Serum 25(OH) Vitamin D Levels in Polish Women during Pregnancies Complicated by Hypertensive Disorders and Gestational Diabetes
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Methods
4.2. Statistical Analyses
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Karras, S.; Paschou, S.A.; Kandaraki, E.; Anagnostis, P.; Annweiler, C.; Tarlatzis, B.C.; Hollis, B.W.; Grant, W.B.; Goulis, D.G. Hypovitaminosis D in pregnancy in the Mediterranean region: A systematic review. Eur. J. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D and sunlight: Strategies for cancer prevention and other health benefits. Clin. J. Am. Soc. Nephrol. 2008, 3, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; MacDonald, P. Vitamin D: More than a “bone-a-fide” hormone. Mol. Endocrinol. 2003, 17, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Blázquez-García, S.; Pérez-Sacristán, B. Simultaneous determination of vitamins A, E and 25-OH-vitamin D: Application in clinical assessments. Clin. Biochem. 2006, 39, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Fried, D.A.; Rhyu, J.; Odato, K.; Blunt, H.; Karagas, M.R.; Gilbert-Diamond, D. Maternal and cord blood vitamin D status and childhood infection and allergic disease: A systematic review. Nutr. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Walentowicz-Sadlecka, M.; Grabiec, M.; Sadlecki, P.; Gotowska, M.; Walentowicz, P.; Krintus, M.; Mankowska-Cyl, A.; Sypniewska, G. 25(OH)D3 in patients with ovarian cancer and its correlation with survival. Clin. Biochem. 2012, 45, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int. Suppl. 2011. [Google Scholar] [CrossRef] [PubMed]
- Walicka, M.; Marcinowska-Suchowierska, E. Vitamin D deficiency during pregnancy and lactation. Ginekol. Pol. 2008, 79, 780–784. [Google Scholar] [PubMed]
- Bener, A.; Al-Hamaq, A.O.; Saleh, N.M. Association between vitamin D insufficiency and adverse pregnancy outcome: Global comparisons. Int. J. Womens Health 2013, 5, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Rajagambeeram, R.; Abu Raghavan, S.; Ghosh, S.; Basu, S.; Ramasamy, R.; Murugaiyan, S.B. Diagnostic utility of heat stable alkaline phosphatase in hypertensive disorders of pregnancy. J. Clin. Diagn. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Gillon, T.E.R.; Pels, A.; von Dadelszen, P.; MacDonell, K.; Magee, L.A. Hypertensive disorders of pregnancy: A systematic review of international clinical practice guidelines. PLoS ONE 2014, 9, e113715. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Bärebring, L.; Bullarbo, M.; Glantz, A.; LeuAgelii, M.; Jagner, Å.; Ellis, J.; Hulthén, L.; Schoenmakers, I.; Augustin, H. Preeclampsia and blood pressure trajectory during pregnancy in relation to vitamin D status. PLoS ONE 2016, 11, e0152198. [Google Scholar] [CrossRef] [PubMed]
- Kazimierak, W.; Kowalska-Koprek, U.; Karowicz-Bilińska, A.; Berner-Trąbska, M.; Lenczowska-Wężyk, M.; Brzozowska, M.; Kuś, E. 24-hour Holter measurement of blood pressure in pregnant women and effectiveness of the treatment. Ginekol. Pol. 2008, 79, 174–176. [Google Scholar] [PubMed]
