Multistep Model of Cervical Cancer: Participation of miRNAs and Coding Genes
Abstract
:1. Introduction
2. Altered miRNAs Expression in Cervical Carcinomas
| Name of miRNA | Expression Level | Technic | Type of Tissue | Reference |
|---|---|---|---|---|
| miR-1 | Down | Microarray | Cancer | [23] |
| Down | Cloning and Sequencing | Cancer | [35] | |
| miR-7 | Up | Microarray | Cancer | [23] |
| Down | RT-PCR | Cancer | [36] | |
| Up | Cloning and Sequencing | Cancer | [35] | |
| miR-9 | Up | RT-PCR | Cancer | [25] |
| Up | RT-PCR | CIN 2, 3 and cancer | [37] | |
| miR-10a | Up | Microarray | CIN 1, 3 and Cancer | [38] |
| Up | RT-PCR | CIN 2, 3 and Cancer | [37] | |
| Up | RT-PCR | Cancer | [39] | |
| miR-10b | Down | Microarray | Cancer | [23] |
| Up | Microarray | Cancer | [32] | |
| Down | Cloning and Sequencing | Cancer | [35] | |
| miR-15a | Up | Microarray | Cancer | [32] |
| Up | Microarray and RT-PCR | Cancer | [40] | |
| miR-15b | Up | Cloning and Sequencing | Cancer | [28] |
| Up | Microarray | Cancer | [33] | |
| Up | Microarray | Cancer | [34] | |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-16 | Up | Cloning and Sequencing | Cancer | [28] |
| Down | Microarray | CIN 1, 3 and Cancer | [38] | |
| Up | Microarray | Cancer | [32] | |
| Up | RT-PCR | CIN 1, 2, 3 and Cancer | [41] | |
| miR-17-5p | Up | Microarray | Cancer | [32] |
| Down | RT-PCR | Cancer | [37] | |
| Down | RT-PCR | Cancer | [42] | |
| miR-19a/b | Up | Microarray | CIN 2, 3 and Cancer | [22] |
| Up | RT-PCR | Cancer | [43] | |
| miR-20a | Up | Microarray | Cancer | [23] |
| Up | RT-PCR | Cancer | [44] | |
| Up | Microarray and RT-PCR | Cancer | [45] | |
| Up | RT-PCR | Cancer | [46] | |
| miR-20b | Up | Microarray | Cancer | [23] |
| Up | Microarray | Cancer | [32] | |
| Up | Microarray and RT-PCR | Cancer | [40] | |
| Up | RT-PCR | CIN 2, 3 and Cancer | [37] | |
| miR-21 | Up | Cloning | Cancer | [27] |
| Up | Microarrays and RT-PCR | Cancer | [33] | |
| Up | Northern blot and Microarray | Cancer | [47] | |
| Up | Microarray | Cancer | [32] | |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-23b | Down | Cloning | Cancer | [27] |
| Down | RT-PCR | Cancer | [48] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-26a | Down | Microarray | CIN 1, 3 and Cancer | [38] |
| Down | Microarray and RT-PCR | Cancer | [45] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-27a | Down | Microarray and RT-PCR | CIN 1, 3 and Cancer | [38] |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| Up | RT-PCR | CIN 1, 2, 3 and Cancer | [41] | |
| miR-27b | Down | Microarray | CIN 2, 3 and Cancer | [22] |
| Down | Microarray | Cancer | [45] | |
| miR-29a | Down | Microarray and RT-PCR | CIN 2, 3 and Cancer | [49] |
| Down | Microarray | CIN 1, 3 and Cancer | [38] | |
| Down | RT-PCR | CIN 1, 2, 3 and Cancer | [41] | |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-31 | Up | Northern blot and Microarray | Cancer | [47] |
| Up | Microarray and RT-PCR | Cancer | [33] | |
| Up | Microarray and Northern Blot | Cancer | [34] | |
| Up | Microarray | Cancer | [23] | |
| Up | Cloning and Sequencing | Cancer | [35] | |
| miR-34a | Down | Northern Blot | Cancer | [21] |
| Down | Microarray and RT-PCR | Cancer | [45] | |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| Down | RT-PCR | CIN 1, 2, 3 and Cancer | [50] | |
| miR-92a | Up | Microarray and RT-PCR | CIN 2, 3 and Cancer | [49] |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| Up | RT-PCR | CIN 1, 2, 3 and Cancer | [41] | |
| miR-93 | Up | Microarray | Cancer | [23] |
| Up | Microarray | Cancer | [32] | |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| Up | RT-PCR | Cancer | [31] | |
| miR-99a | Down | Microarrays | CIN 1, 3 and Cancer | [38] |
| Down | Microarray and RT-PCR | CIN 2, 3 and Cancer | [49] | |
| Down | Microarray | Cancer | [23] | |
| Down | Microarray | Cancer | [32] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-99b | Down | Microarray | Cancer | [23] |
| Down | Cloning and Sequencing | Cancer | [35] | |
| miR-100 | Down | RT-PCR | CIN 1, 2, 3 and Cancer | [51] |
| Down | Microarray | Cancer | [32] | |
| Down | Microarray | Cancer | [23] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| Down | RT-PCR | CIN 1, 2, 3 and Cancer | [41] | |
| miR-106b | Up | Microarray | Cancer | [32] |
| Up | Microarray | CIN 2,3 and Cancer | [22] | |
| Up | Microarray and RT-PCR | Cancer | [40] | |
| miR-125a-5p | Up | Microarray | Cancer | [34] |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| Down | Microarray and RT-PCR | Cancer | [45] | |
| miR-125b | Down | Microarray | Cancer | [32] |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| Down | Microarray and RT-PCR | Cancer | [45] | |
| Down | Cloning and Sequencing | Cancer | [35] | |
| miR-133a | Up | RT-PCR | Cancer | [25] |
| Up | Microarray, in situ Hybridization and RT-PCR | CIN 2, 3 and Cancer | [52] | |
| Up | RT-PCR | Cancer | [25] | |
| miR-133b | Up | RT-PCR | Cancer | [25] |
| Up | Microarray, in situ Hybridization and RT-PCR | CIN 2, 3 and Cancer | [52] | |
| Up | RT-PCR | Cancer | [25] | |
