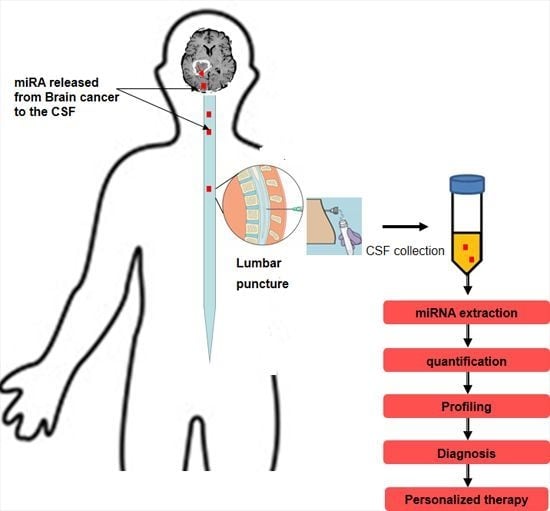
Tumor-Associated CSF MicroRNAs for the Prediction and Evaluation of CNS Malignancies
Abstract
:1. Background

2. miRNAs Circulating in Body Fluids

3. Cerebrospinal Fluid (CSF) as Diagnostic Window for the Pathological State of the Central Nervous System (CNS)
3.1. CSF Characteristics
3.2. CSF Analysis for Brain Cancer Markers Detection
| Marker | Sample | Type of Brain Cancer | References |
|---|---|---|---|
| * AT III | * CSF | * CNS lymphoma | [68,81,82] |
| EGFR | CSF | Brain metastases from lung adenocarcinoma | [83] |
| Pro-inflammatory (* IL-1β, IL-6, IL-8, IL-12, * GM-and * TNF-α) and anti-inflammatory cytokines (IL-4, IL-10), and (* VEGF, * bFGF) | Blood Serum | Glioblastoma | [84] |
| * CYFRA 21-1, * NSE and * CEA | CSF | Meningeal carcinomas | [85] |
| * CXCL13 plus interleukin 10 | CSF | CNS lymphoma | [74] |
| * VEGF receptor 1 and 2 | CSF | Leukemia CNS metastasis | [86] |
| miR-21 and miR-15b | CSF | Glioblastoma | [25] |
| miR-19, miR-21, and miR-92a | CSF | * PCNSL | [25] |
| miR-10b and miR-21 | CSF | Glioblastoma and brain Metastasis | [2] |
| Members of miR-200 family | CSF | Brain metastases from lung and breast cancers | [2] |
| miR 210 | Serum | Gliomas | [81] |
| miRNA-205 | Serum | Glioma | [87] |
| miR-21 | CSF | Glioma | [60] |
| MiR-451, -711, -223 and -125b | CSF | Glioblastoma, medulloblastoma, brain metastasis and lymphoma | [61] |
| MiR-935 | CSF | Only brain metastasis | [61] |
| * GFAP and * EGFR | Serum | Gliomas | [88] |
| Interleukin-10 | CSF | PCNSL | [89] |
| * PGD2 | CSF | Medulloblastoma | [90] |
| * IgG levels | CSF | Cerebral low-grade lymphoma | [91] |
| CXCL13 and CXCL12 | CSF | CNS lymphoma | [92] |
| * MIC-1/* GDF15 | CSF | Glioblastoma | [93] |
| Vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 | CSF | Brain metastases from lung and breast cancers | [94] |
| β-2 microglobulin | CSF | Myeloma of the central nervous system | [94] |
| Apolipoprotein A-II | CSF | Pediatric brain tumors (medulloblastoma , high-grade glioma, atypical rhabdoid tumor, astrocytoma, plexus carcinoma and anaplastic ependymoma, germ cell tumor) | [95] |
| VEGF | CSF | Leptomeningeal metastasis | [96] |
| VEGF and serologic (recoverin) | CSF and serum | Malignant glioma | [97] |
| Mitochondrial DNA mutations | CSF | Medulloblastoma | [98] |
| c-kit | CSF | Germ cell tumors | [99] |
| Human chorionic gonadotropin (hCG) and α-fetoprotein (AFP) | Serum and CSF | Intracranial germ cell tumors | [100] |
| Prostaglandin D synthase (β-trace) | CSF | Meningeal hemangiopericytoma | [101,102] |
| CD27 | CSF | CNS lymphoma | [103] |
| β-hCG | CSF and serum | Brain metastases from gestational trophoblastic tumors | [104] |
4. miRNAs as Potential Novel Candidates for Brain Cancer Markers in the CSF
5. Diagnostic and Prognostic Value of CSF miRNAs in Brain Cancers
6. Considerations and Concerns about the Use of Circulating miRNAs as Biomarkers

6.1. Issues Associated with CSF Collection and Preparation
6.2. Issues Associated with miRNA Isolation and Assessment
6.3. Issues Associated with miRNA-Profiling/Detection Methods
6.4. Issues Associated with Data Normalization