- Szczepaniak-Chicheł, L.; Tykarski, A. Treatment of arterial hypertension in pregnancy in relation to current guidelines of the Polish Society of Arterial Hypertension from 2011. Ginekol. Pol. 2012, 83, 778–783. [Google Scholar] [PubMed]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Głuchowska, M.; Kowalska-Koprek, U.; Karowicz-Bilińska, A. Evaluation of the usefulness of endoglin level as a predictor of preeclampsia in pregnant women with hypertension. Ginekol. Pol. 2013, 84, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sepulveda, A.; Monteiro, L.J.; Dobierzewska, A.; España-Perrot, P.P.; Venegas-Araneda, P.; Guzmán-Rojas, A.M.; González, M.I.; Palominos-Rivera, M.; Irarrazabal, C.E.; Figueroa-Diesel, H.; et al. Placental aromatase is deficient in placental ischemia andpreeclampsia. PLoS ONE 2015, 10, e0139682. [Google Scholar] [CrossRef] [PubMed]
- Kornacki, J.; Skrzypczak, J. Preeclampsia—Two manifestations of the same disease. Ginekol. Pol. 2008, 79, 432–437. [Google Scholar] [PubMed]
- Weinert, L.S. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: Comment to the international association of diabetes and pregnancy study groups consensus panel. Diabetes Care 2010, 33, e97. [Google Scholar] [CrossRef] [PubMed]
- Wojcikowski, C.; Lech, M.; Chećka, Z.; Wierzchowska, J.; Lukaszuk, K. Large-scale screening for early diagnosis of gestational diabetes mellitus (GDM). Ginekol. Pol. 1997, 68, 297–301. [Google Scholar] [PubMed]
- Genuis, S.J. Maternal and pediatric health outcomes in relation to gestational vitamin D sufficiency. Obst. Gynecol. Int. 2015, 2015, 501829. [Google Scholar] [CrossRef] [PubMed]
- Pleskačová, A.; Bartáková, V.; Pácal, L.; Kuricová, K.; Bělobrádková, J.; Tomandl, J.; Kaňková, K. Vitamin D status in women with gestational diabetes mellitus during pregnancy and postpartum. BioMed Res. Int. 2015, 2015, 260624. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Sangha, R.; Cassidy-Bushrow, A.E.; Wegienka, G. Maternal-cord blood vitamin D correlations vary by maternal levels. J. Pregnancy 2016, 2016, 7474192. [Google Scholar]
- Ali, M.M.; Vaidya, V. Vitamin D and cancer. J. Cancer Res. Ther. 2007, 3, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Dawodu, A.; Wagner, C.L. Prevention of vitamin D deficiency in mothers and infants worldwide—A paradigm shift. Paediatr. Int. Child Health 2012, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and vitamin D: Necessary for public health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Su, L.; Liu, M.; Liu, Y.; Cao, X.; Wang, Z.; Xiao, H. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: A prospective observational study in southern China. Eur. J. Clin. Nutr. 2014, 68, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Rifas-Shiman, S.L.; Huh, S.Y.; Kleinman, K.; Litonjua, A.A.; Oken, E.; Rich-Edwards, J.W.; Camargo, C.A.; Gillman, M.W. Vitamin D status and hypertensive disorders in pregnancy. Ann. Epidemiol. 