 92, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Valiente, C.; Arrigoni, C.; Esteban, R.M.; Amado, R. Grape pomace as a potential food fiber. J. Food Sci. 1995, 60, 818–820. [Google Scholar] [CrossRef]
- Cao, X.; Ito, Y. Supercritical fluid extraction of grape seed oil and subsequentseparation of free fatty acids by high-speed counter-current chromatography. J. Chromatogr. A 2003, 1021, 117–124. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kalogeropoulos, N.; Karathanos, V.T. Deployment of response surface methodology to optimise recovery of grape (Vitis vinifera) stem polyphenols. Talanta 2009, 79, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Boskou, G.; Chiou, A.; Andrikopoulos, N.K. An investigation on factors affecting recovery of antioxidant phenolics and anthocyanins from red grape (Vitis vinifera L.) pomace employing water/ethanol-based solutions. Am. J. Food Technol. 2008, 3, 164–173. [Google Scholar]
- Bombardelli, E.; Morrazzoni, P. Vitis vinifera L. Fitoterapia 1995, 66, 291–317. [Google Scholar]
- Felício, J.D.; Rossi, M.H.; Park, H.R.; Gonçalez, E.; Braggio, M.M.; David, J.M.; Cordeiro, I. Biflavonoids from Ouratea multiflora. Fitoterapia 2001, 72, 453–455. [Google Scholar]
- Hebash, K.A.H.; Fadel, H.M.; Soliman, M.M.A. Volatile components of grape leaves. J. Isl. Acad. Sci. 1991, 4, 26–28. [Google Scholar]
- Xia, E.-Q.; Deng, G.-F.; Guom, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P.; Pandurang, A.; Banerjee, K. Phenolic composition and antioxidant activity in grapevine parts and berries (Vitis vinifera L.) cv. Kishmish Chornyi (Sharad Seedless) during maturation. Int. J. Food Sci. Technol. 2006, 41, 1–9. [Google Scholar]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Gurbuz, Y. Determination of nutritive value of leaves of several Vitis vinifera varieties as a source of alternative feedstuff for sheep using in vitro and in situ measurements. Small Rumin. Res. 2007, 71, 59–66. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging. II. Analyses of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar]
- Vattem, D.A.; Shetty, K. Ellagic acid production and phenolic antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus edodes using a solid-state system. Process Biochem. 2003, 39, 367–379. [Google Scholar] [CrossRef]
- Tao, Y.; García, J.F.; Sun, D.W. Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. Food Sci. Technol. 2012, 48, 316–322. [Google Scholar]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chrom. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in food: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71S–88S. [Google Scholar]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals: Sources, Applications and Health Effects; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Pridham, J.B. Phenolics in Plants in Health and Disease; Pergamon Press: New York, NY, USA, 1960. [Google Scholar]
- Bengoechea, M.L.; Sancho, A.I.; Bartolomé, B.; Estrella, I.; Gómez-Cordovés, C.; Hernández, T. Phenolic composition of industrially manufactured Purées and concentrates from peach and apple fruits. J. Agric. Food Chem. 1997, 45, 4071–4075. [Google Scholar]
- Gharras, H.E. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar]
- Berrin, B.; Kassel, G.; Derya, T. Study of polyphenol content in the seeds of red grape (Vitis vinifera L.) verities cultivated in Turkey and their antiradical activity. Food Chem. 2008, 56, 312–327. [Google Scholar]
- Nilgün, G.B.; Gülcan, O.; Osman, S. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 2004, 15, 335–339. [Google Scholar]
- De Freitas, V.A.P.; Glories, Y. Concentration and compositional changes of procyanidins in grape seeds and skin of white Vitis vinifera varieties. J. Sci. Food Agric. 1999, 79, 1601–1606. [Google Scholar]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Changes in grape seed polyphenols during ripening. Phytochemistry 2008, 55, 77–85. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Comp. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Li, D.; Wang, L.J.; Ozkan, N.; Chen, X.D.; Mao, Z.H.; Yang, H.Z. Optimization of ethanol–water extraction of lignans from flaxseed. Sep. Purif. Technol. 2007, 57, 17–24. [Google Scholar] [CrossRef]