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Teplyuk, N.M.; Mollenhauer, B.; Gabriely, G.; Giese, A.; Kim, E.; Smolsky, M.; Kim, R.Y.; Saria, M.G.; Pastorino, S.; Kesari, S.; et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro-Oncology 2012, 14, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Astrakas, L.G.; Zurakowski, D.; Tzika, A.A.; Zarifi, M.K.; Anthony, D.C.; de Girolami, U.; Tarbell, N.J.; Black, P.M. Noninvasive magnetic resonance spectroscopic imaging biomarkers to predict the clinical grade of pediatric brain tumors. Clin. Cancer Res. 2004, 10, 8220–8228. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.R. Cerebrospinal fluid approach on neuro-oncology. Arq. Neuropsiquiatr. 2013, 71, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Yotsukura, S.; Mamitsuka, H. Evaluation of serum-based cancer biomarkers: A brief review from a clinical and computational viewpoint. Crit. Rev. Oncol. Hematol. 2015, 93, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsidawi, S.; Malek, E.; Driscoll, J.J. MicroRNAs in brain metastases: Potential role as diagnostics and therapeutics. Int. J. Mol. Sci. 2014, 15, 10508–10526. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.J. The miRNA-drug resistance connection: A new era of personalized medicine using noncoding RNA begins. Pharmacogenomics 2012, 13, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids—The mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Alonci, A.; Campo, S.; Penna, G.; Petrungaro, A.; Gerace, D.; Musolino, C. Circulating microRNAs: New biomarkers in diagnosis, prognosis and treatment of cancer (review). Int. J. Oncol. 2012, 41, 1897–1912. [Google Scholar] [PubMed]
- D'Asti, E.; Garnier, D.; Lee, T.H.; Montermini, L.; Meehan, B.; Rak, J. Oncogenic extracellular vesicles in brain tumor progression. Front. Physiol. 2012, 3, 294. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, J.P.; Ward, J.; Taylor, I.A.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimer's Dis. 2008, 14, 27–41. [Google Scholar]
- Melkonyan, H.S.; Feaver, W.J.; Meyer, E.; Scheinker, V.; Shekhtman, E.M.; Xin, Z.; Umansky, S.R. Transrenal nucleic acids: From proof of principle to clinical tests. Ann. N. Y. Acad. Sci. 2008, 1137, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, S.; Weber, J.; Baxter, D.; Galas, D.J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010, 38, 7248–7259. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Alexandrov, P.N.; Zhao, Y.; Hill, J.M.; Bhattacharjee, S. Spreading of Alzheimer’s disease inflammatory signaling through soluble micro-RNA. NeuroReport 2010, 23, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.F.G.; Baulande, S.; Gerber, N.U.; Baumgartner, M.; Grotzer, M.A. Detection and quantification of extracellular microRNAs in medulloblastoma. J. Cancer Metastasis Treat. 2015, 1, 67–75. [Google Scholar]
- Denk, J.; Boelmans, K.; Siegismund, C.; Lassner, D.; Arlt, S.; Jahn, H. MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s disease. PLoS ONE 2015, 10, e0126423. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.F.; Wu, N.; Wang, L.; Li, J. Circulating microRNAs: A novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell. Mol. Neurobiol. 2013, 33, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Baraniskin, A.; Kuhnhenn, J.; Schlegel, U.; Maghnouj, A.; Zollner, H.; Schmiegel, W.; Hahn, S.; Schroers, R. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro-Oncology 2012, 14, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.; Fiaschetti, G.; Baumgartner, M.; Grotzer, M.A. Significance and therapeutic value of miRNAs in embryonal neural tumors. Molecules 2014, 19, 5821–5862. [Google Scholar] [CrossRef] [PubMed]