| miR-143 | Down | Cloning and Northern Blot | Cancer | [28] |
| Down | Microarrays, RT-PCR and Northern Blot | CIN 3 and Cancer | [26] | |
| Down | Microarrays | Cancer | [23] | |
| Down | Microarray and RT-PCR | Cancer | [45] | |
| Down | Microarray and RT-PCR | Cancer | [53] | |
| Down | Cloning and Sequencing | Cancer | [35] | |
| Down | Microarray | CIN 1, 3 and cancer | [38] | |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-145 | Up | RT-PCR | Cancer | [25] |
| Down | Microarray and Northern Blot | CIN 3 and Cancer | [26] | |
| Down | Cloning, Microarray and Northern Blot | Cancer | [28] | |
| Down | Microarray | Cancer | [32] | |
| Down | Microarray | Cancer | [23] | |
| Down | Microarray and RT-PCR | Cancer | [45] | |
| Down | RT-PCR | Cancer | [54] | |
| Down | Microarray | CIN 1, 3 and cancer | [38] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-146a | Up | Cloning and Northern Blot | Cancer | [28] |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| Up | RT-PCR | Cancer | [55] | |
| miR-146b-5p | Up | Microarray | Cancer | [23] |
| Up | Microarray | Cancer | [32] | |
| miR-155 | Up | Cloning | Cancer | [28] |
| Up | Microarray and RT-PCR | CIN 2, 3 and Cancer | [49] | |
| Up | Microarray | Cancer | [32] | |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| Up | Cloning and Sequencing | Cancer | [35] | |
| miR-191 | Down | Northern Blot and Microarray | Cancer | [47] |
| Up | Microarray | Cancer | [34] | |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-193b | Down | RT-PCR and Microarray | Cancer | [33] |
| Down | RT-PCR | CIN 2, 3 and Cancer | [37] | |
| Up | RT-PCR, Microarray and Northern Blot | CIN 3 and Cancer | [26] | |
| miR-195 | Down | RT-PCR, Microarray and Northern Blot | CIN 3 and Cancer | [26] |
| Down | Microarray and RT-PCR | CIN 2, 3 and Cancer | [49] | |
| Down | Microarray | Cancer | [32] | |
| Down | Microarray | Cancer | [23] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-196b | Down | RT-PCR | Cancer | [56] |
| Down | Cloning | Cancer | [27] | |
| miR-199a | Up | RT-PCR | Cancer | [25] |
| Down | Microarray | CIN 1, 3 and cancer | [38] | |
| Down | Microarray | Cancer | [32] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| Up | RT-PCR | Cancer | [25] | |
| miR-200a | Up | Microarray | Cancer | [23] |
| Up | RT-PCR | Cancer | [31] | |
| miR-200a* | Up | Microarray | CIN 2, 3 and Cancer | [22] |
| Up | Cloning and Sequencing | Cancer | [35] | |
| miR-200c | Up | Microarray | Cancer | [34] |
| Up | Microarray | CIN 2, 3 and Cancer | [22] | |
| Down | RT-PCR | Cancer | [45] | |
| Up | Microarray | Cancer | [23] | |
| miR-203 | Down | RT-PCR | Cancer | [28] |
| Up | Microarray | Cancer | [33] | |
| Down | Microarrays | CIN 1, 3 and cancer | [38] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| Down | RT-PCR | Cancer | [57] | |
| Down | RT-PCR | Cancer | [46] | |
| Down | RT-PCR | CIN 2, 3 and Cancer | [37] | |
| Up | Cloning and Sequencing | Cancer | [35] | |
| Down | RT-PCR | Cancer | [25] | |
| miR-205 | Down | Microarray | CIN 1, 3 and cancer | [38] |
| Up | RT-PCR | Cancer | [58] | |
| Up | RT-PCR | Cancer | [35] | |
| Up | RT-PCR, Microarray and Northern Blot | CIN 3 and Cancer | [26] | |
| miR-210 | Up | RT-PCR, Microarray and Northern Blot | CIN 3 and Cancer | [26] |
| Up | Microarray | Cancer | [23] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| miR-214 | Down | Northern Blot and Microarray | Cancer | [47] |
| Down | Microarray | Cancer | [23] | |
| Down | RT-PCR | Cancer | [59] | |
| Up | RT-PCR | Cancer | [25] | |
| miR-218 | Down | RT-PCR, Microarray and Northern Blot | CIN 3 and Cancer | [26] |
| Down | Microarray | Cancer | [23] | |
| Down | RT-PCR | Cancer | [60] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| Down | RT-PCR | Cancer | [61] | |
| Down | RT-PCR | CIN 1, 2, 3 and Cancer | [62] | |
| miR-224 | Up | Microarray | Cancer | [23] |
| Up | RT-PCR | Cancer | [63] | |
| miR-375 | Down | Microarray | Cancer | [32] |
| Down | Microarrays and RT-PCR | CIN 2, 3 and Cancer | [49] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] | |
| Down | RT-PCR | CIN 2, 3 and Cancer | [64] | |
| Down | Microarray and RT-PCR | Cancer | [45] | |
| miR-424 | Down | RT-PCR | CIN 1, 2, 3 and Cancer | [65] |
| Down | RT-PCR | Cancer | [66] | |
| Down | RT-PCR | Cancer | [37] | |
| miR-497 | Down | Microarray | Cancer | [23] |
| Down | Microarray | Cancer | [32] | |
| Down | Microarray | CIN 2, 3 and Cancer | [22] |
3. miRNAs Implicated in Cervical Cancer Progression
4. miRNAs Regulated by HPV Oncoproteins
5. Aberrant miRNAs Expression in Cellular Processes
5.1. MicroRNAs Involved in Proliferation
5.2. Cell Cycle and Apoptosis
5.3. Migration and Invasion
6. Construction of a Multistep Model of Carcinogenesis by Expression of miRNAs and Their Targets
6.1. MicroRNAs Misregulated in Step 1
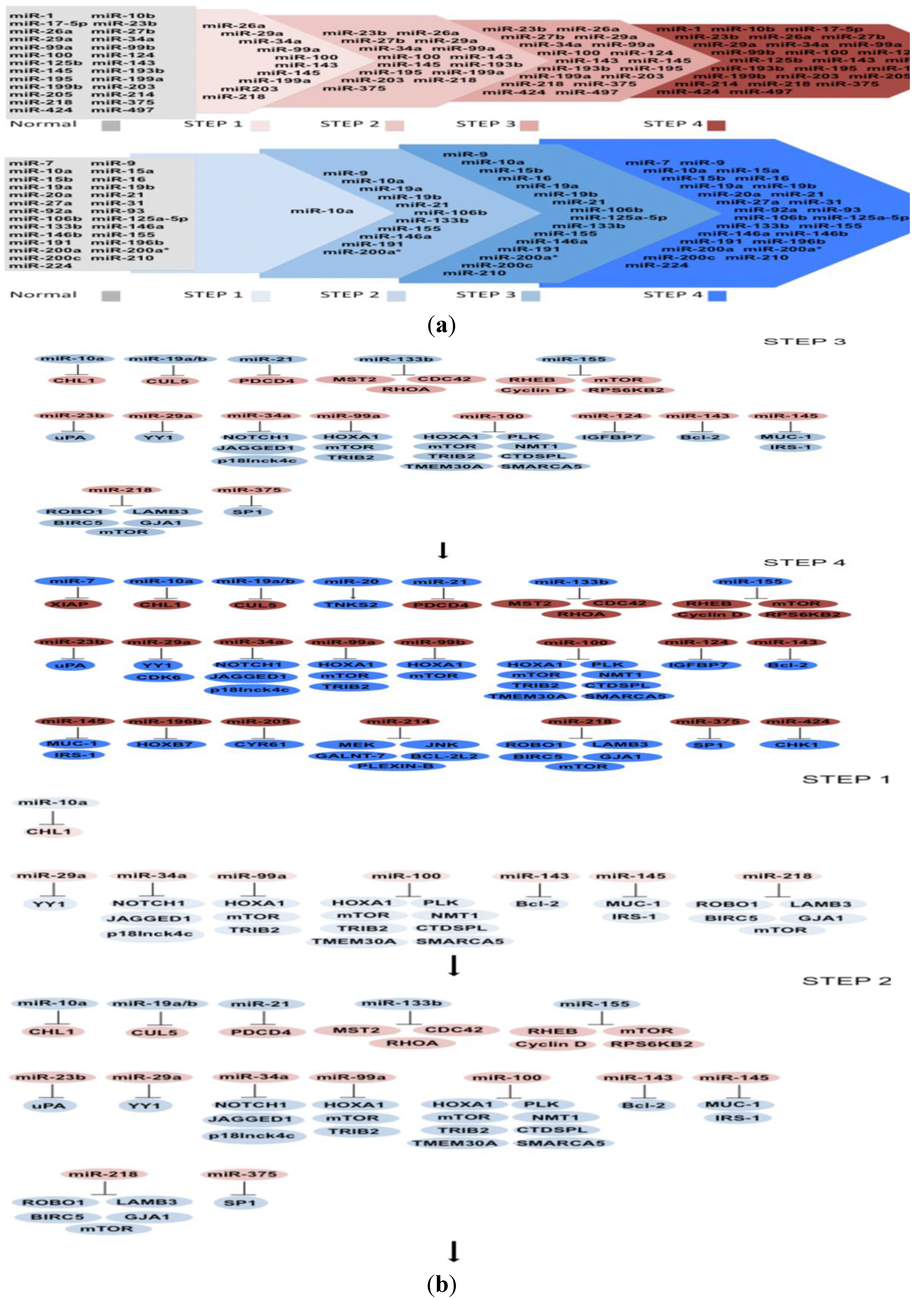
6.2. MicroRNAs Misregulated in Step 2
6.3. MicroRNAs Misregulated in Step 3
6.4. MicroRNAs Misregulated in Step 4
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 2001, 94, 153–156. [Google Scholar] [CrossRef]
