Synthesis and Evaluation of Neuroprotective Selenoflavanones
Abstract
:1. Introduction
2. Results and Discussion

2.1. Synthesis of the Selenoflavanones and Flavanones


2.2. Physicochemical Properties of the Selenoflavanones and Flavanones
| Selenoflavanone | Physicochemical Properties | Flavanone | Physicochemical Properties | ||
|---|---|---|---|---|---|
| tPSA a (Å2) | ClogP a | tPSA a (Å2) | ClogP a | ||
| 1a | 26.3 | 4.20 | 2a | 35.5 | 3.50 |
| 1b | 35.5 | 3.88 | 2b | 44.8 | 3.11 |
2.3. Biological Activities of the Selenoflavanones and Flavanones
2.3.1. Antioxidant Effect and Cytotoxicity of the Selenoflavanones and Flavanones

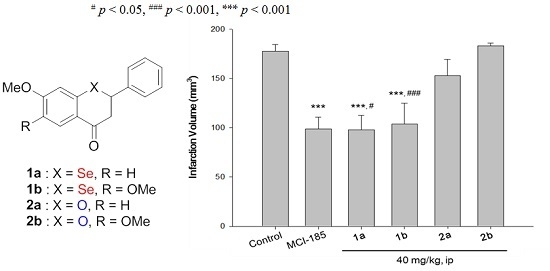
2.3.2. Neuroprotective Activity of the Selenoflavanones and Flavanones

3. Experimental Section
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for the Acylation of Bromobenzenes
3.1.3. General Procedure for the Formation of Selenoflavanones
3.1.4. General Procedure for the Acylation of Phenols
3.1.5. General Procedure for the Formation of Flavanones
3.2. In Vitro Cell Viability
3.2.1. Cell Culture
3.2.2. MTT Assay
3.3. In Vivo Neuroprotective Activity
3.3.1. Transient Ischemia Model
3.3.2. Drug Administration
3.3.3. Morphometric Measurement of Infarct Volume and Edema
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, S67–S90. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Pratap, R. The biological potential of flavones. Nat. Prod. Rep. 2010, 27, 1571–1593. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Carlson, S.H.; Wyss, J.M. Flavonoids and age-related disease: Risk, benefits and critical windows. Maturitas 2010, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Horakova, L. Flavonoids in prevention of diseases with respect to modulation of Ca-pump function. Interdiscip. Toxicol. 2011, 4, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. Oxygen radicals in focal cerebral ischemia. Brain Pathol. 1994, 4, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kondo, T.; Kawase, M.; Li, Y.; Sato, S.; Chen, S.F.; Chan, P.H. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J. Neurosci. 1998, 18, 205–213. [Google Scholar] [PubMed]
- Fujimura, M.; Morita-Fujimura, Y.; Kawase, M.; Copin, J.-C.; Calagui, B.; Epstein, C.J.; Chan, P.H. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J. Neurosci. 1999, 19, 3414–3422. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Sugawara, T.; Chan, P.H. Involvement of oxidative stress and caspase-3 in cortical infarction after photothrombotic ischemia in mice. J. Cereb. Blood Flow Metab. 2000, 20, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Gehrke, S.; Yang, Y.; Yang, L.; Beal, M.F.; Lu, B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Merino, C.; Lopez-Sanchez, C.; Lagoa, R.; Samhan-Arias, A.K.; Bueno, C.; Garcia-Martinez, V. Neuroprotective actions of flavonoids. Curr. Med. Chem. 2011, 18, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Kim, Y.-J.; Lee, J.Y.; Lee, J.; Jeong, J.-H. Synthesis and evaluation of selenoflavones that have potential neuroprotective effects. Heterocycles 2014, 89, 2794–2805. [Google Scholar]
- Batt, D.G.; Goodman, R.; Jones, D.G.; Kerr, J.S.; Mantegna, L.R.; McAllister, C.; Newton, R.C.; Nurnberg, S.; Welch, P.K.; Covington, M.B. 2’-Substituted chalcone derivatives as inhibitors of interleukin-1 biosynthesis. J. Med. Chem. 1993, 36, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.S.; McCullough, J.D. The dissociation constants of some monosubstituted benzeneseleninic acids. II. A new synthesis of diaryl diselenides. J. Am. Chem. Soc. 1951, 73, 1109–1112. [Google Scholar] [CrossRef]
- Huang, W.-H.; Chien, P.-Y.; Yang, C.-H.; Lee, A.-R. Novel synthesis of flavonoids of Scutellaria baicalensis Georgi. Chem. Pharm. Bull. 2003, 51, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Esmati, N.; Ardestani, S.K.; Emami, S.; Ajdari, S.; Davoodi, J.; Shafiee, A.; Foroumadi, A. Halogenated flavanones as potential apoptosis-inducing agents: Synthesis and biological activity evaluation. Eur. J. Med. Chem. 2012, 58, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, S.A.; Pennington, L.D. Structure-brain exposure relationships. J. Med. Chem. 2006, 49, 7559–7583. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Kilic, E.; Bahr, M.; Hermann, D.M. Effects of recombinant tissue plasminogen activator after intraluminal thread occlusion in mice. Stroke 2001, 32, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Lee, P.; Park, J.A.; Oh, H.R.; Lee, S.Y.; Park, J.-H.; Lee, E.H.; Ryu, J.H.; Lee, K.R.; Kim, S.Y. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem. Int. 2008, 52, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Namiki, Y.; Fukatsu-Sasaki, K.; Furutani, N.; Tada, N. Neuroprotective effects of edaravone: A novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006, 12, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Pitts, L.H.; Germano, S.M.; Nishimura, M.C.; Davis, R.L.; Bartowski, H.M. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986, 17, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-N.; He, Y.Y.; Wu, G.; Khan, M.; Hsu, C.Y. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 1993, 24, 117–121. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-S.; Kim, D.-M.; Kim, Y.-J.; Yang, S.; Lee, K.-T.; Ryu, J.H.; Jeong, J.-H. Synthesis and Evaluation of Neuroprotective Selenoflavanones. Int. J. Mol. Sci. 2015, 16, 29574-29582. https://doi.org/10.3390/ijms161226188
Choi Y-S, Kim D-M, Kim Y-J, Yang S, Lee K-T, Ryu JH, Jeong J-H. Synthesis and Evaluation of Neuroprotective Selenoflavanones. International Journal of Molecular Sciences. 2015; 16(12):29574-29582. https://doi.org/10.3390/ijms161226188
Chicago/Turabian StyleChoi, Yong-Sung, Dong-Myung Kim, Yoon-Jung Kim, Sai Yang, Kyung-Tae Lee, Jong Hoon Ryu, and Jin-Hyun Jeong. 2015. "Synthesis and Evaluation of Neuroprotective Selenoflavanones" International Journal of Molecular Sciences 16, no. 12: 29574-29582. https://doi.org/10.3390/ijms161226188