- Basha, S.M.; Musingo, M.; Colova Hasna, V.S. Compositional differences in the phenolics compounds of muscadine and bunch grape wines. Afr. J. Biotechnol. 2004, 3, 523–528. [Google Scholar]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Hayes, A.W.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Di Lecce, G.; Arranz, S.; Jáuregui, O.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Lamuela-Raventós, R.M. Phenolic profiling of the skin, pulp and seeds of Albariño grapes using hybrid quadrupole time-of-flight and triple-quadrupole mass spectrometry. Food Chem. 2014, 145, 874–882. [Google Scholar]
- Kallithraka, S.; Salachaa, M.I.; Tzouroua, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Montealegre, P.R.; Peces, R.R.; Vozmediano, J.L.C.; Gascueña, J.M.; Romero, G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Comp. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Singleton, V.L.; Timberlake, C.F.; Lea, A.G.H. The phenolic cinnamates of white grape. J. Sci. Food Agric. 1978, 29, 403–410. [Google Scholar]
- Schoedl, K.; Schumacher, R.; Forneck, A. Studying the polyphenols of grapevine leaves according to age and insertion level under controlled conditions. Sci. Hortic. 2012, 141, 37–41. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agroindustrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Baydar, N.G. Chemical composition of grape canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar]
- Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Han, L.; Suna, B.S. Extraction yields and anti-oxidant activity of proanthocyanidins from different parts of grape pomace: Effect of mechanical treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiaka, R.; Góreckac, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar]
- Jeffery, D.W.; Parker, M.; Smith, P.A. Flavonol composition of Australian red and white wines determined by high-performance liquid chromatography. Aust. J. Grape Wine R. 2008, 14, 153–161. [Google Scholar]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; Tommasi, N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Amico, V.; Napoli, E.M.; Renda, A.; Ruberto, G.; Spatafora, C.; Tringali, C. Constituents of grape pomace from the Sicilian cultivar “Nerello Mascalese”. Food Chem. 2004, 88, 599–607. [Google Scholar] [CrossRef]
- Amico, V.; Chillemi, R.; Mangiafico, S.; Spatafora, C.; Tringali, C. Polyphenol-enriched fractions from Sicilian grape pomace: HPLC–DAD analysis and antioxidant activity. Bioresour. Technol. 2008, 99, 5960–5966. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.; Mayen, M.; Merida, J.; Medina, M. Extraction of phenolic compounds from red grape marc for use as food lipid antioxidants. Food Chem. 1999, 66, 209–215. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Gómez, M.V.; Velders, A.H.; Hermosín-Gutiérrez, I. Flavonol 3-O-glycosides series of Vitis vinifera cv. Petit Verdot red wine grapes. J. Agric. Food Chem. 2009, 57, 209–219. [Google Scholar]
- Spatafora, C.; Barbagallo, E.; Amico, V.; Tringali, C. Grape stems from Sicilian Vitis vinifera cultivars as a source of polyphenol-enriched fractions with enhanced antioxidant activity. Food Sci. Technol. 2013, 54, 542–548. [Google Scholar]
- González-Centeno, M.R.; Jourdes, M.; Fermenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.-L. Proanthocyanidin composition and antioxidant potential of the stem winemaking byproducts from 10 different grape varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different french grape varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vanzela, E.S.; Pierotti Procópio, D.; Filomeno Fontes, E.A.; Mota Ramos, A.; Stringheta, P.C.; da-Silva, R.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Aging of red wines made from hybrid grape cv. BRS Violeta: Effects of accelerated aging conditions on phenolic composition, color and antioxidant activity. Food Res. Int. 2014, 56, 182–189. [Google Scholar]