- Chakrabarti, O.; Krishna, S. Molecular interactions of “high risk” human papillomaviruses E6 and E7 oncoproteins: implications for tumour progression. J. Biosci. 2003, 28, 337–348. [Google Scholar] [CrossRef]
- DiMaio, D.; Liao, J.B. Human papillomaviruses and cervical cancer. Adv. Virus Res. 2006, 66, 125–159. [Google Scholar] [CrossRef]
- Dyson, N.; Howley, P.M.; Munger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Miller, D.M.; Blume, S.; Borst, M.; Gee, J.; Polansky, D.; Ray, R.; Rodu, B.; Shrestha, K.; Snyder, R.; Thomas, S.; et al. Oncogenes, malignant transformation, and modern medicine. Am. J. Med. Sci. 1990, 300, 59–69. [Google Scholar] [CrossRef]
- Weinberg, R.A. Tumor suppressor genes. Science 1991, 254, 1138–1146. [Google Scholar]
- Lim, P.K.; Bliss, S.A.; Patel, S.A.; Taborga, M.; Dave, M.A.; Gregory, L.A.; Greco, S.J.; Bryan, M.; Patel, P.S.; Rameshwar, P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011, 71, 1550–1560. [Google Scholar] [CrossRef]
- Rechavi, O.; Erlich, Y.; Amram, H.; Flomenblit, L.; Karginov, F.V.; Goldstein, I.; Hannon, G.J.; Kloog, Y. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev. 2009, 23, 1971–1979. [Google Scholar]
- Dalmay, T.; Edwards, D.R. MicroRNAs and the hallmarks of cancer. Oncogene 2006, 25, 6170–6175. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. MiRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar]
- Grimson, A.; Farh, K.K.; Johnston, W.K.; Garrett-Engele, P.; Lim, L.P.; Bartel, D.P. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol. Cell 2007, 27, 91–105. [Google Scholar] [CrossRef]
- Baek, D.; Villen, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Selbach, M.; Schwanhausser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef]
- Lopez, J.A.; Alvarez-Salas, L.M. Differential effects of miR-34c-3p and miR-34c-5p on SiHa cells proliferation apoptosis, migration and invasion. Biochem. Biophys. Res. Commun. 2011, 409, 513–519. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.; Luan, X.; Li, Y.; Liu, M.; Li, X.; Liu, T.; Tang, H. MicroRNA-214 is aberrantly expressed in cervical cancers and inhibits the growth of HeLa cells. IUBMB Life 2009, 61, 1075–1082. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.K.; McCoy, J.P.; Banerjee, N.S.; Rader, J.S.; Broker, T.R.; Meyers, C.; Chow, L.T.; Zheng, Z.M. Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA 2009, 15, 637–647. [Google Scholar] [CrossRef]
- Wilting, S.M.; Snijders, P.J.; Verlaat, W.; Jaspers, A.; van de Wiel, M.A.; van Wieringen, W.N.; Meijer, G.A.; Kenter, G.G.; Yi, Y.; le Sage, C.; et al. Altered microRNA expression associated with chromosomal changes contributes to cervical carcinogenesis. Oncogene 2013, 32, 106–116. [Google Scholar] [CrossRef]
- Rao, Q.; Shen, Q.; Zhou, H.; Peng, Y.; Li, J.; Lin, Z. Aberrant microRNA expression in human cervical carcinomas. Med. Oncol. 2012, 29, 1242–1248. [Google Scholar] [CrossRef]
- Kumar, M.S.; Lu, J.; Mercer, K.L.; Golub, T.R.; Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 2007, 39, 673–677. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, C.H.; Choi, J.J.; Park, Y.A.; Kim, S.J.; Hwang, S.Y.; Kim, W.Y.; Kim, T.J.; Lee, J.H.; Kim, B.G.; et al. Altered microRNA expression in cervical carcinomas. Clin. Cancer Res. 2008, 14, 2535–2542. [Google Scholar]
- Martinez, I.; Gardiner, A.S.; Board, K.F.; Monzon, F.A.; Edwards, R.P.; Khan, S.A. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene 2008, 27, 2575–2582. [Google Scholar] [CrossRef]
- Lui, W.O.; Pourmand, N.; Patterson, B.K.; Fire, A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007, 67, 6031–6043. [Google Scholar] [CrossRef]
- Wang, X.; Tang, S.; Le, S.Y.; Lu, R.; Rader, J.S.; Meyers, C.; Zheng, Z.M. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One 2008, 3, e2557. [Google Scholar]
- Reshmi, G.; Chandra, S.S.; Babu, V.J.; Babu, P.S.; Santhi, W.S.; Ramachandran, S.; Lakshmi, S.; Nair, A.S.; Pillai, M.R. Identification and analysis of novel microRNAs from fragile sites of human cervical cancer: computational and experimental approach. Genomics 2011, 97, 333–340. [Google Scholar]
- Li, J.H.; Xiao, X.; Zhang, Y.N.; Wang, Y.M.; Feng, L.M.; Wu, Y.M.; Zhang, Y.X. MicroRNA miR-886-5p inhibits apoptosis by down-regulating Bax expression in human cervical carcinoma cells. Gynecol. Oncol. 2011, 120, 145–151. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Li, H.L.; Han, L.Y. Expression of miR-200a, miR-93, metastasis-related gene RECK and MMP2/MMP9 in human cervical carcinoma—Relationship with prognosis. Asian Pac. J. Cancer Prev. 2013, 14, 2113–2118. [Google Scholar] [CrossRef]
- Lajer, C.B.; Garnaes, E.; Friis-Hansen, L.; Norrild, B.; Therkildsen, H.M.; Glud, M.; Rossing, M.; Lajer, H.; Svane, D.; Skotte, L.; et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: Bridging between HPV-related head and neck cancer and cervical cancer. Br. J. Cancer 2012, 106, 1526–1534. [Google Scholar] [CrossRef]
