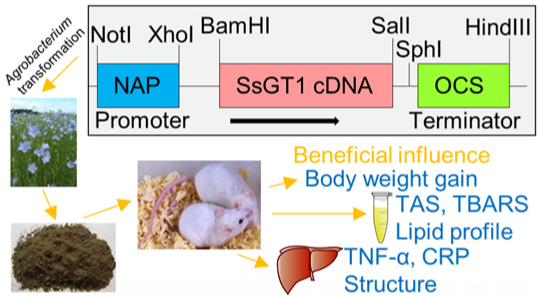
Genetically Modified Flax Expressing NAP-SsGT1 Transgene: Examination of Anti-Inflammatory Action
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Feeding with GM (Genetically Modified) GT#4 Flaxseed Cake on Body Weight Gain of Mice and Food Intake

2.2. Effects of Administration of GM GT#4 Flaxseed Cake on Red-ox State Indices in Mice Serum

2.3. Influence of Consumption of GM Flax GT#4 Seed Cake on Mice Serum Lipid Profile

2.4. Effects of GM GT#4 Flaxseed Cake Consumption on Inflammatory State Biomarkers in Mice Liver

2.5. Effects of Administration of GM GT#4 Flaxseed Cake on Liver Histological Changes in Mice

2.6. Influence of GM GT#4 Flaxseed Cake Feeding on Liver Ultrastructure in Mice

3. Experimental Section
3.1. Plant Material and Transformation
3.2. Nutritional Study and Preparation of Animal Material
| Component | Experimental Diet | |||
|---|---|---|---|---|
| SD | HFD | Linola | GT#4 | |
| Flaxseed cake (%) | - | - | 30.0 | 30.0 |
| Nutritional value (% dry matter) | ||||
| Crude protein | 20.0 | 20.1 | 19.7 | 19.5 |
| Crude fat | 2.8 | 12.1 | 11.6 | 11.9 |
| Crude fiber | 4.0 | 3.8 | 4.9 | 4.6 |
| Gross energy (kcal) | 465 | 515 | 512 | 514 |
| Gross energy from crude fat (kcal/100 g) | 27 | 115 | 110 | 113 |
3.3. Total Antioxidant Status
3.4. Lipid Peroxidation
3.5. Serum Lipid Profile
3.6. Inflammatory State Markers
3.7. Hepatic Histological Evaluation
3.8. Transmission Electron Microscopic Examination of Liver
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Association for the Study of Obesity. Available online: http://www.aso.org.uk (accessed on 14 July 2014).
- Popkin, B.M. The World Is Fat: The Fads, Trends, Policies, and Products that Are Fattening the Human Race; Avery Trade/Penguin Group: New York, NY, USA, 2009. [Google Scholar]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar]
- Christiansen, T.; Richelsen, B.; Bruun, J.M. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int. J. Obes. 2005, 29, 146–150. [Google Scholar]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar]
- Makowski, L.; Hotamisligil, G.S. Fatty acid binding proteins—The evolutionary crossroads of inflammatory and metabolic responses. J. Nutr. 2004, 134, 2464S–2468S. [Google Scholar]
- Farrante, A.W., Jr. Obesity induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007, 262, 408–414. [Google Scholar]
- Park, T.; Kim, Y. Phytochemicals as potential agents for prevention and treatment of obesity and metabolic diseases. In Anti-obesity Drug Discovery and Development; Bentham Science: Sharjah, United Arab Emirates, 2011; p. 1. [Google Scholar]
- Santos, A.P.; Rogero, M.M.; Bastos, D.H. Edible Plants, their Secondary Metabolites and Antiobesogenic Potential. Recent Pat. Food Nutr. Agric. 2010, 2, 195–212. [Google Scholar]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Phytochemicals and regulation of the adipocyte life cycle. J. Nutr. Biochem. 2008, 19, 717–726. [Google Scholar]
- Bravo, I. Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and atherosclerosis: Human trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar]
- Mulvihill, E.E.; Huff, M.W. Antiatherogenic properties of flavonoids: Implications for cardiovascular health. Can. J. Cardiol. 2010, 26 (Suppl. A), 17A–21A. [Google Scholar]
- Moon, H.K.; Yang, E.S.; Park, J.W. Protection of peroxynitrite-induced DNA damage by dietary antioxidants. Arch. Pharm. Res. 2006, 29, 213–217. [Google Scholar]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; Sánchez de Medina, F. Effects of Flavonoids and other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar]
