Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy
Abstract
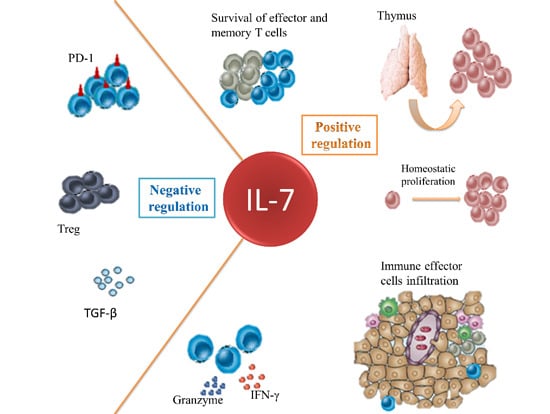
:1. Introduction
2. Biology of IL-7 and Its Signaling
3. Function of IL-7 in T Cell-Mediated Immune Response and the Underlying Mechanisms
3.1. IL-7 Signaling Is Essential for Thymopoiesis
3.2. IL-7 for Peripheral Homeostasis of T Cells
3.3. IL-7 in SLOs Is Important for Adaptive Immune Responses
4. Application of IL-7 in Cancer Immunotherapy
4.1. IL-7 for Immune Reconstitution in Cancer Patients
4.2. IL-7 Enhances the Function of Effector Immune Cells
4.3. IL-7 Antagonizes the Immunosuppressive Network
5. Potential Caveat of the Use of IL-7
6. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Puel, A.; Ziegler, S.F.; Buckley, R.H.; Leonard, W.J. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat. Genet. 1998, 20, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Surh, C.D.; Sprent, J. Homeostasis of naive and memory T cells. Immunity 2008, 29, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Kittipatarin, C.; Khaled, A.R. Interlinking interleukin-7. Cytokine 2007, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- ElKassar, N.; Gress, R.E. An overview of IL-7 biology and its use in immunotherapy. J. Immunotoxicol. 2010, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, W.; Fewkes, N.M.; Mackall, C.L. IL-7 in human health and disease. Semin. Immunol. 2012, 24, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, R.I.; Warming, S.; Lawrence, S.M.; Ishii, M.; Abshari, M.; Washington, A.V.; Feigenbaum, L.; Warner, A.C.; Sims, D.J.; Li, W.Q.; et al. Visualization and identification of IL-7 producing cells in reporter mice. PLoS ONE 2009, 4, e7637. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Vogt, T.K.; Favre, S.; Britschgi, M.R.; Acha-Orbea, H.; Hinz, B.; Cyster, J.G.; Luther, S.A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 2007, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Onder, L.; Narang, P.; Scandella, E.; Chai, Q.; Iolyeva, M.; Hoorweg, K.; Halin, C.; Richie, E.; Kaye, P.; Westermann, J.; et al. IL-7-producing stromal cells are critical for lymph node remodeling. Blood 2012, 120, 4675–4683. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, E.; DʼAntuono, T.; Pompa, P.; Giuliani, R.; Rosini, S.; Stuppia, L.; Musiani, P.; Sorrentino, C. The lack of epithelial interleukin-7 and BAFF/BLyS gene expression in prostate cancer as a possible mechanism of tumor escape from immunosurveillance. Clin. Cancer Res. 2009, 15, 2979–2987. [Google Scholar]
- Guimond, M.; Veenstra, R.G.; Grindler, D.J.; Zhang, H.; Cui, Y.; Murphy, R.D.; Kim, S.Y.; Na, R.; Hennighausen, L.; Kurtulus, S.; et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat. Immunol. 2009, 10, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Golden-Mason, L.; Kelly, A.M.; Traynor, O.; McEntee, G.; Kelly, J.; Hegarty, J.E.; OʼFarrelly, C. Expression of interleukin 7 (IL-7) mRNA and protein in the normal adult human liver: Implications for extrathymic T cell development. Cytokine 2001, 14, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sawa, Y.; Arima, Y.; Ogura, H.; Kitabayashi, C.; Jiang, J.J.; Fukushima, T.; Kamimura, D.; Hirano, T.; Murakami, M. Hepatic interleukin-7 expression regulates T cell responses. Immunity 2009, 30, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, W.Q.; Aiello, F.B.; Mazzucchelli, R.; Asefa, B.; Khaled, A.R.; Durum, S.K. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005, 16, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Coles, M. IL-7: The global builder of the innate lymphoid network and beyond, one niche at a time. Semin. Immunol. 