- Gómez-Plaza, E.; Gil-Munoz, R.; Lopez-Roca, J.M.; Martínez-Cutillas, A.; Fernández-Fernández, J.I. Phenolic compounds and color stability of red wines: Effect of skin maceration time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar]
- Jara-Palacios, M.J.; González-Manzano, S.; Escudero-Gilete, M.L.; Hernanz, D.; Dueñas, M.; González-Paramás, A.M.; Heredia, F.J.; Santos-Buelga, C. Study of zalema grape pomace: Phenolic composition and biological effects in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 5114–5121. [Google Scholar]
- Ratnasooriya, C.C.; Rupasinghe, H.P.V. Extraction of phenolic compounds from grapes and their pomace using β-cyclodextrin. Food Chem. 2012, 134, 625–631. [Google Scholar] [CrossRef]
- Guendez, R.; Kallithraka, S.; Makris, D.P.; Kefalas, P. Determination of low molecular weight phenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chem. 2005, 89, 1–9. [Google Scholar]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4'-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Weber, H.A.; Hodges, A.E.; Guthrie, J.R.; O’Brien, B.M.; Robaugh, D.; Clark, A.P.; Harris, R.K.; Algaier, J.W.; Smith, C.S. Comparison of proanthocyanidins in commercial antioxidants: Grape seed and pine bark extracts. J. Agric. Food Chem. 2007, 55, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Mez-Cordoveä, C.G.; Bartolomeä, B.; Laureano, O.; Ricardo da Silva, J.M. Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis Vinifera L. cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6781. [Google Scholar]
- De Freitas, V.A.P.; Glories, Y.; Monique, A. Development changes of procyanidines in grapes of red Vitis vinifera varieties and their composition in respective wines. Am. J. Enol. Vitic. 2000, 51, 397–403. [Google Scholar]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 13, 859–871. [Google Scholar]
- Teixeira Barcia, M.; Becker Pertuzatti, P.; Rodrigues, D.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; Teixeira Godoy, H. Occurrence of low molecular weight phenolics in Vitis vinifera red grape cultivars and their winemaking by-products from São Paulo (Brazil). Food Res. Int. 2014, 62, 500–513. [Google Scholar]
- Pérez-Serradilla, J.A.; Castro de Luque, M.D. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar]
- Liu, C.; Wang, L.; Wang, J.; Wu, B.; Liu, W.M.; Fan, P.; Liang, Z.; Li, S. Resveratrols in Vitis berry skins and leaves: Their extraction and analysis by HPLC. Food Chem. 2013, 136, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.T.R.; Waffo-Téguo, P.; Teissedre, P.L.; Pujolas, A.; Vercauteren, J.; Cabanis, J.C.; Mérillon, J.M. Determination of stilbenes (trans-astringin, cis- and trans-piceid, and cis- and trans-resveratrol) in Portuguese wines. J. Agric. Food Chem. 1999, 47, 2666–2670. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Labanda, J.F.; Mallavia, R.; Pérez-Fons, L.; Lizama, V.; Saura, D.; Micol, V. Determination of piceid and resveratrol in Spanish wines deriving from Monastrell (Vitis vinifera L.) grape variety. J. Agric. Food Chem. 2004, 52, 5396–5403. [Google Scholar]
- Püssa, T.; Floren, J.; Kuldkepp, P.; Raal, A. Survey of grapevine Vitis vinifera stem polyphenols by liquid chromatography-diode array detection-tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 7488–7494. [Google Scholar]
- Gonzalez-Barrio, R.; Beltran, D.; Cantos, E.; Gil, M.; Espin, J.; Tomas-Barberan, F. Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. “Superior” white table grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar]