- Deka, A.; Vita, J.A. Tea and cardiovascular disease. Pharmacol. Res. 2011, 64, 136–145. [Google Scholar]
- Hoensch, H.P.; Oertel, R. Emerging role of bioflavonoids in gastroenterology: Especially their effects on intestinal neoplasia. World J. Gastrointest. Oncol. 2011, 3, 71–74. [Google Scholar]
- Jäger, A.K.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar]
- Laranjinha, J.A.N.; Almeida, L.M.; Madeira, V.M.C. Reactivity of dietary phenolic acids with peroxyl radicals: Antioxidant activity upon low density lipoprotein peroxidation. Biochem. Pharmacol. 1994, 48, 487–494. [Google Scholar]
- Chimi, M.; Cillard, J.; Cillard, P.; Rahmani, M. Peroxyl and hydroxyl radical scavenging activity of some natural phenolic extracts. J. Am. Oil Chem. Soc. 1991, 68, 307–312. [Google Scholar]
- Adlercreutz, H. Lignans and Human Health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar]
- Prasad, K. Antioxidant activity of secoisolariciresinoldiglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int. J. Angiol. 2000, 9, 220–225. [Google Scholar]
- Lorenc-Kukuła, K.; Żuk, M.; Kulma, A.; Czemplik, M.; Kostyń, K.; Skała, J.; Starzycki, M.; Szopa, J. Engineering Flax with the GT Family 1 Solanum sogarandinum Glycosyltransferase SsGT1 Confers Increased Resistance to Fusarium Infection. J. Agric. Food Chem. 2009, 57, 6698–6705. [Google Scholar]
- EFSA Panel on Genetically Modified Organisms (GMO). Guidance for risk assessment of food and feed from genetically modified plants. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Guidance on conducting repeated-dose 90-day oral toxicity study in rodents on whole food/feed. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- Rivera, L.; Moron, R.; Sanchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar]
- Yamamoto, Y.; Oue, E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci. Biotechnol. Biochem. 2006, 70, 933–939. [Google Scholar]
- Stewart, L.K.; Soileau, J.L.; Ribnicky, D.; Wang, Z.Q.; Raskin, I.; Poulev, A.; Majewski, M.; Cefalu, W.T.; Gettys, T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism 2008, 57, S39–S46. [Google Scholar]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar]
- Egert, S.; Boesch-Saadatmandi, C.; Wolffram, S.; Rimbach, G.; Muller, M.J. Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. J. Nutr. 2010, 140, 278–284. [Google Scholar]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Influence of oryzanol and ferulic acid on the lipid metabolism and antioxidative status in high fat-fed mice. J. Clin. Biochem. Nutr. 2010, 46, 150–156. [Google Scholar]
- Wilson, T.A.; Nicolosi, R.J.; Woolfrey, B.; Kritchevsky, D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypocholesterolemic hamsters. J. Nutr. Biochem. 2007, 18, 105–112. [Google Scholar]
- Żuk, M.; Kulma, A.; Dymińska, L.; Szołtysek, K.; Prescha, A.; Hanuza, J.; Szopa, J. Flavonoid engineering of flax potentiate its biotechnological application. BMC Biotech. 2011, 11. [Google Scholar] [CrossRef]
- Beltowski, J.; Wojcicka, G.; Gorny, D.; Marciniak, A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J. Physiol. Pharmacol. 2000, 51, 883–896. [Google Scholar]
- Spiteller, G. Lipid peroxidation in aging and age-dependent diseases. Exp. Gerontol. 2001, 36, 1425–1457. [Google Scholar]
- Arts, M.J.T.J.; Dallinga, J.S.; Voss, H.P.; Haenen, G.R.M.M.; Bast, A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 2004, 88, 567–570. [Google Scholar]