2012, 24, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Kerdiles, Y.M.; Beisner, D.R.; Tinoco, R.; Dejean, A.S.; Castrillon, D.H.; DePinho, R.A.; Hedrick, S.M. Foxo1 links homing and survival of naive T cells by regulating l-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 2009, 10, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Beckett, O.; Flavell, R.A.; Li, M.O. An essential role of the forkhead-box transcription factor foxo1 in control of T cell homeostasis and tolerance. Immunity 2009, 30, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.H.; Bollenbacher, J.; Rovella, V.; Tripuraneni, R.; Du, Y.B.; Liu, C.Y.; Williams, A.; McCoy, J.P.; Leonard, W.J. GA binding protein regulates interleukin 7 receptor α-chain gene expression in T cells. Nat. Immunol. 2004, 5, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yu, Q.; Erman, B.; Appelbaum, J.S.; Montoya-Durango, D.; Grimes, H.L.; Singer, A. Suppression of IL7Rα transcription by IL-7 and other prosurvival cytokines: A novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 2004, 21, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Oh, S.A.; Ma, Q.; Bivona, M.R.; Zhu, J.; Li, M.O. TGF-β cytokine signaling promotes CD8+ t cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor α expression. Immunity 2013, 39, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, W.; Highfill, S.; Walsh, S.T.; Beq, S.; Morse, E.; Kockum, I.; Alfredsson, L.; Olsson, T.; Hillert, J.; Mackall, C.L. Soluble IL7Rα potentiates IL-7 bioactivity and promotes autoimmunity. Proc. Natl. Acad. Sci. USA 2013, 110, E1761–E1770. [Google Scholar] [CrossRef] [PubMed]
- Swainson, L.; Kinet, S.; Mongellaz, C.; Sourisseau, M.; Henriques, T.; Taylor, N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood 2007, 109, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Sato, S.; Katayama, K.; Tsuruo, T. Akt-dependent phosphorylation of p27kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 2002, 277, 28706–28713. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T.; Silva, A.; Brandao, J.G.; Nadler, L.M.; Cardoso, A.A.; Boussiotis, V.A. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 2004, 200, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Avots, A.; Zahedi, R.P.; Schuler, T.; Sickmann, A.; Bommhardt, U.; Serfling, E. An alternative NFAT-activation pathway mediated by IL-7 is critical for early thymocyte development. Nat. Immunol. 2013, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, R.; Durum, S.K. Interleukin-7 receptor expression: Intelligent design. Nat. Rev. Immunol. 2007, 7, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Schlissel, M.S.; Durum, S.D.; Muegge, K. The interleukin 7 receptor is required for t cell receptor gamma locus accessibility to the V(D)J recombinase. J. Exp. Med. 2000, 191, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, E.; Annett, G.; Parkman, R.; Weinberg, K. Serum levels of IL-7 in bone marrow transplant recipients: Relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999, 23, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Jameson, S.C. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2002, 2, 547–556. [Google Scholar] [PubMed]
- Bream, J.H.; Hodge, D.L.; Gonsky, R.; Spolski, R.; Leonard, W.J.; Krebs, S.; Targan, S.; Morinobu, A.; OʼShea, J.J.; Young, H.A. A distal region in the interferon-γ gene is a site of epigenetic remodeling and transcriptional regulation by interleukin-2. J. Biol. Chem. 2004, 279, 41249–41257. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.Y.; Pobezinsky, L.A.; Guinter, T.I.; Thomas, J.; Adams, A.; Park, J.H.; Tai, X.; Singer, A. IL-7 signaling must be intermittent, not continuous, during CD8+ T cell homeostasis to promote cell survival instead of cell death. Nat. Immunol. 2013, 14, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Sarin, A.; Aman, M.J.; Nakajima, H.; Shores, E.W.; Henkart, P.A.; Leonard, W.J. Functional cleavage of the common cytokine receptor γ chain (γc) by calpain. Proc. Natl. Acad. Sci. USA 1997, 94, 11534–11539. [Google Scholar] [CrossRef] [PubMed]