- Burgos, K.L.; Javaherian, A.; Bomprezzi, R.; Ghaffari, L.; Rhodes, S.; Courtright, A.; Tembe, W.; Kim, S.; Metpally, R.; van Keuren-Jensen, K. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA 2013, 19, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Arenz, C. miRNAs as novel therapeutic targets and diagnostic biomarkers for Parkinson’s disease: A patent evaluation of WO2014018650. Expert Opin. Ther. Pat. 2014, 24, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.J.; Douglas, V.C.; Tihan, T.; Rubenstein, J.L.; Josephson, S.A. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013, 70, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, S.S.; Nygaard, A.B.; Nielsen, M.Y.; Jensen, K.; Christensen, T. miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl. Stroke Res. 2014, 5, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Gal, S.; Dunlop, H.M.; Pushkaran, B.; Liggins, A.P.; Pulford, K.; Banham, A.H.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br. J. Haematol. 2008, 141, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, X.; Zhao, Y.; Tian, T.; Jin, G.; Shu, Y.; Chen, Y.; Xu, L.; Zen, K.; Zhang, C.; et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.K.; Chong, W.W.; Jin, H.; Lam, E.K.; Shin, V.Y.; Yu, J.; Poon, T.C.; Ng, S.S.; Sung, J.J. Differential expression of microRNAs in plasma of patients with colorectal cancer: A potential marker for colorectal cancer screening. Gut 2009, 58, 1375–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Kosaka, N.; Tanaka, M.; Koizumi, F.; Kanai, Y.; Mizutani, T.; Murakami, Y.; Kuroda, M.; Miyajima, A.; Kato, T.; et al. MicroRNA-500 as a potential diagnostic marker for hepatocellular carcinoma. Biomarkers 2009, 14, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Liu, X.B.; Wong, B.Y.; Ng, R.W.; Yuen, A.P.; Wei, W.I. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin. Cancer Res. 2008, 14, 2588–2592. [Google Scholar] [CrossRef] [PubMed]
- Grunder, E.; D’Ambrosio, R.; Fiaschetti, G.; Abela, L.; Arcaro, A.; Zuzak, T.; Ohgaki, H.; Lv, S.Q.; Shalaby, T.; Grotzer, M. MicroRNA-21 suppression impedes medulloblastoma cell migration. Eur. J. Cancer 2011, 47, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.; Fiaschetti, G.; Baumgartner, M.; Grotzer, M.A. MicroRNA signatures as biomarkers and therapeutic target for CNS embryonal tumors: the pros and the cons. Int. J. Mol. Sci. 2014, 15, 21554–21586. [Google Scholar] [CrossRef] [PubMed]
- Fiaschetti, G.; Abela, L.; Nonoguchi, N.; Dubuc, A.M.; Remke, M.; Boro, A.; Grunder, E.; Siler, U.; Ohgaki, H.; Taylor, M.D.; et al. Epigenetic silencing of miRNA-9 is associated with HES1 oncogenic activity and poor prognosis of medulloblastoma. Br. J. Cancer 2014, 110, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, P.T.; Sanford, T.; Choudhary, A.; Mydlarz, W.W.; Brown, D.; Adai, A.T.; Ochs, M.F.; Ahrendt, S.A.; Mambo, E.; Califano, J.A. Serum microRNA biomarkers for detection of non-small cell lung cancer. PLoS ONE 2012, 7, e32307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuk, K.; Zucknick, M.; Heil, J.; Madhavan, D.; Schott, S.; Turchinovich, A.; Arlt, D.; Rath, M.; Sohn, C.; Benner, A.; et al. Circulating microRNAs in plasma as early detection markers for breast cancer. Int. J. Cancer 2013, 132, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Xi, Q.H.; Ge, W.L.; Zhang, X.Q. Identification of serum microRNA-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pac. J. Cancer Prev. 2013, 14, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Dong, R.; Huang, X.; Ding, S.; Qiu, H. Circulating microRNA-218 was reduced in cervical cancer and correlated with tumor invasion. J. Cancer Res. Clin. Oncol. 2012, 138, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Mitchell, P.S.; Kroh, E.M.; Dowell, A.E.; Chery, L.; Siddiqui, J.; Nelson, P.S.; Vessella, R.L.; Knudsen, B.S.; Chinnaiyan, A.M.; et al. Circulating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxia. PLoS ONE 2013, 8, e69239. [Google Scholar] [CrossRef] [PubMed]