- Muralidhar, B.; Goldstein, L.D.; Ng, G.; Winder, D.M.; Palmer, R.D.; Gooding, E.L.; Barbosa-Morais, N.L.; Mukherjee, G.; Thorne, N.P.; Roberts, I.; et al. Global microRNA profiles in cervical squamous cell carcinoma depend on Drosha expression levels. J. Pathol. 2007, 212, 368–377. [Google Scholar] [CrossRef]
- Muralidhar, B.; Winder, D.; Murray, M.; Palmer, R.; Barbosa-Morais, N.; Saini, H.; Roberts, I.; Pett, M.; Coleman, N. Functional evidence that Drosha overexpression in cervical squamous cell carcinoma affects cell phenotype and microRNA profiles. J. Pathol. 2011, 224, 496–507. [Google Scholar] [CrossRef]
- Witten, D.; Tibshirani, R.; Gu, S.G.; Fire, A.; Lui, W.O. Ultra-high throughput sequencing-based small RNA discovery and discrete statistical biomarker analysis in a collection of cervical tumours and matched controls. BMC Biol. 2010, 8, 58. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, P.; Chen, Z.; Liu, M.; Li, X.; Tang, H. MicroRNA-7 downregulates XIAP expression to suppress cell growth and promote apoptosis in cervical cancer cells. FEBS Lett. 2013, 587, 2247–2253. [Google Scholar]
- Cheung, T.H.; Man, K.N.; Yu, M.Y.; Yim, S.F.; Siu, N.S.; Lo, K.W.; Doran, G.; Wong, R.R.; Wang, V.W.; Smith, D.I.; et al. Dysregulated microRNAs in the pathogenesis and progression of cervical neoplasm. Cell Cycle 2012, 11, 2876–2884. [Google Scholar] [CrossRef]
- Pereira, P.M.; Marques, J.P.; Soares, A.R.; Carreto, L.; Santos, M.A. MicroRNA expression variability in human cervical tissues. PLoS One 2010, 5, e11780. [Google Scholar]
- Long, M.J.; Wu, F.X.; Li, P.; Liu, M.; Li, X.; Tang, H. MicroRNA-10a targets CHL1 and promotes cell growth, migration and invasion in human cervical cancer cells. Cancer Lett. 2012, 324, 186–196. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, Y.Y.; Guo, Y.L.; Li, Z.J.; Geng, L. Profiling of microRNA-mRNA reveals roles of microRNAs in cervical cancer. Chin. Med. J. (Engl.) 2012, 125, 4270–4276. [Google Scholar]
- Wang, X.; Wang, H.K.; Li, Y.; Hafner, M.; Banerjee, N.S.; Tang, S.; Briskin, D.; Meyers, C.; Chow, L.T.; Xie, X.; et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc. Natl. Acad. Sci. USA 2014, 111, 4262–4267. [Google Scholar] [CrossRef]
- Wei, Q.; Li, Y.X.; Liu, M.; Li, X.; Tang, H. MiR-17–5p targets TP53INP1 and regulates cell proliferation and apoptosis of cervical cancer cells. IUBMB Life 2012, 64, 697–704. [Google Scholar] [CrossRef]
- Xu, X.M.; Wang, X.B.; Chen, M.M.; Liu, T.; Li, Y.X.; Jia, W.H.; Liu, M.; Li, X.; Tang, H. MicroRNA-19a and -19b regulate cervical carcinoma cell proliferation and invasion by targeting CUL5. Cancer Lett. 2012, 322, 148–158. [Google Scholar] [CrossRef]
- Kang, H.W.; Wang, F.; Wei, Q.; Zhao, Y.F.; Liu, M.; Li, X.; Tang, H. MiR-20a promotes migration and invasion by regulating TNKS2 in human cervical cancer cells. FEBS Lett. 2012, 586, 897–904. [Google Scholar]
- Chen, J.; Yao, D.; Li, Y.; Chen, H.; He, C.; Ding, N.; Lu, Y.; Ou, T.; Zhao, S.; Li, L.; et al. Serum microRNA expression levels can predict lymph node metastasis in patients with early-stage cervical squamous cell carcinoma. Int. J. Mol. Med. 2013, 32, 557–567. [Google Scholar]
- Zhao, S.; Yao, D.S.; Chen, J.Y.; Ding, N. Aberrant expression of miR-20a and miR-203 in cervical cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2289–2293. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Huang, Y.; Ma, L.; Yin, Y.; Tang, M.; Hu, C. Microarray profile of micro-ribonucleic acid in tumor tissue from cervical squamous cell carcinoma without human papillomavirus. J. Obstet. Gynaecol. Res. 2009, 35, 842–849. [Google Scholar]
- Yeung, C.L.A.; Tsang, T.Y.; Yau, P.L.; Kwok, T.T. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene 2011, 30, 2401–2410. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Xu, J.; Ye, F.; Shen, Y.; Zhou, J.; Lu, W.; Wan, X.; Ma, D.; Xie, X. Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV-related target genes for miR-29. J. Pathol. 2011, 224, 484–495. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Ye, F.; Li, Y.; Lv, W.; Xie, X. Reduced miR-34a expression in normal cervical tissues and cervical lesions with high-risk human papillomavirus infection. Int. J. Gynecol. Cancer 2010, 20, 597–604. [Google Scholar] [CrossRef]
- Li, B.H.; Zhou, J.S.; Ye, F.; Cheng, X.D.; Zhou, C.Y.; Lu, W.G.; Xie, X. Reduced miR-100 expression in cervical cancer and precursors and its carcinogenic effect through targeting PLK1 protein. Eur. J. Cancer 2011, 47, 2166–2174. [Google Scholar]
- Qin, W.; Dong, P.; Ma, C.; Mitchelson, K.; Deng, T.; Zhang, L.; Sun, Y.; Feng, X.; Ding, Y.; Lu, X.; et al. MicroRNA-133b is a key promoter of cervical carcinoma development through the activation of the ERK and AKT1 pathways. Oncogene 2012, 31, 4067–4075. [Google Scholar] [CrossRef]
- Liu, L.; Yu, X.; Guo, X.; Tian, Z.; Su, M.; Long, Y.; Huang, C.; Zhou, F.; Liu, M.; Wu, X.; et al. MiR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol. Med. Rep. 2012, 5, 753–760. [Google Scholar]