- Katz, G.; Pobezinsky, L.A.; Jeurling, S.; Shinzawa, M.; van Laethem, F.; Singer, A. T cell receptor stimulation impairs IL-7 receptor signaling by inducing expression of the microrna miR-17 to target janus kinase 1. Sci. Signal. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Fletcher, A.L.; Turley, S.J. Stromal and hematopoietic cells in secondary lymphoid organs: Partners in immunity. Immunol. Rev. 2013, 251, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.J.; Fletcher, A.L.; Elpek, K.G. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol. 2010, 10, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Bajenoff, M.; Egen, J.G.; Koo, L.Y.; Laugier, J.P.; Brau, F.; Glaichenhaus, N.; Germain, R.N. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006, 25, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, L.; Gao, J.; Yang, Y.; Hu, C.; Guo, B.; Zhu, B. T lymphocytes maintain structure and function of fibroblastic reticular cells via lymphotoxin (LT)-B. BMC Immunol. 2014, 15. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Liu, L.; Gao, J.; Guo, B.; Zhu, B. Essential role of TNF-α in development of spleen fibroblastic reticular cells. Cell. Immunol. 2015, 293, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Anne, L.; Fletcher, D.M.; Shannon, J.T. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol. 2011, 32, 6. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.W.; Duraes, F.V.; Hirosue, S.; Raghavan, V.R.; Nembrini, C.; Thomas, S.N.; Issa, A.; Hugues, S.; Swartz, M.A. VEGF-c promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012, 1, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: Implications for cancer therapy. Cancer Immunol. Res. 2015, 3, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Lampaki, S.; Yarmus, L.; Kioumis, I.; Pitsiou, G.; Katsikogiannis, N.; Hohenforst-Schmidt, W.; Li, Q.; Huang, H.; Sakkas, A.; et al. Interleukin-7 and interleukin-15 for cancer. J. Cancer 2014, 5, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Kmieciak, M.; Graham, L.; Morales, J.K.; Bear, H.D.; Manjili, M.H. Radiofrequency thermal ablation of breast tumors combined with intralesional administration of IL-7 and IL-15 augments anti-tumor immune responses and inhibits tumor development and metastasis. Breast Cancer Res. Treat. 2009, 114, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Le, H.K.; Graham, L.; Miller, C.H.; Kmieciak, M.; Manjili, M.H.; Bear, H.D. Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1 + ionomycin in IL-7 + IL-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in IL-2. Cancer Immunol. Immunother. 2009, 58, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.; Graham, L.; Manjili, M.H.; Bear, H.D. IL-7 + IL-15 are superior to IL-2 for the ex vivo expansion of 4T1 mammary carcinoma-specific T cells with greater efficacy against tumors in vivo. Breast Cancer Res. Treat. 2010, 122, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.W.; Sharma, S.; Stolina, M.; Butterfield, L.H.; Luo, J.; Lin, Y.; Dohadwala, M.; Batra, R.K.; Wu, L.; Economou, J.S.; et al. Intratumoral administration of adenoviral interleukin 7 gene-modified dendritic cells augments specific antitumor immunity and achieves tumor eradication. Hum. Gene Ther. 2000, 11, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Schroten, C.; Scheffer, R.; Boon, L.; de Ridder, C.M.; Bangma, C.H.; Kraaij, R. Tumor protection by IL-7 secreting whole cell vaccine is merely mediated by NK1.1-positive cells. J. Immunother. 2012, 35, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Schroten-Loef, C.; de Ridder, C.M.; Reneman, S.; Crezee, M.; Dalgleish, A.; Todryk, S.M.; Bangma, C.H.; Kraaij, R. A prostate cancer vaccine comprising whole cells secreting IL-7, effective against subcutaneous challenge, requires local GM-CSF for intra-prostatic efficacy. Cancer Immunol. Immunother. 