- Redova, M.; Poprach, A.; Nekvindova, J.; Iliev, R.; Radova, L.; Lakomy, R.; Svoboda, M.; Vyzula, R.; Slaby, O. Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J. Transl. Med. 2012, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, Z.; Roberts, H.; Eichenberger, M.R.; Billeter, A.; Ocheretner, G.; Pan, J.; Rai, S.N.; Jorden, J.; Williford, A.; Galandiuk, S. A plasma microRNA panel for detection of colorectal adenomas: A step toward more precise screening for colorectal cancer. Ann. Surg. 2013, 258, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, L.; Liu, B.; Yang, L.; Meng, X.; Zhang, W.; Ma, Y.; Xiao, H. Genome-wide microRNA profiles identify miR-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012, 316, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ge, G.; Pan, T.; Wen, D.; Chen, L.; Yu, X.; Zhou, X.; Gan, J. A serum microRNA panel as potential biomarkers for hepatocellular carcinoma related with hepatitis B virus. PLoS ONE 2014, 9, e107986. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, X.; Du, Y.; Yao, W.; Shen, L.; Wang, C.; Hu, Z.; Zhuang, R.; Ning, G.; Zhang, C.; et al. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin. Chem. 2012, 58, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, J.; Kamohara, H.; Watanabe, M.; Tanaka, Y.; Kinoshita, K.; Saito, S.; Hiyoshi, Y.; Iwatsuki, M.; Baba, Y.; Baba, H. Serum microRNA-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J. Surg. Oncol. 2012, 106, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Lin, P.M.; Wang, Y.M.; Chen, Z.J.; Lin, S.F.; Yang, M.Y. Circulating miRNA is a novel marker for head and neck squamous cell carcinoma. Tumour Biol. 2012, 33, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Cantara, S.; Pilli, T.; Sebastiani, G.; Cevenini, G.; Busonero, G.; Cardinale, S.; Dotta, F.; Pacini, F. Circulating miRNA95 and miRNA190 are sensitive markers for the differential diagnosis of thyroid nodules in a Caucasian population. J. Clin. Endocrinol. Metab. 2014, 99, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, H.; Fukushima, S.; Yamashita, J.; Honda, N.; Oyama, R.; Kakimoto, A.; Masuguchi, S.; Ishihara, T.; Inoue, Y.; Jinnin, M.; et al. The circulating microRNA-221 level in patients with malignant melanoma as a new tumor marker. J. Dermatol. Sci. 2011, 61, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, L.; Gorur, A.; Yaroglu, H.Y.; Ozcan, C.; Tamer, L. Differential expression of microRNAs in plasma of patients with laryngeal squamous cell carcinoma: Potential early-detection markers for laryngeal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2013, 139, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Q.; Huang, G.L.; Guo, C.C.; Pu, X.X.; Lin, T.Y. Diagnostic and prognostic value of circulating miR-221 for extranodal natural killer/T-cell lymphoma. Dis. Markers 2010, 29, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ferrajoli, A.; Shanafelt, T.D.; Ivan, C.; Shimizu, M.; Rabe, K.G.; Nouraee, N.; Ikuo, M.; Ghosh, A.K.; Lerner, S.; Rassenti, L.Z.; et al. Prognostic value of miR-155 in individuals with monoclonal B-cell lymphocytosis and patients with B chronic lymphocytic leukemia. Blood 2013, 122, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, P.; Li, A.; Jiang, W.; Wang, H.; Wang, J.; Xie, K. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J. Exp. Clin. Cancer Res. 2012, 31, 97. [Google Scholar] [CrossRef] [PubMed]
