Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery
Abstract
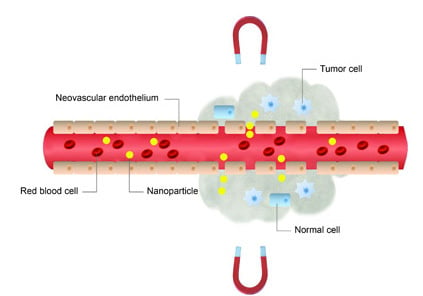
:1. Introduction
- (1)
- Magnetic contrast agents for magnetic resonance imaging (MRI).
- (2)
- Magnetic separation. For this, magnetic beads are functionalized with a biological or chemical agent known to bind to a specific target and mixed in a beaker with a solution believed to contain the target. After a specific time of contact between the beads and the solution, a permanent magnet placed alongside the solution beaker induces a magnetic moment and sets up a field gradient across the solution. The targets that have become bound to the magnetized beads are thus separated from the bulk solution.
- (3)
- Immunoassays, where MNPs bound to primary or secondary antibodies are used to separate and quantify antigens.
- (4)
- Hyperthermia agents, where the MNPs are selectively heated by application of a high-frequency alternating magnetic field.
- (5)
- Magnetic vectors that can be directed by means of magnetic field gradients towards a certain location, as in the case of the targeted drug delivery.

2. Physical Principles of Magnetism


- (1)
- Organic polymers, such as dextran, chitosan, polyethylene glycol (PEG), polysorbate and polyaniline.
- (2)
- Organic surfactants, such as sodium oleate and dodecylamine.
- (3)
- Inorganic metals, such as gold.
- (4)
- Inorganic, such as silica and carbon.
- (5)
- Bioactive molecules and structures, such as liposomes, peptides and ligands/receptors.
3. Iron Oxide Nanoparticles
4. Magnetically-Guided Drug Targeting
4.1. In Vitro Studies
4.2. In Vivo Studies: Animal Models
4.2.1. Chemotherapy


4.2.2. Other Biomedical Applications

4.3. In Vivo Studies in Humans
5. Magnetofection
6. Magnetically-Induced Drug Release


7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chatterjee, K.; Sarkar, S.; Jagajjanani Rao, K.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, S.F.; Santos, A.M.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lai, B.; Cheng, J.; Xia, G.; Gao, F.; Xu, W.; Ding, J.; Gao, C.; Sun, X.; Xu, C.; et al. Daunorubicin-loaded magnetic nanoparticles of Fe3O4 overcome multidrug resistance and induce apoptosis of K562-n/VCR cells in vivo. Int. J. Nanomed. 2008, 4, 201–208. [Google Scholar]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Seo, J.W.; Cheon, J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Craik, D. Magnetism: Principles and Applications; Wiley: Chichester, NY, USA, 1995. [Google Scholar]
- Fang, C.; Zhang, M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009, 19, 6258–6266. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.; Connolly, J.; Jones, S.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Bogart, L.K.; Pourroy, G.; Murphy, C.J.; Puntes, V.; Pellegrino, T.; Rosenblum, D.; Peer, D.; Levy, R. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano 2014, 8, 3107–3122. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.; Santamaría, J. Magnetic nanoparticles for drug delivery. NanoToday 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R., 2nd; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol. 2008, 3, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides. Structure, Properties, Reactions, Occurrence and Uses; VCH: Weinheim, Germany, 1996. [Google Scholar]
- Rebodos, R.L.; Vikesland, P.J. Effects of oxidation on the magnetization of nanoparticulate magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef] [PubMed]