- Xing, A.Y.; Wang, B.; Shi, D.B.; Zhang, X.F.; Gao, C.; He, X.Q.; Liu, W.J.; Gao, P. Deregulated expression of miR-145 in manifold human cancer cells. Exp. Mol. Pathol. 2013, 95, 91–97. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Wang, X.; Yu, Q.; Li, S.; Yu, X.; Gong, W. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int. J. Mol. Sci. 2014, 15, 758–773. [Google Scholar] [CrossRef]
- How, C.; Hui, A.B.; Alajez, N.M.; Shi, W.; Boutros, P.C.; Clarke, B.A.; Yan, R.; Pintilie, M.; Fyles, A.; Hedley, D.W.; et al. MicroRNA-196b regulates the homeobox B7-vascular endothelial growth factor axis in cervical cancer. PLoS One 2013, 8, e67846. [Google Scholar]
- Zhu, X.; Er, K.; Mao, C.; Yan, Q.; Xu, H.; Zhang, Y.; Zhu, J.; Cui, F.; Zhao, W.; Shi, H. miR-203 suppresses tumor growth and angiogenesis by targeting VEGFA in cervical cancer. Cell Phys. Biochem. 2013, 32, 64–73. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Y.; Caramuta, S.; Larsson, C.; Lui, W.O. MiR-205 expression promotes cell proliferation and migration of human cervical cancer cells. PLoS One 2012, 7, e46990. [Google Scholar]
- Wang, F.; Liu, M.; Li, X.; Tang, H. MiR-214 reduces cell survival and enhances cisplatin-inducedcytotoxicity via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett. 2013, 587, 488–495. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Dong, R.; Huang, X.; Ding, S.; Qiu, H. Circulating microRNA-218 was reduced in cervical cancer and correlated with tumor invasion. J. Cancer Res. Clin. Oncol. 2012, 138, 671–674. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kinoshita, T.; Nohata, N.; Itesako, T.; Yoshino, H.; Enokida, H.; Nakagawa, M.; Shozu, M.; Seki, N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int. J. Oncol. 2013, 42, 1523–1532. [Google Scholar]
- Li, Y.; Liu, J.; Yuan, C.; Cui, B.; Zou, X.; Qiao, Y. High-risk human papillomavirus reduces the expression of microRNA-218 in women with cervical intraepithelial neoplasia. J. Int. Med. Res. 2010, 38, 1730–1736. [Google Scholar]
- Shen, S.N.; Wang, L.F.; Jia, Y.F.; Hao, Y.Q.; Zhang, L.; Wang, H. Upregulation of microRNA-224 is associated with aggressive progression and poor prognosis in human cervical cancer. Diagn. Pathol. 2013. [Google Scholar] [CrossRef]
- Bierkens, M.; Krijgsman, O.; Wilting, S.M.; Bosch, L.; Jaspers, A.; Meijer, G.A.; Meijer, C.J.; Snijders, P.J.; Ylstra, B.; Steenbergen, R.D. Focal aberrations indicate EYA2 and hsa-miR-375 as oncogene and tumor suppressor in cervical carcinogenesis. Genes Chromosom. Cancer 2013, 52, 56–68. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Ye, F.; Wang, F.; Wan, X.; Lu, W.; Xie, X. Identification of miR-23a as a novel microRNA normalizer for relative quantification in human uterine cervical tissues. Exp. Mol. Med. 2011, 43, 358–366. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, F.; Wang, X.; Cheng, B.; Ye, F.; Xie, X.; Zhou, C.; Lu, W. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene 2013, 32, 976–987. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Yan, Q.; Chen, X.; Yang, Y.; Liu, X.; Wan, X. Interferon-β induced microRNA-129-5p down-regulates HPV-18 E6 and E7 viral gene expression by targeting SP1 in cervical cancer cells. PLoS One 2013, 8, e81366. [Google Scholar]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef]
- Schmitz, M.; Driesch, C.; Jansen, L.; Runnebaum, I.B.; Durst, M. Non-random integration of the HPV genome in cervical cancer. PLoS One 2012, 7, e39632. [Google Scholar]
- Kraus, I.; Driesch, C.; Vinokurova, S.; Hovig, E.; Schneider, A.; von Knebel Doeberitz, M.; Durst, M. The majority of viral-cellular fusion transcripts in cervical carcinomas cotranscribe cellular sequences of known or predicted genes. Cancer Res. 2008, 68, 2514–2522. [Google Scholar]
- Nuovo, G.J.; Wu, X.; Volinia, S.; Yan, F.; di Leva, G.; Chin, N.; Nicol, A.F.; Jiang, J.; Otterson, G.; Schmittgen, T.D.; et al. Strong inverse correlation between microRNA-125b and human papillomavirus DNA in productive infection. Diagn. Mol. Pathol. 2010, 19, 135–143. [Google Scholar] [CrossRef]
- Soto-Reyes, E.; Gonzalez-Barrios, R.; Cisneros-Soberanis, F.; Herrera-Goepfert, R.; Perez, V.; Cantu, D.; Prada, D.; Castro, C.; Recillas-Targa, F.; Herrera, L.A. Disruption of CTCF at the miR-125b1 locus in gynecological cancers. BMC Cancer 2012. [Google Scholar] [CrossRef]
- Greco, D.; Kivi, N.; Qian, K.; Leivonen, S.K.; Auvinen, P.; Auvinen, E. Human papillomavirus 16 E5 modulates the expression of host microRNAs. PLoS One 2011, 6, e21646. [Google Scholar]
- Yue, C.; Wang, M.; Ding, B.; Wang, W.; Fu, S.; Zhou, D.; Zhang, Z.; Han, S. Polymorphism of the pre-miR-146a is associated with risk of cervical cancer in a Chinese population. Gynecol. Oncol. 2011, 122, 33–37. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Zhou, J.; Xu, J.; Peng, C.; Ye, F.; Shen, Y.; Lu, W.; Wan, X.; Xie, X. MiR-375 is down-regulated in squamous cervical cancer and inhibits cell migration and invasion via targeting transcription factor SP1. Am. J. Pathol. 2011, 179, 2580–2588. [Google Scholar]
- Jazdzewski, K.; Murray, E.L.; Franssila, K.; Jarzab, B.; Schoenberg, D.R.; de la Chapelle, A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA 2008, 105, 7269–7274. [Google Scholar]