- Ilhan-Mutlu, A.; Wagner, L.; Wohrer, A.; Furtner, J.; Widhalm, G.; Marosi, C.; Preusser, M. Plasma MicroRNA-21 concentration may be a useful biomarker in glioblastoma patients. Cancer Investig. 2012, 30, 1532–4192. [Google Scholar] [CrossRef] [PubMed]
- Ilhan-Mutlu, A.; Wagner, L.; Wohrer, A.; Jungwirth, S.; Marosi, C.; Fischer, P.; Preusser, M. Blood alterations preceding clinical manifestation of glioblastoma. Cancer Investig. 2012, 30, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, C.; Chen, X.; Chen, S.; Zhang, Y.; Zhi, F.; Wang, J.; Li, L.; Zhou, X.; Li, N.; et al. Identification of seven serum microRNAs from a genome-wide serum microRNA expression profile as potential noninvasive biomarkers for malignant astrocytomas. Int. J. Cancer 2013, 132, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wang, P.Y.; Li, X.Y.; Chen, J.X.; Li, Y.; Zhang, X.Z.; Zhang, C.G.; Jiang, T.; Li, W.B.; Ding, W.; et al. Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget 2015, 6, 26971–26981. [Google Scholar] [CrossRef] [PubMed]
- Drusco, A.; Bottoni, A.; Lagana, A.; Acunzo, M.; Fassan, M.; Cascione, L.; Antenucci, A.; Kumchala, P.; Vicentini, C.; Gardiman, M.P.; et al. A differentially expressed set of microRNAs in cerebro-spinal fluid (CSF) can diagnose CNS malignancies. Oncotarget 2015, 6, 20829–20839. [Google Scholar] [CrossRef] [PubMed]
- Baraniskin, A.; Kuhnhenn, J.; Schlegel, U.; Chan, A.; Deckert, M.; Gold, R.; Maghnouj, A.; Zollner, H.; Reinacher-Schick, A.; Schmiegel, W.; et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 2011, 117, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Machida, A.; Ohkubo, T.; Yokota, T. Circulating microRNAs in the cerebrospinal fluid of patients with brain diseases. Methods Mol. Biol. 2013, 1024, 203–209. [Google Scholar] [PubMed]
- Samuel, N.; Remke, M.; Rutka, J.T.; Raught, B.; Malkin, D. Proteomic analyses of CSF aimed at biomarker development for pediatric brain tumors. J. Neurooncol. 2014, 118, 225–238. [Google Scholar] [PubMed]
- Weston, C.L.; Glantz, M.J.; Connor, J.R. Detection of cancer cells in the cerebrospinal fluid: Current methods and future directions. Fluids Barriers CNS 2011, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Allen, J.E.; Dicker, D.T.; Peters, K.L.; Sheehan, J.M.; Glantz, M.J.; El-Deiry, W.S. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget 2011, 2, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Baraniskin, A.; Schroers, R. Modern cerebrospinal fluid analyses for the diagnosis of diffuse large B-cell lymphoma of the CNS. CNS Oncol. 2014, 3, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Josephson, S.A.; Fridlyand, J.; Karch, J.; Kadoch, C.; Karrim, J.; Damon, L.; Treseler, P.; Kunwar, S.; Shuman, M.A.; et al. Protein biomarker identification in the CSF of patients with CNS lymphoma. J. Clin. Oncol. 2008, 26, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Schroers, R.; Baraniskin, A.; Heute, C.; Kuhnhenn, J.; Alekseyev, A.; Schmiegel, W.; Schlegel, U.; Pels, H.J. Detection of free immunoglobulin light chains in cerebrospinal fluids of patients with central nervous system lymphomas. Eur. J. Haematol. 2010, 85, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Bougel, S.; Lhermitte, B.; Gallagher, G.; de Flaugergues, J.C.; Janzer, R.C.; Benhattar, J. Methylation of the hTERT promoter: A novel cancer biomarker for leptomeningeal metastasis detection in cerebrospinal fluids. Clin. Cancer Res. 2013, 19, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.; di Noto, R.; del Vecchio, L. Diagnostic strategies to investigate cerebrospinal fluid involvement in haematological malignancies. Leuk. Res. 2013, 37, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A. Overview of primary brain tumors: Pathologic classification, epidemiology, molecular biology, and prognostic markers. Hematol. Oncol. Clin. N. Am. 2012, 26, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Kersten, M.J.; Evers, L.M.; Dellemijn, P.L.; van den Berg, H.; Portegies, P.; Hintzen, R.Q.; van Lier, R.A.; von dem Borne, A.E.; van Oers, R.H. Elevation of cerebrospinal fluid soluble CD27 levels in patients with meningeal localization of lymphoid malignancies. Blood 1996, 87, 1985–1989. [Google Scholar] [PubMed]