- Haneda, K.; Morrish, A.H. Vacancy ordering in γ-Fe2O3 small particles. Solid State Commun. 1977, 22, 779–782. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Goya, G.F.; Berquo, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. App. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Roca, A.G.; Marco, J.F.; Morales, M.P.; Serna, C.J. Effect of nature and particle size on properties of uniform magnetite and maghemite nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Roca, A.G.; Morales, M.P.; O’Grady, K.; Serna, C.J. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology 2006, 17, 2783–2788. [Google Scholar] [CrossRef]
- Dunlop, D.J. Superparamagnetic and single-domain threshold sizes in magnetite. J. Geophys. Res. 1973, 78, 1780–1792. [Google Scholar] [CrossRef]
- Filippousi, M.; Angelakeris, M.; Katsikini, M.; Paloura, E.; Efthimiopoulos, I.; Wang, Y.; Zamboulis, D.; van Tendeloo, G. Surfactant effects on the structural and magnetic properties of iron oxide nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 16209–16217. [Google Scholar] [CrossRef]
- Freeman, M.W.; Arrot, A.; Watson, H.H.L. Magnetism in medicine. J. Appl. Phys. 1960, 31, S404. [Google Scholar] [CrossRef]
- Goodwin, S.; Peterson, C.; Hoh, C.; Bittner, C. Targeting and retention of magnetic targeted carriers (MTCs) enhancing intra-arterial chemotherapy. J. Magn. Magn. Mater. 1999, 194, 132–139. [Google Scholar] [CrossRef]
- Dobson, I. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Saiyed, Z.; Telang, S.; Ramchand, C. Application of magnetic techniques in the field of drug discovery and biomedicine. Biomagn. Res. Technol. 2003, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.M.; Kent, T.; Wolfe, C.; Ficco, C.; Johnson, J. MTC™: A magnetically targetable drug carrier for paclitaxel. In Scientific and Clinical Applications of Magnetic Carriers; Häfeli, U., Schütt, W., Teller, J., Zbororowski, M., Eds.; Plenum Press: New York, NY, USA, 1997; pp. 481–494. [Google Scholar]
- Tietze, R.; Jurgons, R.; Lyer, S.; Schreiber, E.; Wiekhorst, F.; Eberbeck, D.; Richter, H.; Steinhoff, U.; Trahms, L.; Alexiou, C. Quantification of drug-loaded magnetic nanoparticles in rabbit liver and tumor after in vivo administration. J. Magn. Magn. Mater. 2009, 321, 1465–1468. [Google Scholar] [CrossRef]
- Voltairas, P.A.; Fotiadis, D.I.; Michalis, L.K. Hydrodynamics of magnetic drug targeting. J. Biomech. 2002, 35, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Ruuge, E.K.; Rusetski, A.N. Magnetic fluids as drug carriers: Targeted transport of drugs by a magnetic field. J. Magn. Magn. Mater. 1993, 122, 335–339. [Google Scholar] [CrossRef]
- Grief, A.D.; Richardson, G. Mathematical modelling of magnetically targeted drug delivery. J. Magn. Magn. Mater. 2005, 293, 455–463. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic micro- and nano-particle-based targeting for drug and gene delivery. Nanomedicine (Lond.) 2006, 1, 31–37. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.K.T.; Jones, S.K.; Dobson, I. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001:1–224001:15. [Google Scholar] [CrossRef]
- Martel, S.; Mathieu, J.B.; Felfoul, O.; Chanu, A.; Aboussouan, E.; Tamaz, S.; Pouponneau, P.; Beaudoin, G.; Soulez, G.; Yahia, L.; et al. Automatic navigation of an untethered device in the artery of a living animal using a conventional clinical magnetic resonance imaging system. Appl. Phys. Lett. 2007, 90, 114105–114111. [Google Scholar] [CrossRef]
- Pouponneau, P.; Leroux, J.C.; Martel, S. Magnetic nanoparticles encapsulated into biodegradable microparticles steered with an upgraded magnetic resonance imaging system for tumor chemoembolization. Biomaterials 2009, 30, 6327–6332. [Google Scholar] [CrossRef] [PubMed]
- Pouponneau, P.; Savadogo, O.; Napporn, T.; Yahia, L.; Martel, S. Corrosion study of iron-cobalt alloys for MRI-based propulsion embedded in untethered microdevices operating in the vascular network. J. Biomater. Mater. Res. B Appl. Biomater. 2010, 93, 203–211. [Google Scholar]