- Prasad, K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinoldiglucoside (SDG) isolated from flax seed. Circulation 1999, 99, 1355–1362. [Google Scholar]
- Rhee, Y.; Brunt, A. Flax seed supplementation improved insulin resistance in obese glucose intolerant people: A randomized crossover design. Nutr. J. 2011, 10. [Google Scholar] [CrossRef]
- Kamada, C.; da Silva, E.L.; Ohnishi-Kameyama, M.; Moon, J.H.; Terao, J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radic. Res. 2005, 39, 185–194. [Google Scholar]
- Bloedon, L.T.; Szapary, P.O. Flax seed and Cardiovascular Risk. Nutr. Rev. 2004, 62, 18–27. [Google Scholar]
- Tacke, H.; Luedde, T.; Trautwein, C. Inflammatory Pathways in Liver Homeostasis and Liver Injury. Clin. Rev. Allerg. Immunol. 2009, 36, 4–12. [Google Scholar]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar]
- Ouchi, N.; Kihara, S.; Arita, Y.; Okamoto, Y.; Maeda, K.; Kuriyama, H.; Hotta, K.; Nishida, M.; Takahashi, M.; Muraguchi, M.; et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 2000, 102, 1296–1301. [Google Scholar]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar]
- Vasseur, F.; Meyre, D.; Froguel, P. Adiponectin, type 2 diabetes and the metabolic syndrome: Lessons from human genetic studies. Expert Rev. Mol. Med. 2006, 8, 1–12. [Google Scholar]
- Manjeet, K.R.; Ghosh, B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int. J. Immunopharmacol. 1999, 21, 435–443. [Google Scholar]
- Overman, A.; Chuang, C.-C.; McIntosh, M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2011, 35, 1165–1172. [Google Scholar]
- Chuang, C.-C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungpert, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-α-mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 2010, 92, 1511–1521. [Google Scholar]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar]
- Rahman, I. Oxidative stress, transcription factors and chromatin remodeling in lung inflammation. Biochem. Pharmacol. 2002, 64, 935–942. [Google Scholar]
- Rein, D.; Schijlen, E.; Kooistra, T.; Herbers, K.; Verschuren, L.; Hall, R.; Sonnewald, U.; Bovy, A.; Kleeman, R. Transgenic flavonoid tomato intake reduces C-reactive protein in human C-reactive protein transgenic mice more than wild-type tomato. J. Nutr. 2006, 136, 2331–2337. [Google Scholar]
- Song, Y.; Manson, J.E.; Buringm, J.E.; Sesso, H.D.; Liu, S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar]
- Garcia-Mediavilla, M.V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar]
- Hutchins, A.M.; Brown, B.B.; Cunnane, S.C.; Domitrovich, S.G.; Adams, E.R.; Bobowiec, C.E. Daily flax seed consumption improves glycemic control in obese men and women with pre-diabetes: A randomized study. Nutr. Res. 2013, 33, 367–375. [Google Scholar]
- Dodin, S.D.; Cunnane, S.C.; Mâsse, B.; Lemay, A.; Jacques, H.; Asselin, G.; Tremblay-Mercier, J.; Marc, I.; Lamarche, B.; Légaré, F.; et al. Flax seed on cardiovascular disease markers in healthy menopausal women: A randomized, double-blind, placebo-controlled trial. Nutrition 2008, 24, 23–30. [Google Scholar]
- Aoun, M.; Michel, F.; Fouret, G.; Casas, F.; Jullien, M.; Wrutniak-Cabello, C.; Ramos, J.; Cristol, J.-P.; Coudray, C.; Carbonneau, M.-A.; et al. A polyphenol extract modifies quantity but not quality of liver fatty acid content in high-fat-high-sucrose diet-fed rats: Possible implication of the sirtuin pathway. Br. J. Nutr. 2010, 104, 1760–1770. [Google Scholar]
- Felmlee, M.A.; Woo, G.; Simko, E.; Krol, E.S.; Muir, A.D.; Alcorn, J. Effects of the flax seed lignan ssecoisolariciresinoldiglucoside and its aglycone on serum and hepatic lipids in hyperlipidaemic rats. Br. J. Nutr. 2009, 102, 361–369. [Google Scholar]