- Rubenstein, J.L.; Wong, V.S.; Kadoch, C.; Gao, H.X.; Barajas, R.; Chen, L.; Josephson, S.A.; Scott, B.; Douglas, V.; Maiti, M.; et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 2013, 121, 4740–4748. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Johnston, S.K. Neoplastic meningitis: Survival as a function of cerebrospinal fluid cytology. Cancer 2009, 115, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Kros, J.M.; Mustafa, D.M.; Dekker, L.J.; Sillevis Smitt, P.A.; Luider, T.M.; Zheng, P.P. Circulating glioma biomarkers. Neuro-Oncology 2015, 17, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Cheng, J.X.; Zhang, W.; Zhang, X.; Wang, R.; Lin, H.; Huo, J.L.; Cheng, H. Quantitative detection of multiple gene promoter hypermethylation in tumor tissue, serum, and cerebrospinal fluid predicts prognosis of malignant gliomas. Neuro-Oncology 2010, 12, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Leptomeningeal metastasis. Curr. Opin. Oncol. 2010, 22, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Gleissner, B.; Chamberlain, M.C. Neoplastic meningitis. Lancet Neurol. 2006, 5, 443–452. [Google Scholar] [CrossRef]
- Le Rhun, E.; Taillibert, S.; Chamberlain, M.C. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg. Neurol. Int. 2013, 4, S265–S288. [Google Scholar] [PubMed]
- Lai, N.S.; Wu, D.G.; Fang, X.G.; Lin, Y.C.; Chen, S.S.; Li, Z.B.; Xu, S.S. Serum microRNA-210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br. J. Cancer 2015, 112, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Andreasson, U.; Blennow, K. CSF antithrombin III and disruption of the blood-brain barrier. J. Clin. Oncol. 2009, 27, 2302–2303. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cai, L.; Zhang, Y.; Tan, H.; Deng, Q.; Zhao, M.; Xu, X. Sensitive detection of EGFR mutations in cerebrospinal fluid from lung adenocarcinoma patients with brain metastases. J. Mol. Diagn. 2014, 16, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, R.; Codrici, E.; Popescu, I.D.; Mihai, S.; Necula, L.G.; Petrescu, D.; Teodoru, M.; Tanase, C.P. Cytokine patterns in brain tumour progression. Mediat. Inflamm. 2013, 2013, 979748. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Piao, Y.; Zhang, X.; Li, W.; Hao, X. The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer Biomark. 2013, 13, 123–130. [Google Scholar] [PubMed]
- Tang, Y.T.; Jiang, F.; Guo, L.; Si, M.Y.; Jiao, X.Y. The soluble VEGF receptor 1 and 2 expression in cerebral spinal fluid as an indicator for leukemia central nervous system metastasis. J. Neurooncol. 2013, 112, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Lan, F.; Hu, M.; Pan, Q.; Wang, Q.; Wang, J. Downregulation of serum microRNA-205 as a potential diagnostic and prognostic biomarker for human glioma. J. Neurosurg. 2015, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, A.; Gardberg, M.; Frantzen, J.; Parkkola, R.; Vuorinen, V.; Pesola, M.; Minn, H. Serum levels of GFAP and EGFR in primary and recurrent high-grade gliomas: Correlation to tumor volume, molecular markers, and progression-free survival. J. Neurooncol. 