- Mathieu, J.B.; Martel, S. Steering of aggregating magnetic microparticles using propulsion gradients coils in an MRI scanner. Magn. Reson. Med. 2010, 63, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.B.; Martel, S. Magnetic microparticle steering within the constraints of an MRI system: Proof of concept of a novel targeting approach. Biomed. Microdevices 2007, 9, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Seliger, C.; Jurgons, R.; Wiekhorst, F.; Eberbeck, D.; Trahms, L.; Iro, H.; Alexiou, C. In vitro investigation of the behavior of magnetic particles by circulating artery model. J. Magn. Magn. Mater. 2007, 311, 358–362. [Google Scholar] [CrossRef]
- Kettering, M.; Winter, J.; Zeisberger, M.; Bremer-Streck, S.; Oehring, H.; Bergemann, C.; Alexiou, C.; Hergt, R.; Halbhuber, K.J.; Kaiser, W.A.; et al. Magnetic nanoparticles as bimodal tools in magnetically induced labelling and magnetic heating of tumour cells: An in vitro study. Nanotechnology 2007, 18, 175101:1–175101:9. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Tsai, S.C.; Yang, C.Y.; Kuo, C.Y.; Chan, T.Y.; Zou, H.M.; Lian, W.N.; et al. Magnetic liposomes for colorectal cancer cells therapy by high-frequency magnetic field treatment. Nanoscale Res. Lett. 2014, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Chorny, M.; Hood, E.; Levy, R.J.; Muzykantov, V.R. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J. Control. Release 2010, 146, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Lee, E.J.; Son, M.; Lee, J.H.; Yoo, D.; Kim, J.W.; Park, S.W.; Shin, J.S.; Cheon, J. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat. Mater. 2012, 11, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; He, Y.J.; Chen, X.W.; Wang, J.H. Quantum dots conjugated with Fe3O4-filled carbon nanotubes for cancer-targeted imaging and magnetically guided drug delivery. Langmuir 2012, 28, 16469–16476. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.H.; Ho, V.T.; Chen, H.H.; Huang, W.C.; Huang, Y.F.; Lin, S.C.; Chern, C.S.; Chiu, H.C. Superparamagnetic hollow hybrid nanogels as a potential guidable vehicle system of stimuli-mediated MR imaging and multiple cancer therapeutics. Langmuir 2013, 29, 6434–6443. [Google Scholar] [CrossRef] [PubMed]
- Child, H.W.; del Pino, P.A.; de la Fuente, J.M.; Hursthouse, A.S.; Stirling, D.; Mullen, M.; McPhee, G.M.; Nixon, C.; Jayawarna, V.; Berry, C.C. Working together: The combined application of a magnetic field and penetratin for the delivery of magnetic nanoparticles to cells in 3D. ACS Nano 2011, 5, 7910–7919. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Ehrlich, H.P.; Buttle, D.J.; Nakatsuji, T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 1981, 211, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hayashida, M.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 2004, 10, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hibino, E.; Kobayashi, C.; Terasaki, H.; Kagami, H.; Ueda, M.; Kobayashi, T.; Honda, H. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Takizawa, Y.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. Tissue engineering using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004, 10, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hibino, E.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. A new methodology of mesenchymal stem cell expansion using magnetic nanoparticles. Biochem. Eng. J. 2004, 20, 119–125. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Linemann, T.; Pondman, K.M.; Lichota, J.; Kim, K.S.; Pieters, R.J.; Visser, G. M.; Moos, T. Uptake and transport of superparamagnetic iron oxide nanoparticles through human brain capillary endothelial cells. ACS Chem. Neurosci. 2013, 4, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Scherer, C.; Figueiredo Neo, A.M. Ferrofluids: Properties and applications. Braz. J. Phys. 2005, 35, 718–727. [Google Scholar] [CrossRef]