2014, 24, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Van Weert, B.; van den Berg, D.; Hrudey, E.J.; Oostvogels, A.J.; de Miranda, E.; Vrijkotte, T.G. Is first trimester vitamin D status in nulliparous women associated with pregnancy related hypertensive disorders? Midwifery 2016, 34, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Seely, E.W.; Rana, S.; Bhan, I.; Ecker, J.; Karumanchi, S.A.; Thadhani, R. First trimester vitamin D, vitamin D binding protein and subsequent preeclampsia. Hypertension 2010, 56, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E. Vitamin D for the prevention of preeclampsia? A hypothesis. Nutr. Rev. 2005, 63, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.M.; Hämäläinen, E.; Mäki, A.; Räikkönen, K.; Pesonen, A.-K.; Taipale, P.; Kajantie, E.; Laivuori, H. Vasoactive agents for the prediction of early- and late-onset preeclampsia in a high-risk kohort. BMC Pregnancy Childbirth 2013, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, H.; Mosblech, N.; Reineke, T.; Puchooa, A.; Menke-Möllers, I.; Zechner, U.; Gembruch, U. Prenatal clinical assessment ofsFlt-1(soluble fms-like tyrosine kinase-1)/PlGF (placental growth factor) ratio as a diagnostic tool for preeclampsia, pregnancy-inducedhypertension and proteinuria. Geburtshilfe Frauenheilkd. 2013, 73, 440–445. [Google Scholar] [PubMed]
- Holmes, V.A.; Young, I.S.; Patterson, C.C.; Maresh, M.J.; Pearson, D.W.; Walker, J.D.; McCance, D.R.; diabetesand preeclampsia intervention trial (dapit) study group. The role of angiogenic and antiangiogenic factors in the second trimester in the prediction of preeclampsia in pregnant women with type 1diabetes. Diabetes Care 2013, 36, 3671–3677. [Google Scholar] [CrossRef] [PubMed]
- Zabul, P.; Wozniak, M.; Slominski, A.T.; Preis, K.; Gorska, M.; Korozan, M.; Wieruszewski, J.; Zmijewski, M.A.; Zabul, E.; Tuckey, R.; et al. A Proposed molecular mechanism of high-dose vitamin D3 supplementation in prevention and treatment of preeclampsia. Int. J. Mol. Sci. 2015, 16, 13043–13064. [Google Scholar] [CrossRef] [PubMed]
- Mutter, W.P.; Karumanchi, S.A. Molecular mechanisms ofpreeclampsia. Microvasc. Res. 2008, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Cavadino, A.; Williams, D.; Fraser, A.; Vereczkey, A.; Fraser, W.D.; Banhidy, F.; Lawlor, D.; Czeizel, A.E. Vitamin D and Pre-Eclampsia: Original data, systematic review and meta-analysis. Ann. Nutr. Metab. 2013, 63, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Haugen, M.; Brantsaeter, A.L.; Trogstad, L.; Aleksander, J.; Roth, C.; Magnus, P.; Meltzer, H.M. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 2009, 20, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.; Salehi-Abargouei, A.; Tabesh, M.; Esmaillzadeh, A. Maternal vitamin D status and risk of pre-eclampsia: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Pena, H.R.; de Lima, M.C.; Brandt, K.G.; de Antunes, M.M.; da Silva, G.A. Influence of preeclampsia and gestational obesity in maternal and newborn levels of vitamin D. BMC Pregnancy