2015, 124, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, T.; Nakamizo, S.; Nishihara, M.; Kawamura, A.; Tanaka, H.; Mizukawa, K.; Miyake, S.; Taniguchi, M.; Hosoda, K.; Kohmura, E. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro-Oncology 2012, 14, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, M.U.; Hathout, Y.; MacDonald, T.J.; Kieran, M.W.; Gururangan, S.; Blaney, S.M.; Phillips, P.; Packer, R.; Gordish-Dressman, H.; Rood, B.R. Proteomic profiling of cerebrospinal fluid identifies prostaglandin D2 synthase as a putative biomarker for pediatric medulloblastoma: A pediatric brain tumor consortium study. Proteomics 2011, 11, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, G.; Psaras, T.; Krope, K.; von Coelln, R.; Fend, F.; Bock, T.; Schittenhelm, J.; Melms, A.; Meyermann, R.; Bornemann, A. Cerebral low-grade lymphoma and light chain deposition disease: Exceedingly high IgG levels in the cerebrospinal fluid as a diagnostic clue. Clin. Neuropathol. 2010, 29, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Korfel, A.; Pfeiffer, S.; Kiewe, P.; Volk, H.D.; Cakiroglu, H.; Widmann, T.; Thiel, E. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin. Cancer Res. 2009, 15, 5968–5973. [Google Scholar] [CrossRef] [PubMed]
- Shnaper, S.; Desbaillets, I.; Brown, D.A.; Murat, A.; Migliavacca, E.; Schluep, M.; Ostermann, S.; Hamou, M.F.; Stupp, R.; Breit, S.N.; et al. Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int. J. Cancer 2009, 125, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.D.; Hess, K.R.; Puduvalli, V.K.; Colman, H.; Conrad, C.A.; Gilbert, M.R.; Weinberg, J.; Cristofanilli, M.; Yung, W.K.; Liu, T.J. Biomarkers of disease: Cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J. Neurooncol. 2009, 94, 229–234. [Google Scholar] [CrossRef] [PubMed]
- De Bont, J.M.; den Boer, M.L.; Reddingius, R.E.; Jansen, J.; Passier, M.; van Schaik, R.H.; Kros, J.M.; Sillevis Smitt, P.A.; Luider, T.H.; Pieters, R. Identification of apolipoprotein A-II in cerebrospinal fluid of pediatric brain tumor patients by protein expression profiling. Clin. Chem. 2006, 52, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Wiendl, H.; Renninger, M.; Forschler, H.; Dichgans, J.; Weller, M. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: Diagnostic and prognostic value. Br. J. Cancer 2004, 91, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Sampath, P.; Weaver, C.E.; Sungarian, A.; Cortez, S.; Alderson, L.; Stopa, E.G. Cerebrospinal fluid (vascular endothelial growth factor) and serologic (recoverin) tumor markers for malignant glioma. Cancer Control 2004, 11, 174–180. [Google Scholar] [PubMed]
- Wong, L.J.; Lueth, M.; Li, X.N.; Lau, C.C.; Vogel, H. Detection of mitochondrial DNA mutations in the tumor and cerebrospinal fluid of medulloblastoma patients. Cancer Res. 2003, 63, 3866–3871. [Google Scholar] [PubMed]
- Miyanohara, O.; Takeshima, H.; Kaji, M.; Hirano, H.; Sawamura, Y.; Kochi, M.; Kuratsu, J. Diagnostic significance of soluble c-kit in the cerebrospinal fluid of patients with germ cell tumors. J. Neurosurg. 2002, 97, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Seregni, E.; Massimino, M.; Nerini Molteni, S.; Pallotti, F.; van der Hiel, B.; Cefalo, G.; Spreafico, F.; Fossati, F.; Bombardieri, E. Serum and cerebrospinal fluid human chorionic gonadotropin (hCG) and α-fetoprotein (AFP) in intracranial germ cell tumors. Int. J. Biol. Markers 2002, 17, 112–118. [Google Scholar] [PubMed]