- Widder, K.J.; Senyei, A.E.; Scarpelli, D.G. Magnetic microspheres: A model system for site specific drug delivery in vivo. Proc. Soc. Exp. Biol. Med. 1978, 158, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Mosbach, K.; Schröder, U. Preparation and application of magnetic polymers for targeting of drugs. FEBS Lett. 1979, 102, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Senyei, A.; Widder, K.J.; Czerlinski, C. Magnetic guidance of drug carrying microspheres. J. Appl. Phys. 1978, 49, 3578–3583. [Google Scholar] [CrossRef]
- Widder, K.J.; Morris, R.M.; Poore, G.; Howard, D.P., Jr.; Senyei, A.E. Tumor remission in Yoshida sarcoma-bearing rts by selective targeting of magnetic albumin microspheres containing doxorubicin. Proc. Natl. Acad. Sci. USA 1981, 78, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Widder, K.J.; Marino, P.A.; Morris, R.M.; Howard, D.P.; Poore, G.A.; Senyei, A.E. Selective targeting of magnetic albumin microspheres to the Yoshida sarcoma: Ultrastructural evaluation of microsphere disposition. Eur. J. Cancer Clin. Oncol. 1983, 19, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.F.; Morris, R.M.; Senyei, A.E.; Widder, K.J.; Heller, G.S. Magnetic targeting of microspheres in blood flow. Microvasc. Res. 1984, 27, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Hung, C.T. Magnetically controlled targeted micro-carrier systems. Life Sci. 1989, 44, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Hung, C.T.; Rao, N.S. Ultrastructural disposition of adriamycin-associated magnetic albumin microspheres in rats. J. Pharm. Sci. 1989, 78, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Hung, C.T. Effect of carrier dose on the multiple tissue disposition of doxorubicin hydrochloride administered via magnetic albumin microspheres in rats. J. Pharm. Sci. 1989, 78, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Hung, C.T. Targeted delivery of low dose doxorubicin hydrochloride administered via magnetic albumin microspheres in rats. J. Microencapsul. 1990, 7, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Hung, C.T. Comparative disposition of adriamycin delivered via magnetic albumin microspheres in presence and absence of magnetic field in rats. Life Sci. 1990, 46, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.C.; Bittner, C.A.; Peterson, C.L.; Wong, G. Single-dose toxicity study of hepatic intra-arterial infusion of doxorubicin coupled to a novel magnetically targeted drug carrier. Toxicol. Sci. 2001, 60, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, A.S.; Bergemann, C.; Huhnt, W.; Fricke, T.; Riess, H.; Brock, J.W.; Huhn, D. Preclinical experiences with magnetic drug targeting: Tolerance and efficacy. Cancer Res. 1996, 56, 4694–4701. [Google Scholar] [PubMed]
- Pulfer, S.K.; Gallo, J.M. Enhanced brain tumor selectivity of cationic magnetic polysaccharide microspheres. J. Drug Target. 1998, 6, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Pulfer, S.K.; Ciccotto, S.L.; Gallo, J.M. Distribution of small magnetic particles in brain tumor-bearing rats. J. Neurooncol. 1999, 41, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylik, O.; Cherchenko, A.; Ilkin, A.; Dudchenko, N.; Ruditsa, V.; Novoseletz, M.; Zozulya, Y. Glial brain tumor targeting of magnetite nanoparticles in rats. J. Magn. Magn. Mater. 2001, 225, 241–247. [Google Scholar] [CrossRef]