- Brosh, R.; Shalgi, R.; Liran, A.; Landan, G.; Korotayev, K.; Nguyen, G.H.; Enerly, E.; Johnsen, H.; Buganim, Y.; Solomon, H.; et al. Rotter 53-Repressed miRNAs are involved with E2F in a feed-forward loop promoting proliferation. Mol. Syst. Biol. 2008, 4, 229. [Google Scholar]
- Zheng, Z.M.; Wang, X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim. Biophys. Acta 1809, 668–677. [Google Scholar]
- Cannell, I.G.; Kong, Y.W.; Johnston, S.J.; Chen, M.L.; Collins, H.M.; Dobbyn, H.C.; Elia, A.; Kress, T.R.; Dickens, M.; Clemens, M.J.; et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc. Natl. Acad. Sci. USA 2010, 107, 5375–5380. [Google Scholar] [CrossRef]
- Bueno, M.J.; de Cedron, M.G.; Laresgoiti, U.; Fernandez-Piqueras, J.; Zubiaga, A.M.; Malumbres, M. Multiple E2F-induced microRNAs prevent replicative stress in response to mitogenic signaling. Mol. Cell. Biol. 2010, 30, 2983–2995. [Google Scholar] [CrossRef]
- Woods, K.; Thomson, J.M.; Hammond, S.M. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J. Biol. Chem. 2007, 282, 2130–2134. [Google Scholar]
- O’Donnell, K.A.; Wentzel, E.A.; Zeller, K.I.; Dang, C.V.; Mendell, J.T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005, 435, 839–843. [Google Scholar]
- Myklebust, M.P.; Bruland, O.; Fluge, O.; Skarstein, A.; Balteskard, L.; Dahl, O. microRNA-15b is induced with E2F-controlled genes in HPV-related cancer. Br. J. Cancer 2011, 105, 1719–1725. [Google Scholar]
- Melar-New, M.; Laimins, L.A. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. J. Virol. 2010, 84, 5212–5221. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Wilting, S.M.; van Boerdonk, R.A.; Henken, F.E.; Meijer, C.J.; Diosdado, B.; Meijer, G.A.; le Sage, C.; Agami, R.; Snijders, P.J.; Steenbergen, R.D. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol. Cancer 2010, 9, 167. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Z.; Jin, Y.; Dragas, D.; Zhang, L.; Adjei, B.S.; Wang, A.; Dai, Y.; Zhou, X. MicroRNA-99 family members suppress Homeobox A1 expression in epithelial cells. PLoS One 2013, 8, e80625. [Google Scholar]
- Cui, F.; Li, X.; Zhu, X.; Huang, L.; Huang, Y.; Mao, C.; Yan, Q.; Zhu, J.; Zhao, W.; Shi, H. MiR-125b inhibits tumor growth and promotes apoptosis of cervical cancer cells by targeting phosphoinositide 3-kinase catalytic subunit delta. Cell Physiol. Biochem. 2012, 30, 1310–1318. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Y.; Huang, Y.; Yu, H.; Huang, Y.; Wu, L.; Huang, L. Up-regulated miR155 reverses the epithelial-mesenchymal transition induced by EGF and increases chemo-sensitivity to cisplatin in human Caski cervical cancer cells. PLoS One 2012, 7, e52310. [Google Scholar]
- Wan, G.; Xie, W.; Liu, Z.; Xu, W.; Lao, Y.; Huang, N.; Cui, K.; Liao, M.; He, J.; Jiang, Y.; et al. Hypoxia-induced MIR155 is a potent autophagy inducer by targeting multiple players in the MTOR pathway. Autophagy 2014, 10, 70–79. [Google Scholar] [CrossRef]
- Qiang, R.; Wang, F.; Shi, L.Y.; Liu, M.; Chen, S.; Wan, H.Y.; Li, Y.X.; Li, X.; Gao, S.Y.; Sun, B.C.; et al. Plexin-B1 is a target of miR-214 in cervical cancer and promotes the growth and invasion of HeLa cells. Int. J. Biochem. Cell. Biol. 2011, 43, 632–641. [Google Scholar]
- Li, J.; Ping, Z.; Ning, H. MiR-218 Impairs Tumor Growth and Increases Chemo-Sensitivity to Cisplatin in Cervical Cancer. Int. J. Mol. Sci. 2012, 13, 16053–16064. [Google Scholar] [CrossRef]
- Cai, N.; Wang, Y.D.; Zheng, P.S. The microRNA-302–367 cluster suppresses the proliferation of cervical carcinoma cells through the novel target AKT1. RNA 2013, 19, 85–95. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, H.; Zhang, Q.Q.; Zhou, H.; Qu, L.H. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 539–542. [Google Scholar]
- Tian, R.Q.; Wang, X.H.; Hou, L.J.; Jia, W.H.; Yang, Q.; Li, Y.X.; Liu, M.; Li, X.; Tang, H. MicroRNA-372 is down-regulated and targets cyclin-dependent kinase 2 (CDK2) and cyclin A1 in human cervical cancer, which may contribute to tumorigenesis. J. Biol. Chem. 2011, 286, 25556–25563. [Google Scholar]
- Tang, T.; Wong, H.K.; Gu, W.; Yu, M.Y.; To, K.F.; Wang, C.C.; Wong, Y.F.; Cheung, T.H.; Chung, T.K.; Choy, K.W. MicroRNA-182 plays an onco-miRNA role in cervical cancer. Gynecol. Oncol. 2013, 129, 199–208. [Google Scholar]
- Pang, R.T.; Leung, C.O.; Ye, T.M.; Liu, W.; Chiu, P.C.; Lam, K.K.; Lee, K.F.; Yeung, W.S. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis 2010, 31, 1037–1044. [Google Scholar] [CrossRef]
- Shi, M.; Du, L.; Liu, D.; Qian, L.; Hu, M.; Yu, M.; Yang, Z.; Zhao, M.; Chen, C.; Guo, L.; et al. Glucocorticoid regulation of a novel HPV-E6-p53-miR-145 pathway modulates invasion and therapy resistance of cervical cancer cells. J. Pathol. 2012, 228, 148–157. [Google Scholar] [CrossRef]
- Hu, X.; Schwarz, J.K.; Lewis, J.S., Jr.; Huettner, P.C.; Rader, J.S.; Deasy, J.O.; Grigsby, P.W.; Wang, X. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010, 70, 1441–1448. [Google Scholar] [CrossRef]