- Kawashima, M.; Suzuki, S.O.; Yamashima, T.; Fukui, M.; Iwaki, T. Prostaglandin D synthase (β-trace) in meningeal hemangiopericytoma. Mod. Pathol. 2001, 14, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Saso, L.; Leone, M.G.; Sorrentino, C.; Giacomelli, S.; Silvestrini, B.; Grima, J.; Li, J.C.; Samy, E.; Mruk, D.; Cheng, C.Y. Quantification of prostaglandin D synthetase in cerebrospinal fluid: A potential marker for brain tumor. Biochem. Mol. Biol. Int. 1998, 46, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Saio, M.; Andoh, H.; Takenaka, K.; Shinoda, J.; Nishimura, Y.; Sakai, N.; Takami, T. Diagnostic utility of CSF soluble CD27 for primary central nervous system lymphoma in immunocompetent patients. Neurol. Res. 2000, 22, 434–442. [Google Scholar] [PubMed]
- Bakri, Y.; al-Hawashim, N.; Berkowitz, R. CSF/serum β-hCG ratio in patients with brain metastases of gestational trophoblastic tumor. J. Reprod. Med. 2000, 45, 94–96. [Google Scholar] [PubMed]
- Hannafon, B.N.; Ding, W.Q. Intercellular communication by exosome-derived microRNAs in cancer. Int. J. Mol. Sci. 2013, 14, 14240–14269. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Sanchez-Madrid, F. Intercellular communication: Diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Snuderl, M.; Batista, A.; Kirkpatrick, N.D.; Ruiz de Almodovar, C.; Riedemann, L.; Walsh, E.C.; Anolik, R.; Huang, Y.; Martin, J.D.; Kamoun, W.; et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 2013, 152, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wan, Q.; Li, L.; Jin, H.; Liu, Y.; Wang, Y.; Zhang, G. MicroRNAs as potential biomarkers for diagnosing cancers of central nervous system: A meta-analysis. Mol. Neurobiol. 2015, 51, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Ajit, S.K. Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors 2010, 12, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.D.; Young, A.M.; Karri, S.K. Biomarkers of pediatric brain tumors. Front. Pediatr. 2013, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Leach, P.A.; Estlin, E.J.; Coope, D.J.; Thorne, J.A.; Kamaly-Asl, I.D. Diffuse brainstem gliomas in children: Should we or shouldn’t we biopsy? Br. J. Neurosurg. 2008, 22, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.P.; Ismail, N.; Zhang, X.; Aguda, B.D.; Lee, E.J.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.L.; Schmittgen, T.D.; et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE 2008, 3, e3694. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Graveel, C.R.; Calderone, H.M.; Westerhuis, J.J.; Winn, M.E.; Sempere, L.F. Critical analysis of the potential for microRNA biomarkers in breast cancer management. Breast Cancer 2015, 7, 59–79. [Google Scholar] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2014, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Chevillet, J.R.; Lee, I.; Briggs, H.A.; He, Y.; Wang, K. Issues and prospects of microRNA-based biomarkers in blood and other body fluids. Molecules 2014, 19, 6080–6105. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalaby, T.; Grotzer, M.A. Tumor-Associated CSF MicroRNAs for the Prediction and Evaluation of CNS Malignancies. Int. J. Mol. Sci. 2015, 16, 29103-29119. https://doi.org/10.3390/ijms161226150
Shalaby T, Grotzer MA. Tumor-Associated CSF MicroRNAs for the Prediction and Evaluation of CNS Malignancies. International Journal of Molecular Sciences. 2015; 16(12):29103-29119. https://doi.org/10.3390/ijms161226150
Chicago/Turabian StyleShalaby, Tarek, and Michael A. Grotzer. 2015. "Tumor-Associated CSF MicroRNAs for the Prediction and Evaluation of CNS Malignancies" International Journal of Molecular Sciences 16, no. 12: 29103-29119. https://doi.org/10.3390/ijms161226150