- Viroonchatapan, E.; Sato, H.; Ueno, M.; Adachi, I.; Tazawa, K.; Horikoshi, I. Magnetic targeting of thermosensitive magnetoliposomes to mouse livers in an in situ on-line perfusion system. Life Sci. 1996, 58, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Sugita, T.; Shimose, S.; Nitta, Y.; Ikuta, Y.; Murakami, T. Targeted delivered of anticancer drugs with intravenously administered magnetic liposomes in osteosarcoma-beraing hamsters. Int. J. Oncol. 2000, 17, 309–316. [Google Scholar] [PubMed]
- Yellen, B.B.; Forbes, Z.G.; Halverson, D.S.; Fridman, G.; Barbee, K.A.; Chorny, M.; Levy, R.; Friedman, G. Targeted drug delivery to magnetic implants for therapeutic applications. J. Magn. Magn. Mater. 2005, 293, 647–654. [Google Scholar] [CrossRef]
- Kubo, T.; Sugita, T.; Shimose, S.; Nitta, Y.; Ikuta, Y.; Murakami, T. Targeted systemic chemotherapy using magnetic liposomes with incorporated adriamycin for osteosarcoma in hamsters. Int. J. Oncol. 2001, 18, 121–125. [Google Scholar] [PubMed]
- Nobuto, H.; Sugita, T.; Kubo, T.; Shimose, S.; Yasunaga, Y.; Murakami, T.; Ochi, M. Evaluation of systemic chemotherapy with magnetic liposomal doxorubicin and a dipole external electromagnrt. Int. J. Cancer 2004, 109, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, C.; Arnold, W.; Klein, R.J.; Parak, F.G.; Hulin, P.; Bergemann, C.; Erhardt, W.; Wagenpfeil, S.; Lubbe, A.S. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000, 60, 6641–6648. [Google Scholar] [PubMed]
- Alexiou, C.; Jurgons, R.; Schmid, R.J.; Bergemann, C.; Henke, J.; Erhardt, W.; Huenges, E.; Parak, F. Magnetic drug targeting-biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatment. J. Drug Target. 2003, 11, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Jurgons, R.; Seliger, C.; Hilpert, A.; Trahms, L.; Odenbach, S.; Alexiou, C. Drug loaded magnetic nanoparticles for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2893–S2902. [Google Scholar] [CrossRef]
- Tietze, R.; Lyer, S.; Duerr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Goeen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomedicine 2013, 9, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A novel investigational antimicrotubule agent. J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Dorr, R.T. Pharmacology and toxicology of Cremophor EL diluent. Ann. Pharmacother. 1994, 28 (Suppl 5), S11–s14. [Google Scholar]
- Sharma, A.; Straubinger, N.L.; Straubinger, R.M. Modulation of human ovarian tumor cell sensitivity to N-(phosphonacetyl)-l-aspartate (PALA) by liposome drug carriers. Pharm. Res. 1993, 10, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Zhang, Z.R.; Yang, H.; Tan, Q.Y.; Qin, S.R.; Qiu, X.L. Lyophilized paclitaxel magnetoliposomes as a potential drug delivery system for breast carcinoma via parenteral administration: In vitro and in vivo studies. Pharm. Res. 2005, 22, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Béalle, G.; Di Corato, R.; Kolosnjaj-Tabi, J.; Dupuis, V.; Clement, O.; Gazeau, F.; Wilhelm, C.; Menager, C. Ultra magnetic liposomes for MR imaging, targeting, and hyperthermia. Langmuir 2012, 28, 11834–11842. [Google Scholar] [CrossRef] [PubMed]
- Chertok, B.; David, A.E.; Yang, V.C. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intracarotid administration. Biomaterials 2010, 31, 6317–6324. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-M.; Guan, X.-M.; Liu, X.-Y.; Lan, J.-F.; Cheng, T.; Zhang, H.-X. Luminiscent/magnetic hybrid nanoparticles with folate-conjugated peptide composites for tumor-targeted drug delivery. Bioconj. Chem. 2012, 23, 1010–1021. [Google Scholar] [CrossRef]