- Huang, L.; Lin, J.X.; Yu, Y.H.; Zhang, M.Y.; Wang, H.Y.; Zheng, M. Downregulation of six microRNAs is associated with advanced stage, lymph node metastasis and poor prognosis in small cell carcinoma of the cervix. PLoS One 2012, 7, e33762. [Google Scholar]
- Alajez, N.M.; Lenarduzzi, M.; Ito, E.; Hui, A.B.; Shi, W.; Bruce, J.; Yue, S.; Huang, S.H.; Xu, W.; Waldron, J.; et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 2011, 71, 2381–2391. [Google Scholar] [CrossRef]
- He, G.; Wang, Q.; Zhou, Y.; Wu, X.; Wang, L.; Duru, N.; Kong, X.; Zhang, P.; Wan, B.; Sui, L.; et al. YY1 is a novel potential therapeutic target for the treatment of HPV infection-induced cervical cancer by arsenic trioxide. Int. J. Gynecol. Cancer 2011, 21, 1097–1104. [Google Scholar]
- Baldwin, A.; Li, W.; Grace, M.; Pearlberg, J.; Harlow, E.; Munger, K.; Grueneberg, D.A. Kinase requirements in human cells: II. Genetic interaction screens identify kinase requirements following HPV16 E7 expression in cancer cells. Proc. Natl. Acad. Sci. USA 2008, 105, 16478–16483. [Google Scholar]
- Wang, X.; Meyers, C.; Guo, M.; Zheng, Z.M. Upregulation of p18Ink4c expression by oncogenic HPV E6 via p53-miR-34a pathway. Int. J. Cancer 2011, 129, 1362–1372. [Google Scholar] [CrossRef]
- Bolos, V.; Grego-Bessa, J.; de la Pompa, J.L. Notch signaling in development and cancer. Endocr. Rev. 2007, 28, 339–363. [Google Scholar]
- Naiki, T.; Saijou, E.; Miyaoka, Y.; Sekine, K.; Miyajima, A. TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPβ. J. Biol. Chem. 2007, 282, 24075–24082. [Google Scholar]
- Grandinetti, K.B.; Stevens, T.A.; Ha, S.; Salamone, R.J.; Walker, J.R.; Zhang, J.; Agarwalla, S.; Tenen, D.G.; Peters, E.C.; Reddy, V.A. Overexpression of TRIB2 in human lung cancers contributes to tumorigenesis through downregulation of C/EBPα. Oncogene 2011, 30, 3328–3335. [Google Scholar] [CrossRef]
- Keeshan, K.; He, Y.; Wouters, B.J.; Shestova, O.; Xu, L.; Sai, H.; Rodriguez, C.G.; Maillard, I.; Tobias, J.W.; Valk, P.; et al. Tribbles homolog 2 inactivates C/EBPα and causes acute myelogenous leukemia. Cancer Cell 2006, 10, 401–411. [Google Scholar] [CrossRef]
- Xin, J.X.; Yue, Z.; Zhang, S.; Jiang, Z.H.; Wang, P.Y.; Li, Y.J.; Pang, M.; Xie, S.Y. MiR-99 inhibits cervical carcinoma cell proliferation by targeting TRIB2. Oncol. Lett. 2013, 6, 1025–1030. [Google Scholar]
- Zhang, Y.; Liu, Y.; Yang, Y.X.; Xia, J.H.; Zhang, H.X.; Li, H.B.; Yu, C.Z. The expression of PLK-1 in cervical carcinoma: A possible target for enhancing chemosensitivity. J. Exp. Clin. Cancer Res. 2009. [Google Scholar] [CrossRef]
- Bahassi el, M. Polo-like kinases and DNA damage checkpoint: Beyond the traditional mitotic functions. Exp. Biol. Med. (Maywood) 2011, 236, 648–657. [Google Scholar] [CrossRef]
- Pillai, M.R.; Halabi, S.; McKalip, A.; Jayaprakash, P.G.; Rajalekshmi, T.N.; Nair, M.K.; Herman, B. The presence of human papillomavirus-16/-18 E6, p53, and Bcl-2 protein in cervicovaginal smears from patients with invasive cervical cancer. Cancer Epidemiol. Biomark. Prev. 1996, 5, 329–335. [Google Scholar]
- Dimitrakakis, C.; Kymionis, G.; Diakomanolis, E.; Papaspyrou, I.; Rodolakis, A.; Arzimanoglou, I.; Leandros, E.; Michalas, S. The possible role of p53 and bcl-2 expression in cervical carcinomas and their premalignant lesions. Gynecol. Oncol. 2000, 77, 129–136. [Google Scholar] [CrossRef]
- Baserga, R. The insulin receptor substrate-1: A biomarker for cancer? Exp. Cell Res. 2009, 315, 727–732. [Google Scholar] [CrossRef]
- Wei, X.; Xu, H.; Kufe, D. Human mucin 1 oncoprotein represses transcription of the p53 tumor suppressor gene. Cancer Res. 2007, 67, 1853–1858. [Google Scholar] [CrossRef]
- Shi, T.Y.; Chen, X.J.; Zhu, M.L.; Wang, M.Y.; He, J.; Yu, K.D.; Shao, Z.M.; Sun, M.H.; Zhou, X.Y.; Cheng, X.; et al. A pri-miR-218 variant and risk of cervical carcinoma in Chinese women. BMC Cancer 2013, 10–1186. [Google Scholar]
- Wang, Q.; Shu, R.; He, H.; Wang, L.; Ma, Y.; Zhu, H.; Wang, Z.; Wang, S.; Shen, G.; Lei, P. Co-silencing of Birc5 (survivin) and Hspa5 (Grp78) induces apoptosis in hepatoma cells more efficiently than single gene interference. Int. J. Oncol. 2012, 41, 652–660. [Google Scholar]
- Sukpan, K.; Settakorn, J.; Khunamornpong, S.; Cheewakriangkrai, C.; Srisomboon, J.; Siriaunkgul, S. Expression of survivin, CD117, and C-erbB-2 in neuroendocrine carcinoma of the uterine cervix. Int. J. Gynecol. Cancer 2011, 21, 911–917. [Google Scholar] [CrossRef]
- Narayan, G.; Goparaju, C.; Arias-Pulido, H.; Kaufmann, A.M.; Schneider, A.; Durst, M.; Mansukhani, M.; Pothuri, B.; Murty, V.V. Promoter hypermethylation-mediated inactivation of multiple Slit-Robo pathway genes in cervical cancer progression. Mol. Cancer 2006. [Google Scholar] [CrossRef]
- Yi, S.; Chen, Y.; Wen, L.; Yang, L.; Cui, G. Expression of connexin 32 and connexin 43 in acute myeloid leukemia and their roles in proliferation. Oncol. Lett. 2012, 4, 1003–1007. [Google Scholar]
- Macdonald, A.I.; Sun, P.; Hernandez-Lopez, H.; Aasen, T.; Hodgins, M.B.; Edward, M.; Roberts, S.; Massimi, P.; Thomas, M.; Banks, L; et al. A functional interaction between the MAGUK protein hDlg and the gap junction protein connexin 43 in cervical tumour cells. Biochem. J. 2012, 446, 9–21. [Google Scholar] [CrossRef]