- Li, X.; Wu, B.; Wang, L.; Li, S. Extractable amounts of trans-resveratrol in seed and berry skin in Vitis evaluated at the germplasm level. J. Agric. Food Chem. 2006, 54, 8804–8811. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Das, D.K. Physiological effects of resveratrol. Biofactors 2010, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.E.; Ozvural, E.B.; Vural, H. Extraction and identification of proanthocyanidins from grape seed (Vitis vinifera) using supercritical carbon dioxide. J. Supercrit. Fluids 2011, 55, 924–928. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [PubMed]
- Proestos, C.; Bakogiannis, A.; Psarianos, C.; Koutinas, A.A.; Kanellaki, M.; Komaitis, M. High performance liquid chromatography analyses of phenolic substances in Greek. Food Control. 2005, 16, 319–323. [Google Scholar] [CrossRef]
- Ames, B.N. Endogenous oxidative DNA damage, aging and cancer. Free Rad. Res. Commun. 1989, 7, 121–122. [Google Scholar] [CrossRef]
- Willet, W.C. Diet and health: What should we eat? Science 1994, 264, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Rimm, E.B.; Stampfer, M.J.; Asherio, A.; Giovannucci, E.; Colditz, G.A.; Willett, W.C. Vitamin E consumption and the risk of coronary heart disease in men. N. Engl. J. Med. 1993, 328, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Geibicki, J.; Puhl, H.; Jürgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic. Biol. Med. 1992, 13, 341–390. [Google Scholar] [CrossRef] [PubMed]
- Jalial, I.; Grundy, S.M. Effects of dietary supplementation with a-tocopherol on the oxidative modification of low-density lipoprotein. J. Lipid Res. 1992, 33, 899–906. [Google Scholar] [PubMed]
- Kinsella, J.E.; Frankel, E.; German, B.; Kanner, J. Possible mechanism for the protective role of the antioxidant in wine and plant foods. Food Technol. 1993, 47, 85–89. [Google Scholar]
- Jakobek, L.; Sěruga, M.; Sěruga, B.; Novak, I.; Medvidović-Kosanovic, M. Phenolic compound composition and antioxidant activity of fruits of Rubus and Prunus species from Croatia. Int. J. Food Sci. Technol. 2009, 44, 860–868. [Google Scholar] [CrossRef]
- Jacob, J.K.; Hakimuddin, F.; Paliyath, G.; Fisher, H. Antioxidant and antiproliferative activity of polyphenols in novel high-polyphenol grape lines. Food Res. Int. 2008, 41, 419–428. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheena, V.S.; Luximon-Rammaa, A.; Aruomab, O.I.; Bahoruna, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Di Majo, D.; Guardia, M.; Giammanco, S.; Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar]
- Spranger, I.; Sun, B.S.; Mateus, A.M.; de Freitas, V.; Ricardo-da-Silva, A.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef]
- Dajanta, K.; Janpum, P.; Leksing, W. Antioxidant capacities, total phenolics and flavonoids in black and yellow soybeans fermented by Bacillus subtilis: A comparative study of Thai fermented soybeans (thua nao). Int. Food Res. J. 2013, 20, 3125–3132. [Google Scholar]
- Bobek, P. Dietary tomato and grape pomace in rats: Effect on lipids in serum and liver, and on antioxidant status. Br. J. Biomed. Sci. 1999, 56, 109–113. [Google Scholar] [PubMed]
- Dohadwala, M.M.; Vita, J.A. Grapes and cardiovascular disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Young, C.J.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Moreno, D.A.; Ilic, N.; Poulev, A.; Brasaemle, D.L.; Fried, S.K.; Raskin, I. Inhibitory effects of grape seed extract on lipases. Nutrition 2003, 19, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Oda, E.; Yamashita, T.; Naemura, A.; Yamakoshi, J.; Yamamoto, J. Anti-thrombotic effect of proanthocyanidin, a purified ingredient of grape seed. Thromb. Res. 2005, 115, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuscinski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract, importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Sarala Bai, B.R.; Devaraj, S.N. Cardioprotective effect of grape seed procyanidins on isoproernol-induced myocardial injury in rats. J. Cardiovasc. Pharm. 