- Pouponneau, P.; Leroux, J.P.; Soulez, G.; Gaboury, L.; Martel, S. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials 2011, 32, 3481–3486. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, N.M.; Akchurin, R.S.; Belyaev, A.A.; Smirnov, M.D.; Ragimov, S.E.; Orekhov, A.N. Local prevention of thrombosis in animal arteries by means of magnetic targeting of aspirin-loaded red cell. Thromb. Res. 1990, 57, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P.; Papisov, M.I.; Orekhova, N.M.; Belyaev, A.A.; Petrov, A.D.; Ragimov, S.E. Magnetically driven thrombolytic preparation containing immobilized streptokinase-targeted transport and action. Haemostasis 1988, 18, 113–116. [Google Scholar] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mishra, V.; Singh, P.; Dubey, P.K.; Saraf, D.K.; Vyas, S.P. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting. Int. J. Pharm. 2003, 261, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimeno, S.; Escribano, E.; Queralt, J.; Estelrich, J. External magnetic field-induced selective biodistribution of magnetoliposomes in mice. Nanoscale Res. Lett. 2012, 7, 452. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dorken, B.; Herrmann, F.; Gurtler, R.; et al. Clinical experiences with magnetic drug targeting: A phase I study with 4'-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar] [PubMed]
- Rudge, S.R.; Kurtz, T.L.; Vessely, C.R.; Catterall, L.G.; Williamson, D.L. Preparation, characterization, and performance of magnetic iron carbon composite microparticles for chemotherapy. Biomaterials 2000, 21, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Schutt, W.; Gruttner, C.; Teller, J.; Westphal, F.; Hafeli, U.; Paulke, B.; Goetz, P.; Finck, W. Biocompatible magnetic polymer carriers for in vivo radionuclide delivery. Artif. Organs 1999, 23, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.W.; Kerlan, R.K., Jr.; Fidelman, N.A.; Venook, A.P.; LaBerge, J.M.; Koda, J.; Gordon, R.L. Hepatocellular carcinoma: Regional therapy with a magnetic targeted carrier bound to doxorubicin in a dual MR imaging/conventional angiography suite-initial experience with four patients. Radiology 2004, 230, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Brill, T.; Rudolph, C.; Huth, S.; Gersting, S.; Krötz, F.; Hirschberger, J.; Bergemann, C.; Plank, C. Advances in magnetofection—Magnetically guided nucleic acid delivery. J. Magn. Magn. Mater. 2005, 293, 501–508. [Google Scholar] [CrossRef]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.; Zolotukhin, I.; Fraites, T.J., Jr.; Dobson, J.; Batich, C.; Byrne, B.J. Microsphere-mediated delivery with magnetic particles under the influence of a magnetic field. Mol. Ther. 2000, 1, S239. [Google Scholar] [CrossRef]
- Plank, C.; Scherer, C.; Schillinger, U.; Anton, M. Magnetofection: Enhancement and localization of gene delivery with magnetic particles under the influence of a magnetic field. J. Gene Med. 2000, 2 (Suppl.), 24. [Google Scholar]
- Hughes, C.; Galea-Lauri, J.; Farzaneh, F.; Darling, D. Streptavidin paramagnetic particles provide a choice of three affinity-based capture and magnetic concentration strategies for retroviral vectors. Mol. Ther. 2001, 3, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Kruger, A.; Gansbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.; Fraites, T.J., Jr.; Zolotukhin, I.; Song, S.; Flotte, T.R.; Dobson, J.; Batich, C.; Byrne, B.J. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther. 2002, 6, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Schillinger, U.; Scherer, F.; Bergemann, C.; Remy, J.S.; Krotz, F.; Anton, M.; Lausier, J.; Rosenecker, J. The magnetofection method: Using magnetic force to enhance gene delivery. Biol. Chem. 2003, 384, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, O.; Antequera, Y.S.; Vlaskou, D.; Plank, C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat. Protoc. 2007, 2, 2391–2411. [Google Scholar] [CrossRef] [PubMed]