- Skyldberg, B.; Salo, S.; Eriksson, E.; Aspenblad, U.; Moberger, B.; Tryggvason, K.; Auer, G. Laminin-5 as a marker of invasiveness in cervical lesions. J. Natl. Cancer Inst. 1999, 91, 1882–1887. [Google Scholar] [CrossRef]
- Kohlberger, P.; Beneder, C.; Horvat, R.; Leodolter, S.; Breitenecker, G. Immunohistochemical expression of laminin-5 in cervical intraepithelial neoplasia. Gynecol. Oncol. 2003, 89, 391–394. [Google Scholar] [CrossRef]
- Longworth, M.S.; Wilson, R.; Laimins, L.A. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. EMBO J. 2005, 24, 1821–1830. [Google Scholar] [CrossRef]
- Brehm, A.; Nielsen, S.J.; Miska, E.A.; McCance, D.J.; Reid, J.L.; Bannister, A.J.; Kouzarides, T. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 1999, 18, 2449–2458. [Google Scholar]
- Wada, T.; Kikuchi, J.; Furukawa, Y. Histone deacetylase 1 enhances microRNA processing via deacetylation of DGCR8. EMBO Rep. 2012, 13, 142–149. [Google Scholar] [CrossRef]
- Peralta-Zaragoza, O.; Bermudez-Morales, V.; Gutierrez-Xicotencatl, L.; Alcocer-Gonzalez, J.; Recillas-Targa, F.; Madrid-Marina, V. E6 and E7 oncoproteins from human papillomavirus type 16 induce activation of human transforming growth factor beta1 promoter throughout Sp1 recognition sequence. Viral. Immunol. 2006, 19, 468–480. [Google Scholar]
- Lee, Y.J.; Lee, J.E.; Choi, H.J.; Lim, J.S.; Jung, H.J.; Baek, M.C.; Frokiaer, J.; Nielsen, S.; Kwon, T.H. E3 ubiquitin-protein ligases in rat kidney collecting duct: Response to vasopressin stimulation and withdrawal. Am. J. Physiol. Ren. Physiol. 2011, 301, F883–E896. [Google Scholar] [CrossRef]
- Feng, L.; Allen, N.S.; Simo, S.; Cooper, J.A. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 2007, 21, 2717–2730. [Google Scholar] [CrossRef]
- Lewis, S.P.; Willis, A.N.; Johnson, A.E.; Resau, J.; Burnatowska-Hledin, M.A. Mutational analysis of VACM-1/cul5 exons in cancer cell lines. APMIS 2011, 119, 421–430. [Google Scholar] [CrossRef]
- Lankat-Buttgereit, B.; Goke, R. The tumour suppressor Pdcd4: Recent advances in the elucidation of function and regulation. Biol. Cell 2009, 101, 309–317. [Google Scholar] [CrossRef]
- Deftereos, G.; Corrie, S.R.; Feng, Q.; Morihara, J.; Stern, J.; Hawes, S.E.; Kiviat, N.B. Expression of miR-21 and miR-143 in cervical specimens ranging from histologically normal through to invasive cervical cancer. PLoS One 2011, 6, e28423. [Google Scholar]
- Harris, T.G.; Burk, R.D.; Yu, H.; Minkoff, H.; Massad, L.S.; Watts, D.H.; Zhong, Y.; Gange, S.; Kaplan, R.C.; Anastos, K.; et al. Insulin-like growth factor axis and oncogenic human papillomavirus natural history. Cancer Epidemiol. Biomark. Prev. 2008, 17, 245–248. [Google Scholar] [CrossRef]
- Hirano, S.; Ito, N.; Takahashi, S.; Tamaya, T. Clinical implications of insulin-like growth factors through the presence of their binding proteins and receptors expressed in gynecological cancers. Eur. J. Gynaecol. Oncol. 2004, 25, 187–191. [Google Scholar]
- Tomasini, R.; Samir, A.A.; Carrier, A.; Isnardon, D.; Cecchinelli, B.; Soddu, S.; Malissen, B.; Dagorn, J.C.; Iovanna, J.L.; Dusetti, N.J. TP53INP1s and homeodomain-interacting protein kinase-2 (HIPK2) are partners in regulating p53 activity. J. Biol. Chem. 2003, 278, 37722–37729. [Google Scholar] [CrossRef]
- Yan, H.L.; Xue, G.; Mei, Q.; Wang, Y.Z.; Ding, F.X.; Liu, M.F.; Lu, M.H.; Tang, Y.; Yu, H.Y.; Sun, S.H. Repression of the miR-17–92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J. 2009, 28, 2719–2732. [Google Scholar] [CrossRef]
- Wang, Y.D.; Cai, N.; Wu, X.L.; Cao, H.Z.; Xie, L.L.; Zheng, P.S. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 2013, 4, e760. [Google Scholar] [CrossRef]
- Brockhausen, I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1473, 67–95. [Google Scholar]
- Peng, R.Q.; Wan, H.Y.; Li, H.F.; Liu, M.; Li, X.; Tang, H. MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-α-d galactosamine: Polypeptide N-acetylgalactosaminyltransferase 7. J. Biol. Chem. 2012, 287, 14301–14309. [Google Scholar] [CrossRef]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: Micrornas can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- Noland, C.L.; Doudna, J.A. Multiple sensors ensure guide strand selection in human RNAi pathways. RNA 2013, 19, 639–648. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Granados López, A.J.; López, J.A. Multistep Model of Cervical Cancer: Participation of miRNAs and Coding Genes. Int. J. Mol. Sci. 2014, 15, 15700-15733. https://doi.org/10.3390/ijms150915700
Granados López AJ, López JA. Multistep Model of Cervical Cancer: Participation of miRNAs and Coding Genes. International Journal of Molecular Sciences. 2014; 15(9):15700-15733. https://doi.org/10.3390/ijms150915700
Chicago/Turabian StyleGranados López, Angelica Judith, and Jesús Adrián López. 2014. "Multistep Model of Cervical Cancer: Participation of miRNAs and Coding Genes" International Journal of Molecular Sciences 15, no. 9: 15700-15733. https://doi.org/10.3390/ijms150915700