- Vijaimohan, K.; Jainu, M.; Sabitha, K.E.; Subramaniyam, S.; Anandhan, C.; Shyamala Devi, C.S. Beneficial effects of alpha linolenic acid rich flax seed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci. 2006, 79, 448–454. [Google Scholar]
- Yugarani, T.; Tan, B.K.H.; Teh, M.; Das, N.P. Effects of polyphenolic natural products on the lipid profiles of rats fed high fat diets. Lipids 1992, 27, 181–186. [Google Scholar]
- Ying, H.Z.; Liu, Y.H.; Yu, B.; Wang, Z.Y.; Zang, J.N.; Yu, C.H. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 2013, 52, 53–60. [Google Scholar]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.I.; Ha, T.Y. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Res. 2013, 27, 139–143. [Google Scholar]
- DeLeve, L.D.; Wang, X.; Kanel, G.C.; Atkinson, R.D.; McCuskey, R.S. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am. J. Pathol. 2008, 173, 993–1001. [Google Scholar]
- Jamieson, H.A.; Hilmer, S.N.; Cogger, V.C.; Warren, A.; Cheluvappa, R.; Abernethy, D.R.; Everitt, A.V.; Fraser, R.; de Cabo, R.; le Couteur, D.G. Caloric restriction reduces age-related pseudocapillarization of the hepatic sinusoid. Exp. Gerontol. 2007, 42, 374–378. [Google Scholar]
- Braet, F.; Wisse, E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: A review. Comp. Hepatol. 2002, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zheng, L.; Dong, D.; Xu, L.; Yin, L.; Qi, Y.; Han, X.; Lin, Y.; Liu, K.; Peng, J. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 2013, 141, 2108–2116. [Google Scholar]
- Wróbel, M.; Żebrowski, J.; Szopa, J. Polyhydroxybutyrate synthesis in transgenic flax. J. Biotech. 2004, 107, 41–54. [Google Scholar]
- Art. 30 sec. 1 pt. 1 of the Act on animal experiments dated 21 January 2005 (Journal of Laws No. 33, item 289; Dz.U.05.33.289). Internetowy System Aktów Prawnych. (in Polish). Available online: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20050330289 (accessed on 14 July 2014).
- § 14 sec. 3 of the ordinance of the Minister of Science and Informatization concerning National Ethics Committee on Animal Experiments and local ethics committees on animal experiments dated 19 July 2005 (Journal of Laws No. 153, item 1275; Dz.U.05.153.1275). Internetowy System Aktów Prawnych. (in Polish). Available online: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20051531275 (accessed on 14 July 2014).
- NRC. Nutrient Requirements of Laboratory Animals. National Research Council, 4th ed.; National Academy of Sciences: Washington, DC, USA, 1996. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC: Arlington, TX, USA, 1996. [Google Scholar]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matusiewicz, M.; Kosieradzka, I.; Zuk, M.; Szopa, J. Genetically Modified Flax Expressing NAP-SsGT1 Transgene: Examination of Anti-Inflammatory Action. Int. J. Mol. Sci. 2014, 15, 16741-16759. https://doi.org/10.3390/ijms150916741
Matusiewicz M, Kosieradzka I, Zuk M, Szopa J. Genetically Modified Flax Expressing NAP-SsGT1 Transgene: Examination of Anti-Inflammatory Action. International Journal of Molecular Sciences. 2014; 15(9):16741-16759. https://doi.org/10.3390/ijms150916741
Chicago/Turabian StyleMatusiewicz, Magdalena, Iwona Kosieradzka, Magdalena Zuk, and Jan Szopa. 2014. "Genetically Modified Flax Expressing NAP-SsGT1 Transgene: Examination of Anti-Inflammatory Action" International Journal of Molecular Sciences 15, no. 9: 16741-16759. https://doi.org/10.3390/ijms150916741