- Gersting, S.W.; Schillinger, U.; Lausier, J.; Nicklaus, P.; Rudolph, C.; Plank, C.; Reinhardt, D.; Rosenecker, J. Gene delivery to respiratory epithelial cells by magnetofection. J. Gene Med. 2004, 6, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Krotz, F.; Sohn, H.Y.; Gloe, T.; Plank, C.; Pohl, U. Magnetofection potentiates gene delivery to cultured endothelial cells. J. Vasc. Res. 2003, 40, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Krotz, F.; de Wit, C.; Sohn, H.Y.; Zahler, S.; Gloe, T.; Pohl, U.; Plank, C. Magnetofection—A highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol. Ther. 2003, 7 (5 Pt 1), 700–710. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.P.; El Haj, A.; Dobson, J. Polyethyleneimine functionalized iron oxide nanoparticles as agents for DNA delivery and transfection. J. Mater. Chem. 2007, 17, 2561–2565. [Google Scholar] [CrossRef]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, O.; Zelphati, O.; Rosenecker, J.; Plank, C. siRNA delivery by magnetofection. Curr. Opin. Mol. Ther. 2008, 10, 493–505. [Google Scholar] [PubMed]
- Kamau, S.W.; Hassa, P.O.; Steitz, B.; Petri-Fink, A.; Hofmann, H.; Hofmann-Amtenbrink, M.; von Rechenberg, B.; Hottiger, M.O. Enhancement of the efficiency of non-viral gene delivery by application of pulsed magnetic field. Nucleic Acids Res. 2006, 34, e40. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Griesenbach, U.; Xenariou, S.; Keramane, A.; Batich, C.D.; Alton, E.W.; Dobson, J. Magnetic nanoparticles as gene delivery agents: Enhanced transfection in the presence of oscillating magnet arrays. Nanotechnology 2008, 19, 405102. [Google Scholar] [CrossRef] [PubMed]
- Stride, E.; Porter, C.; Prieto, A.G.; Pankhurst, Q. Enhancement of microbubble mediated gene delivery by simultaneous exposure to ultrasonic and magnetic fields. Ultrasound Med. Biol. 2009, 35, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Xenariou, S.; Griesenbach, U.; Ferrari, S.; Dean, P.; Scheule, R.K.; Cheng, S.H.; Geddes, D.M.; Plank, C.; Alton, E.W. Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene Ther. 2006, 13, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Huttinger, C.; Hirschberger, J.; Jahnke, A.; Kostlin, R.; Brill, T.; Plank, C.; Kuchenhoff, H.; Krieger, S.; Schillinger, U. Neoadjuvant gene delivery of feline granulocyte-macrophage colony-stimulating factor using magnetofection for the treatment of feline fibrosarcomas: A phase I trial. J. Gene Med. 2008, 10, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Furlani, E.P.; Ng, K.C. Nanoscale magnetic biotransport with application to magnetofection. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2008, 77, (6 Pt 1), 061914. [Google Scholar] [CrossRef]
- Laurent, N.; Sapet, C.; le Gourrierec, L.; Bertosio, E.; Zelphati, O. Nucleic acid delivery using magnetic nanoparticles: The magnetofection technology. Ther. Deliv. 2011, 2, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Sapet, C.; Laurent, N.; de Chevigny, A.; le Gourrierec, L.; Bertosio, E.; Zelphati, O.; Beclin, C. High transfection efficiency of neural stem cells with magnetofection. Biotechniques 2011, 50, 187–189. [Google Scholar] [PubMed]
- Kievit, F.M.; Veiseh, O.; Fang, C.; Bhattarai, N.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano 2010, 4, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Wolfrum, J.; Langer, R. Magnetically enhanced insulin release in diabetic rats. J. Biomed. Mater. Res. 1987, 21, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Babincová, M. Microwave induced leakage of magnetoliposomes. Possible clinical Implications. Bioelectrochem. Bioenerg. 1993, 32, 187–189. [Google Scholar] [CrossRef]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. High-frequency, magnetic-field-responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Appl. Mater. Interfaces 2010, 2, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Liu, K.H.; Liu, D.M.; Chen, S.Y.; Chen, I.W. Temperature-sensitive nanocapsules for controlled drug release caused by magnetically triggered structural disruption. Adv. Funct. Mater. 2009, 19, 616–623. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar] [CrossRef]