2009, 58, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sportes, C.; Babb, R.R.; Krumlauf, M.C.; Hakim, F.T.; Steinberg, S.M.; Chow, C.K.; Brown, M.R.; Fleisher, T.A.; Noel, P.; Maric, I.; et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin. Cancer Res. 2010, 16, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Sportes, C.; Ahmadzadeh, M.; Fry, T.J.; Ngo, L.T.; Schwarz, S.L.; Stetler-Stevenson, M.; Morton, K.E.; Mavroukakis, S.A.; Morre, M.; et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 2006, 29, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Sportes, C.; Hakim, F.T.; Memon, S.A.; Zhang, H.; Chua, K.S.; Brown, M.R.; Fleisher, T.A.; Krumlauf, M.C.; Babb, R.R.; Chow, C.K.; et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008, 205, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Cropet, C.; van Glabbeke, M.; Sebban, C.; le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhao, Z.; Kalina, T.; Gillespy, T., III; Liggitt, D.; Andrews, R.G.; Maloney, D.G.; Kiem, H.P.; Storek, J. Interleukin-7 improves reconstitution of antiviral CD4 T cells. Clin. Immunol. 2005, 114, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Beq, S.; Rozlan, S.; Gautier, D.; Parker, R.; Mersseman, V.; Schilte, C.; Assouline, B.; Rance, I.; Lavedan, P.; Morre, M.; et al. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood 2009, 114, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Douek, D.C.; McFarland, R.D.; Koup, R.A. Effects of exogenous interleukin-7 on human thymus function. Blood 2002, 99, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Colombetti, S.; Levy, F.; Chapatte, L. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood 2009, 113, 6629–6637. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Calzascia, T.; Elford, A.R.; Shahinian, A.; Lin, A.E.; Dissanayake, D.; Dhanji, S.; Nguyen, L.T.; Gronski, M.A.; Morre, M.; et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat. Med. 2009, 15, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mei, Y.; Sun, Q.; Guo, L.; Wu, Y.; Yu, X.; Hu, B.; Liu, X.; Liu, H. Autologous tumor vaccine modified with recombinant new castle disease virus expressing IL-7 promotes antitumor immune response. J. Immunol. 2014, 193, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.H.; Yang, X.Q.; Zhu, C.L.; Liu, S.P.; Wang, B.C.; Wang, F.B. Interleukin-7 enhances the in vivo anti-tumor activity of tumor-reactive CD8+ T cells with induction of IFN-γ in a murine breast cancer model. Asian Pac. J. Cancer Prev. 2014, 15, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Jin, J.; Goldschneider, I. In vivo antitumor activity of a recombinant IL-7/HGFβ hybrid cytokine in mice. Cancer Res. 2011, 71, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cieri, N.; Camisa, B.; Cocchiarella, F.; Forcato, M.; Oliveira, G.; Provasi, E.; Bondanza, A.; Bordignon, C.; Peccatori, J.; Ciceri, F.; et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013, 121, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Srivastava, M.K.; Harris-White, M.; Huang, M.; Zhu, L.; Elashoff, D.; Strieter, R.M.; Dubinett, S.M.; Sharma, S. Role of CXCR3 ligands in IL-7/IL-7R α-FC-mediated antitumor activity in lung cancer. Clin. Cancer Res. 2011, 17, 3660–3672. [Google Scholar] [CrossRef] [PubMed]
- Heninger, A.K.; Theil, A.; Wilhelm, C.; Petzold, C.; Huebel, N.; Kretschmer, K.; Bonifacio, E.; Monti, P. IL-7 abrogates suppressive activity of human CD4+CD25+Foxp3+ regulatory T cells and allows expansion of alloreactive and autoreactive t cells. J. Immunol. 2012, 189, 5649–5658. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Yang, S.C.; Huang, M.; Zhu, L.; Kar, U.K.; Batra, R.K.; Elashoff, D.; Strieter, R.M.; Dubinett, S.M.; Sharma, S. IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung cancer. J. Immunol. 2009, 182, 6951–6958. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Laranjeira, A.B.; Martins, L.R.; Cardoso, B.A.; Demengeot, J.; Yunes, J.A.; Seddon, B.; Barata, J.T. IL-7 contributes to the progression of human T-cell acute lymphoblastic leukemias. Cancer Res. 2011, 71, 4780–4789. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T.; Cardoso, A.A.; Boussiotis, V.A. Interleukin-7 in T-cell acute lymphoblastic leukemia: An extrinsic factor supporting leukemogenesis? Leukemia Lymphoma 2005, 46, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, L.; Gloghini, A.; Olivo, K.; di Francia, R.; Lorenzon, D.; de Filippi, R.; Carbone, A.; Colombatti, A.; Pinto, A.; Aldinucci, D. Functional coexpression of interleukin (IL)-7 and its receptor (IL-7R) on hodgkin and reed-sternberg cells: Involvement of IL-7 in tumor cell growth and microenvironmental interactions of Hodgkinʼs lymphoma. Int. J. Cancer 2009, 125, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Clark, R.; Rich, B.; Dowgiert, R.; Hirahara, K.; Hurwitz, D.; Shibata, M.; Mirchandani, N.; Jones, D.A.; Goddard, D.S.; et al. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood 2006, 107, 2440–2445. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, M.A.; Mansel, R.E.; Jiang, W.G. Interleukin-7 (IL-7) and IL-7 receptor (IL-7R) signalling complex in human solid tumours. Histol. Histopathol. 2003, 18, 911–923. [Google Scholar] [PubMed]
- Roato, I.; D’Amelio, P.; Gorassini, E.; Grimaldi, A.; Bonello, L.; Fiori, C.; Delsedime, L.; Tizzani, A.; Libero, A.D.; Isaia, G.; et al. Osteoclasts are active in bone forming metastases of prostate cancer patients. PLoS ONE 2008, 3, e3627. [Google Scholar] [CrossRef] [PubMed]
- Mengus, C.; le Magnen, C.; Trella, E.; Yousef, K.; Bubendorf, L.; Provenzano, M.; Bachmann, A.; Heberer, M.; Spagnoli, G.C.; Wyler, S. Elevated levels of circulating IL-7 and IL-15 in patients with early stage prostate cancer. J. Transl. Med. 2011, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroten, C.; Dits, N.F.; Steyerberg, E.W.; Kranse, R.; van Leenders, A.G.; Bangma, C.H.; Kraaij, R. The additional value of TGFβ1 and IL-7 to predict the course of prostate cancer progression. Cancer Immunol. Immunother. 2012, 61, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Lambeck, A.J.; Crijns, A.P.; Leffers, N.; Sluiter, W.J.; ten Hoor, K.A.; Braid, M.; van der Zee, A.G.; Daemen, T.; Nijman, H.W.; Kast, W.M. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: A potential role for interleukin 7. Clin. Cancer Res. 2007, 13, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Caldo, D.; Godio, L.; DʼAmico, L.; Giannoni, P.; Morello, E.; Quarto, R.; Molfetta, L.; Buracco, P.; Mussa, A.; et al. Bone invading NSCLC cells produce IL-7: Mice model and human histologic data. BMC Cancer 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, L.; Gorgun, G.; Urbano, A.; Foss, F. Interleukin-7 receptor expression and activation in nonhaematopoietic neoplastic cell lines. Cell Signal. 2002, 14, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kadota, K.; Sima, C.S.; Nitadori, J.; Rusch, V.W.; Travis, W.D.; Sadelain, M.; Adusumilli, P.S. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: Tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal Foxp3/CD3 ratio are independent predictors of recurrence. J. Clin. Oncol. 2013, 31, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Capitini, C.M.; Chisti, A.A.; Mackall, C.L. Modulating t-cell homeostasis with IL-7: Preclinical and clinical studies. J. Intern. Med. 2009, 266, 141–153. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhao, L.; Wan, Y.Y.; Zhu, B. Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy. Int. J. Mol. Sci. 2015, 16, 10267-10280. https://doi.org/10.3390/ijms160510267
Gao J, Zhao L, Wan YY, Zhu B. Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy. International Journal of Molecular Sciences. 2015; 16(5):10267-10280. https://doi.org/10.3390/ijms160510267
Chicago/Turabian StyleGao, Jianbao, Lintao Zhao, Yisong Y. Wan, and Bo Zhu. 2015. "Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy" International Journal of Molecular Sciences 16, no. 5: 10267-10280. https://doi.org/10.3390/ijms160510267