2009, 53, 109–115. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B.; Tomczak, A.; Erler, J.; Stochmal, A.; Oleszek, W. Comparative antiplatelet and antioxidant properties of polyphenol-rich extracts from: Berries of Aronia melanocarpa, seeds of grape and bark of Yucca schidigera in vitro. Platelets 2008, 19, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; Rodiño-Janeiro, B.K.; Jerez, M.; Ucieda-Somoza, R.; Núñez, M.J.; González-Juanatey, J.R. Procyanidins from grape pomace are suitable inhibitors of human endothelial NADPH oxidase. J. Cell Biochem. 2012, 113, 1386–1396. [Google Scholar]
- Jayaprakasha, G.K.; Selvi, T.; Sakaria, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Mendoza, L.; Yañez, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Ind. Crops Prod. 2013, 43, 360–364. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Chorianopoulos, N.G.; Nychas, G.J.E.; Haroutounian, S.A. Antilisterial activities of polyphenol-rich extracts of grapes and vinification byproducts. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.J.R.; Alberto, M.R.; Manca de Nadra, M.C. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Rhodes, P.L.; Mitchell, J.W.; Wilson, M.W.; Melton, L.D. Antilisterial activity of grape juice and grape extracts derived from Vitis vinifera variety Ribier. Int. J. Microb. 2006, 107, 281–286. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Mustapha, A. Antimicrobial and antioxidant activities of natural extracts in vitro and in ground beef. J. Food Prot. 2004, 67, 148–155. [Google Scholar] [PubMed]
- Ahn, J.; Grün, I.U.; Mustapha, A. Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Signh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 26, 1533–1538. [Google Scholar]
- Mitjans, M.; del Campo, J.; Abajo, C.; Martínez, V.; Selga, A.; Lozano, C.; Torres, J.L.; Vinardell, M.P. Immunomodulatory activity of a new family of antioxidants obtained from Grape polyphenols. J. Agric. Food Chem. 2004, 52, 7297–7299. [Google Scholar] [PubMed]
- Li, X.L.; Cai, Y.Q.; Qin, H.; Wu, Y.J. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can. J. Physiol. Pharmacol. 2008, 86, 841–849. [Google Scholar] [PubMed]
- Cheah, K.Y.; Bastian, S.E.; Acott, T.M.; Abimosleh, S.M.; Lymn, K.A.; Howarth, G.S. Grape seed extract reduces the severity of selected disease markers in the proximal colon of dextran sulphate sodium-induced colitis in rats. Dig. Dis. Sci. 2013, 58, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Ge, B.; Yang, X.L.; Zhai, J.; Yang, L.N.; Wang, X.X.; Liu, X.; Shi, J.C.; Wu, Y.J. Proanthocyanidins from grape seeds modulates the nuclear factor-κB signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int. Immunopharmacol. 2011, 11, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Yang, X.L.; Wang, L.; Cui, M.X.; Cai, Y.Q.; Li, X.L.; Wu, Y.J. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can. J. Physiol. Pharmacol. 2010, 88, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.W. Hypertension and the pathogenesis of atherosclerosis: Oxidative stress and the mediation of arterial inflammatory response: A new perspective. Hypertension 1995, 25, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, S.B.; Bush, A.J.; Pahor, M.; Gross, M.D. Serum carotenoids and markers of inflammation in nonsmokers. Am. J. Epidemiol. 2000, 152, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, F.; Valtuena, S.; Pellegrini, N.; Ardigo, D.; del Rio, D.; Salvatore, S.; Piatti, P.; Serafini, M.; Zavaroni, I. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br. J. Nutr. 2005, 93, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Y.M.; Fratkins, J.; Leblanc, M. Grape seed extract suppresses lipid peroxidation and reduces hypoxic ischemic brain injury in neonatal rats. Brain Res. Bull. 2005, 66, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Balu, M.; Sangeetha, P.; Murali, G.; Panneerselvam, C. Modulatory role of grape seed extract on age-related oxidative DNA damage in central nervous system of rats. Brain Res. Bull. 2006, 68, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Markus, M.A.; Morris, B.J. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin. Interv. Aging 2008, 3, 331–339. [Google Scholar] [PubMed]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Agustin-Salazar, S.; Medina-Juárez, L.A.; Soto-Valdez, H.; Manzanares-López, F.; Gámez-Meza, N. Influence of the solvent system on the composition of phenolic substances and antioxidant capacity of extracts of grape (Vitis vinifera L.) marc. Aust. J. Grape Wine R. 2014. [Google Scholar] [CrossRef]
- Spigno, G.; de Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the evaluation of the substances currently on the list in the Annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils—Part I of III. EFSA J. 2011, 9, 2482. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Toxicological evaluation of some extraction solvents and certain other substances. In Proceedings of the FAO Nutrition Meetings; WHO: Geneva, Switzerland, 1970. [Google Scholar]
- Cruz, J.M.; Domínguez, H.; Parajó, J.C. Assessment of the production of antioxidants from winemaking waste solids. J. Agric. Food Chem. 2004, 52, 5612–5620. [Google Scholar] [CrossRef] [PubMed]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. Study of solid–liquid extraction kinetics of total polyphenols from grape seeds. J. Food Eng. 2007, 81, 236–242. [Google Scholar]
- Marqués, J.L.; della Porta, G.; Reverchon, E.; Renuncio, J.A.R.; Mainar, A.M. Supercritical antisolvent extraction of antioxidants from grape seeds after vinification. J. Supercrit. Fluid 2013, 82, 238–243. [Google Scholar]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Alonso, E.; Revilla, E.; Kovac, V.; Pekic, B. Study of the extraction of proanthocyanidins from grape seeds. Food Chem. 1998, 61, 201–206. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; de Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Teixeira, A.; Rosa, E.; Barros, A. Assessment of (poly)phenols in grape (Vitis vinifera L.) stems by using food/pharma industry compatible solvents and response surface methodology. Food Chem. 2014, 164, 339–346. [Google Scholar]
- Novak, I.; Janeiro, P.; Seruga, M.; Oliveira-Brett, A.M. Ultrasound extracted flavonoids from four varieties of Portuguese red grape skins determined by reverse-phase high-performance liquid chromatography with electrochemical detection. Anal. Chim. Acta 2008, 630, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Concota, A.; Cincota, A.H. For threapy of atherosclerotic cardiovascular disease. U.S. Patent 10 October 2002. [Google Scholar]
- Bekhit, A.E.D.A.; Cheng, V.J.; McConnell, M.; Zhao, J.H.; Sedcole, R.; Harrison, R. Antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion. Food Chem. 2011, 129, 837–845. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Characterization and Functional and Safety Evaluation of Bioactive Compounds from Iberian-American Fruits for Food Ingredients (CORNUCOPIA). Thematic Network CYTED Ref. 112RT0460. Available online: http://redcornucopia.org (accessed on 2 September 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638-15678. https://doi.org/10.3390/ijms150915638
Teixeira A, Baenas N, Dominguez-Perles R, Barros A, Rosa E, Moreno DA, Garcia-Viguera C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. International Journal of Molecular Sciences. 2014; 15(9):15638-15678. https://doi.org/10.3390/ijms150915638
Chicago/Turabian StyleTeixeira, Ana, Nieves Baenas, Raul Dominguez-Perles, Ana Barros, Eduardo Rosa, Diego A. Moreno, and Cristina Garcia-Viguera. 2014. "Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review" International Journal of Molecular Sciences 15, no. 9: 15638-15678. https://doi.org/10.3390/ijms150915638