- Viroonchatapan, E.; Ueno, M.; Sato, H.; Adachi, I.; Nagae, H.; Tazawa, K.; Horikoshi, I. Preparation and characterization of dextran magnetite-incorporated thermosensitive liposomes: An on-line flow system for quantifying magnetic responsiveness. Pharm. Res. 1995, 12, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Babincova, M.; Cicmanec, P.; Altanerova, V.; Altaner, C.; Babinec, P. AC-magnetic field controlled drug release from magnetoliposomes: Design of a method for site-specific chemotherapy. Bioelectrochemistry 2002, 55, 17–19. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, V.M.; De Paoli Lacerda, S.H.; Spinu, L.; Ingber, B.; Rosenzweig, Z.; Rosenzweig, N. Effect of an oscillating magnetic field on the release properties of magnetic collagen gels. Langmuir 2006, 22, 5894–5899. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.A.; Tsai, P.J.; Wang, Y.C.; Wang, Y.J.; Lo, L.W.; Yang, C.S. Thermosensitive liposomes entrapping iron oxide nanoparticles for controllable drug release. Nanotechnology 2009, 20, 135101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bose, A.; Bothun, D.G. Controlled release from bilayer-decorated magnetoliposomes via electromagnetic heating. ACS Nano 2010, 6, 3215–3221. [Google Scholar] [CrossRef]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Fukuda, H. Preparation of thermo-sensitive magnetic hydrogel microspheres and application to enzyme mobilization. J. Ferment. Bioeng. 1997, 84, 337–341. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.M.; Liu, Y.F.; Ding, G.W.; Pei, Y.; Li, J.; Hua, G.F.; Huang, J. Preparation and characterization of magnetic targeted drug controlled-release hydrogel microspheres. Macromol. Symp. 2005, 225, 71–80. [Google Scholar] [CrossRef]
- Hu, S.-H.; Liu, T.-Y.; Liu, D.-M.; Chen, S.-Y. Controlled pulsatile drug release from a ferrogel by a high-frequancy magnetic field. Macromolecules 2007, 40, 6786–6788. [Google Scholar] [CrossRef]
- Baeza, A.; Guisasola, E.; Ruiz-Hernández, E.; Vallet-Regí, M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Hoare, T.; Santamaria, J.; Goya, G.F.; Irusta, S.; Lin, D.; Lau, S.; Padera, R.; Langer, R.; Kohane, D.S. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 2009, 9, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.; Timko, B.P.; Santamaria, J.; Goya, G.F.; Irusta, S.; Lau, S.; Stefanescu, C.F.; Lin, D.; Langer, R.; Kohane, D.S. Magnetically triggered nanocomposite membranes: A versatile platform for triggered drug release. Nano Lett. 2011, 11, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Thomas, C.R.; Ferris, D.P.; Lee, J.H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.S.; Cheon, J.; Zink, J.I. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070-8101. https://doi.org/10.3390/ijms16048070
Estelrich J, Escribano E, Queralt J, Busquets MA. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. International Journal of Molecular Sciences. 2015; 16(4):8070-8101. https://doi.org/10.3390/ijms16048070
Chicago/Turabian StyleEstelrich, Joan, Elvira Escribano, Josep Queralt, and Maria Antònia Busquets. 2015. "Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery" International Journal of Molecular Sciences 16, no. 4: 8070-8101. https://doi.org/10.3390/ijms16048070