Genipin Derivatives Protect RGC-5 from Sodium Nitroprusside-Induced Nitrosative Stress
Abstract
:1. Introduction

2. Results and Discussion
2.1. Effects of SNP on RGC-5 Cells

2.2. Cytotoxicity and MTT Activity Enhancement of CHR20/21 to RGC-5 Cells

2.3. Effects of CHR20/CHR21 after SNP-Induced Cytotoxicity at Different Duration

2.4. CHR20 and CHR21 Attenuated SNP-Induced Apoptosis of RGC-5 Cells

2.5. The Effect of SNP and CHR21 on the Activities of NOSs
| Groups | tNOS(U/mL) | iNOS(U/mL) | cNOS(U/mL) |
|---|---|---|---|
| ctrl | 8.31 ± 0.44 | 4.63 ± 0.43 | 3.68 ± 0.12 |
| CHR20 | 8.76 ± 0.50 | 4.61 ± 0.27 | 4.15 ± 0.17 & |
| CHR21 | 9.04 ± 0.60 | 4.54 ± 0.33 | 4.50 ± 0.18 & |
| SNP | 7.54 ± 0.27 * | 6.27 ± 0.13 * | 1.27 ± 0.06 * |
| SNP + CHR20 | 9.78 ± 0.31 # | 5.21 ± 0.24 # | 4.57 ± 0.26 # |
| SNP + CHR21 | 9.86 ± 0.35 # | 5.09 ± 0.26 # | 4.77 ± 0.29 # |
2.6. CHR20/CHR21 Increased the Phosphorylation Level of PI3K/Akt and MEK/ERK1/2 in RGC-5 Cells Time- and Dose-Dependently


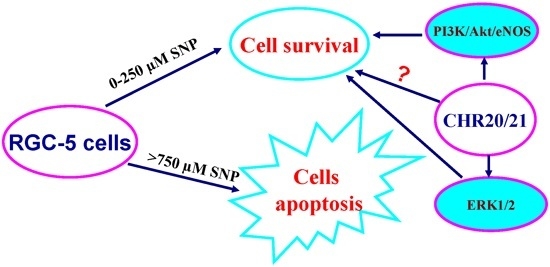
2.7. The PI3K/Akt/eNOS and ERK1/2 Signaling Pathways Are Involved in the Protective Effect of CHR20/21 on SNP-Induced Apoptosis in RGC-5 Cells



2.8. Discussion
3. Experimental Section
3.1. Materials and Reagents
3.2. MTT Assay
3.3. Determination of NOS Activity
3.4. Detection of Apoptosis
3.5. Cell Culture and Specific Signal Pathway Inhibition Treatment
3.6. Western Blotting Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guix, F.X.; Uribesalgo, I.; Coma, M.; Munoz, F.J. The physiology and pathophysiology of nitric oxide in the brain. Progr. Neurobiol. 2005, 76, 126–152. [Google Scholar] [CrossRef] [PubMed]
- Troy, C.M.; Rabacchi, S.A.; Friedman, W.J.; Frappier, T.F.; Brown, K.; Shelanski, M.L. Caspase-2 mediates neuronal cell death induced by β-amyloid. J. Neurosci. 2000, 20, 1386–1392. [Google Scholar] [PubMed]
- Contestabile, A.; Ciani, E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem. Int. 2004, 45, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Casey, R.M.; Ross, M.E.; Iadecola, C. Aminoguanidine ameliorates and l-arginine worsens brain damage from intraluminal middle cerebral artery occlusion. Stroke 1996, 27, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, A.H. Nitric oxide: A potential mediator of retinal ganglion cell damage in glaucoma. Surv. Ophthalmol. 1999, 43, S129–S135. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Lerner, S.F.; Brunzini, R.; Evelson, P.A.; Llesuy, S.F. Oxidative stress markers in aqueous humor of glaucoma patients. Am. J. Ophthalmol. 2004, 137, 62–69. [Google Scholar] [CrossRef]
- Prokai-Tatrai, K.; Xin, H.; Nguyen, V.; Szarka, S.; Blazics, B.; Prokai, L.; Koulen, P. 17β-Estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol. Pharm. 2013, 10, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Jonnala, R.R.; Buccafusco, J.J. Inhibition of nerve growth factor signaling by peroxynitrite. J. Neurosci. Res. 2001, 63, 27–34. [Google Scholar] [CrossRef]
- Chabrier, P.E.; Demerle-Pallardy, C.; Auguet, M. Nitric oxide synthases: Targets for therapeutic strategies in neurological diseases. Cell. Mol. Life Sci. 1999, 55, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Good, P.F.; Hsu, A.; Werner, P.; Perl, D.P.; Olanow, C.W. Protein nitration in Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1998, 57, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Richey Harris, P.L.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [PubMed]
- Good, P.F.; Werner, P.; Hsu, A.; Olanow, C.W.; Perl, D.P. Evidence of neuronal oxidative damage in Alzheimer’s disease. Am. J. Pathol. 1996, 149, 21–28. [Google Scholar] [PubMed]
- Cookson, M.R.; Shaw, P.J. Oxidative stress and motor neurone disease. Brain Pathol. 1999, 9, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A. Light and macular degeneration: A biophysical and clinical perspective. Eye 1987, 1, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.P.; Villegas-Perez, M.P.; Sobrado-Calvo, P.; Garcia-Aviles, A.; Miralles de Imperial, J.; Vidal-Sanz, M. Neuroprotective effects of α(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2074–2084. [Google Scholar]
- Organisciak, D.T.; Darrow, R.M.; Barsalou, L.; Darrow, R.A.; Kutty, R.K.; Kutty, G.; Wiggert, B. Light history and age-related changes in retinal light damage. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1107–1116. [Google Scholar]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes: Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.R.; Clark, A.F.; Daudt, D.; Vishwanatha, J.K.; Yorio, T. A forensic path to RGC-5 cell line identification: Lessons learned. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5712–5719. [Google Scholar] [CrossRef] [PubMed]
- Gabriela, M.; Subhash, C.A.; Jailall, R.; Shawn, P.M.; Suman, R.; John, S.A.; Dongqin, Z.; Frank, P. First-in-class, dual-action, 3,5-disubstituted indole derivatives having human nitric oxide synthase (nNOS) and norepinephrine reuptake inhibitory (NERI) activity for the treatment of neuropathic pain. J. Med. Chem. 2012, 55, 3488–3501. [Google Scholar]
- Patman, J.; Bhardwaj, N.; Ramnauth, J.; Annedi, S.C.; Renton, P.; Maddaford, S.P.; Rakhit, S.; Andrews, J.S. Novel 2-aminobenzothiazoles as selective neuronal nitric oxide synthase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 2540–2544. [Google Scholar] [CrossRef] [PubMed]
- Renton, P.; Speed, J.; Maddaford, S.; Annedi, S.C.; Ramnauth, J.; Rakhit, S.; Andrews, J. 1,5-Disubstituted indole derivatives as selective human neuronal nitric oxide synthase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 5301–5304. [Google Scholar] [CrossRef] [PubMed]
- Ramnauth, J.; Speed, J.; Maddaford, S.P.; Dove, P.; Annedi, S.C.; Renton, P.; Rakhit, S.; Andrews, J.; Silverman, S.; Mladenova, G.; et al. Design, synthesis, and biological evaluation of 3,4-dihydroquinolin-2(1H)-one and 1,2,3,4-tetrahydroquinoline-based selective human neuronal nitric oxide synthase (nNOS) inhibitors. J. Med. Chem. 2011, 54, 5562–5575. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, R.; Huang, Z.; Yang, J.; Yao, X.; Chen, H.; Zhen, W. Synthesis of stable derivatives from genipin and studies of their neuroprotective activity in PC12 cells. ChemMedChem 2012, 7, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, J.; Peng, L.; Zhao, J.; Mu, N.; Huang, J.; Lazarovici, P.; Chen, H.; Zheng, W. Gardenamide A attenuated cell apoptosis induced by serum deprivation via ERK1/2 and PI3K/Akt signaling pathways. Neuroscience 2015, 286, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Peng, L.; Zhao, J.; Zhang, L.; Guo, C.; Zheng, W.; Chen, H. Gardenamide A protects RGC-5 cells from h2o2-induced oxidative stress insults by activating PI3K/Akt/eNOS signaling pathway. Int. J. Mol. Sci. 2015, 16, 22350–22367. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, J.; Liao, S.; Xiao, G.; Luo, J.; Zhang, L.; Little, P.J.; Chen, H.; Zheng, W. Stereoselective reduction of 1-O-isopropyloxygenipin enhances its neuroprotective activity in neuronal cells from apoptosis induced by sodium nitroprusside. ChemMedChem 2014, 9, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Ann. Rev. 2005, 11, 127–152. [Google Scholar]
- Berridge, M.V.; Tan, A.S. Characterisation of the cellular reduction of 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, T.; Morris, R. Cyclic guanosine 3′,5′-monophosphate-mediated neuroprotection by nitric oxide in dissociated cultures of rat dorsal root ganglion neurones. Brain Res. 1997, 774, 116–122. [Google Scholar] [CrossRef]
- Thippeswamy, T.; Morris, R. Evidence that nitric oxide-induced synthesis of cGMP occurs in a paracrine but not an autocrine fashion and that the site of its release can be regulated: Studies in dorsal root ganglia in vivo and in vitro. Nitric Oxide 2001, 5, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, P.; Chen, X.X.; He, G.X. PI3K and ERK/Nrf2 pathways are involved in oleanolic acid-induced heme oxygenase-1 expression in rat vascular smooth muscle cells. J. Cell. Biochem. 2011, 112, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Nakamura, M.; Mukuno, H.; Kanamori, A.; Seigel, G.M.; Negi, A. Latanoprost rescues retinal neuro-glial cells from apoptosis by inhibiting caspase-3, which is mediated by p44/p42 mitogen-activated protein kinase. Exp. Eye Res. 2006, 83, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, B.; Zhu, C.; Feng, Y.; Wang, S.; Shahzad, M.; Hu, C.; Mo, M.; Du, F.; Yu, X. 17β-estradiol impedes Bax-involved mitochondrial apoptosis of retinal nerve cells induced by oxidative damage via the phosphatidylinositol 3-kinase/Akt signal pathway. J. Mol. Neurosci. 2013, 50, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.L.; Guh, J.H.; Chang, Y.L.; Kuo, S.C.; Lee, F.Y.; Teng, C.M. YC-1 prevents sodium nitroprusside-mediated apoptosis in vascular smooth muscle cells. Cardiovasc. Res. 2004, 61, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Z.H.; Chan, Y.M.; Siu, F.K.; Yip, H.K.; Wu, W.; Leung, M.C.; So, K.F. Regulation of caspase activation in axotomized retinal ganglion cells. Mol. Cell. Neurosci. 2004, 25, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Pernet, V.; Hauswirth, W.W.; di Polo, A. Extracellular signal-regulated kinase 1/2 mediates survival, but not axon regeneration, of adult injured central nervous system neurons in vivo. J. Neurochem. 2005, 93, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Zhang, L.; Little, P.J.; Xie, X.; Meng, Q.; Ren, Y.; Zhou, L.; Gao, G.; Quirion, R.; et al. Insulin-like growth factor-1 induces the phosphorylation of PRAS40 via the PI3K/Akt signaling pathway in PC12 cells. Neurosci. Lett. 2012, 516, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sapieha, P.; Kittlerova, P.; Hauswirth, W.W.; di Polo, A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J. Neurosci. 2002, 22, 3977–3986. [Google Scholar] [PubMed]
- Gao, F.; Gao, E.; Yue, T.L.; Ohlstein, E.H.; Lopez, B.L.; Christopher, T.A.; Ma, X.L. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: The roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 2002, 105, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Gordienko, D.V.; Tonshoff, B.; Gelato, M.C.; Goligorsky, M.S. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994, 45, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Caulin-Glaser, T.; Garcia-Cardena, G.; Sarrel, P.; Sessa, W.C.; Bender, J.R. 17β-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ. Res. 1997, 81, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Bauersachs, J.; Fisslthaler, B.; Busse, R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ. Res. 1998, 82, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Bauersachs, J.; Schafer, A.; Scholz, D.; Aldershvile, J.; Busse, R. Isometric contraction induces the Ca2+-independent activation of the endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1996, 96, 1123–1128. [Google Scholar] [CrossRef]
- Montagnani, M.; Chen, H.; Barr, V.A.; Quon, M.J. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J. Biol. Chem. 2001, 276, 30392–30398. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Peng, C.C.; Cheng, Y.M.; Lin, L.Y.; Ker, Y.B.; Chang, C.H.; Chen, K.C.; Peng, R.Y. Quercetin and ferulic acid aggravate renal carcinoma in long-term diabetic victims. J. Agric. Food Chem. 2010, 58, 9273–9280. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.H.; Kar, S.; Quirion, R. Insulin-like growth factor-1-induced phosphorylation of transcription factor FKHRL1 is mediated by phosphatidylinositol 3-kinase/Akt kinase and role of this pathway in insulin-like growth factor-1-induced survival of cultured hippocampal neurons. Mol. Pharmacol. 2002, 62, 225–233. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Zhao, J.; Zhang, L.; Peng, L.; Zhang, X.; Zheng, W.; Chen, H. Genipin Derivatives Protect RGC-5 from Sodium Nitroprusside-Induced Nitrosative Stress. Int. J. Mol. Sci. 2016, 17, 117. https://doi.org/10.3390/ijms17010117
Wang R, Zhao J, Zhang L, Peng L, Zhang X, Zheng W, Chen H. Genipin Derivatives Protect RGC-5 from Sodium Nitroprusside-Induced Nitrosative Stress. International Journal of Molecular Sciences. 2016; 17(1):117. https://doi.org/10.3390/ijms17010117
Chicago/Turabian StyleWang, Rikang, Jiaqiang Zhao, Lei Zhang, Lizhi Peng, Xinyi Zhang, Wenhua Zheng, and Heru Chen. 2016. "Genipin Derivatives Protect RGC-5 from Sodium Nitroprusside-Induced Nitrosative Stress" International Journal of Molecular Sciences 17, no. 1: 117. https://doi.org/10.3390/ijms17010117