Childbirth 2015, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, Z.; Abedi, P.; Dilgouni, T.; Namvar, F.; Ruzafza, S. The relation of preeclampsia and serum level of 25-hydroxyvitamin D in mothers and their neonates: A case control study in Iran. Horm. Metab. Res. 2015, 47, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Halhali, A.; Díaz, L.; Barrera, D.; Avila, E.; Larrea, F. Placental calcitriol synthesis and IGF-I levels in normal and preeclampticpregnancies. J. Steroid Biochem. Mol. Biol. 2014, 144, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Evans, K.N.; Kilby, M.D.; Bulmer, J.N.; Innes, B.A.; Stewart, P.M.; Hewison, M. The ontogeny of 25-hydroxyvitamin D3 1α-hydroxylase expression in human placenta and deciduas. Am. J. Pathol. 2002, 161, 105–114. [Google Scholar] [CrossRef]
- Farrant, H.J.; Krishnaveni, G.V.; Hill, J.C.; Boucher, B.J.; Fisher, D.J.; Noonan, K.; Osmond, C.; Veena, S.R.; Fall, C.H.D. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur. J. Clin. Nutr. 2009, 63, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Loy, S.L.; Lek, N.; Yap, F.; Soh, S.E.; Padmapriya, N.; Tan, K.H.; Biswas, A.; Yeo, G.S.; Kwek, K.; Gluckman, P.D.; et al. Association of maternal vitamin D status with glucose tolerance and caesarean section in a Multi-Ethnic Asian Cohort: The growing up in Singapore towards healthy outcomes study. PLoS ONE 2015, 10, e0142239. [Google Scholar] [CrossRef] [PubMed]
- Nobles, C.J.; Markenson, G.; Chasan-Taber, L. Early pregnancy vitamin D status and risk for adverse maternal and infant outcomes in a bi-ethnic cohort: The behaviors affecting baby and you (baby) study. Br. J. Nutr. 2015, 114, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Pan, G.T.; Guo, J.F.; Li, B.Y.; Qin, L.Q.; Zhang, Z.L. Vitamin D deficiency increases the risk of gestational diabetes mellitus: A meta-analysis of observational studies. Nutrients 2015, 7, 8366–8375. [Google Scholar] [CrossRef] [PubMed]
- White, P. Pregnancy complicating diabetes. Am. J. Med. 1949, 7, 609–616. [Google Scholar] [CrossRef]
| 25(OH)D Concentration (ng/mL) | Study Group (N = 171) | Control Group (N = 36) |
|---|---|---|
| Sufficient concentration: 25(OH)D ≥ 30 | 18 (10.8%) | 11 (30.5%) |
| Insufficientconcentration: 25(OH)D: 20–30 | 75 (43.7%) | 15 (41.7%) |
| Deficiency: 25(OH)D < 20 | 78 (45.6%) | 10 (27.8%) |
| 25(OH)D ng/mL | N | Mean | SD | Minimum | Q1 | Median | Q3 | Maximum | Mann-Whitney U-Text |
|---|---|---|---|---|---|---|---|---|---|
| GH | 45 | 19.01 | 7.36 | 8.00 | 13.70 | 18.20 | 24.30 | 37.50 | NS |
| Controls | 36 | 21.65 | 5.13 | 14.60 | 16.95 | 22.10 | 24.90 | 32.30 | |
| PE | 23 | 21.65 | 5.13 | 14.60 | 16.95 | 22.10 | 24.90 | 32.30 | Z = 3.0759 p = 0.0021 |
| Controls | 36 | 21.65 | 5.13 | 14.60 | 16.95 | 22.10 | 24.90 | 32.30 | |
| GDM | 103 | 21.99 | 7.43 | 9.40 | 15.50 | 22.10 | 27.30 | 47.10 | NS |
| Controls | 36 | 21.65 | 5.13 | 14.60 | 16.95 | 22.10 | 24.90 | 32.30 |
| Parameter | GH | PE | GDM |
|---|---|---|---|
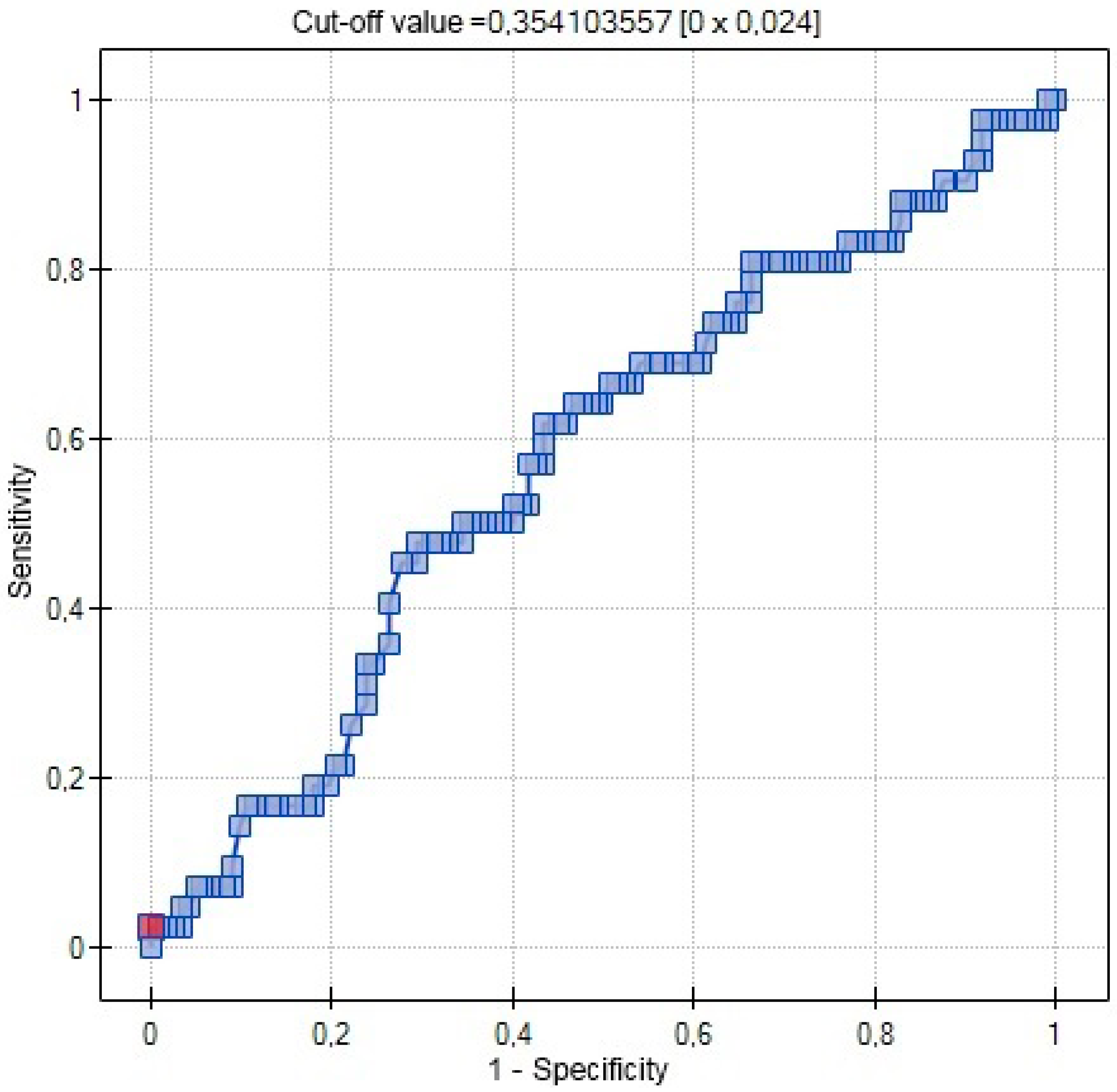
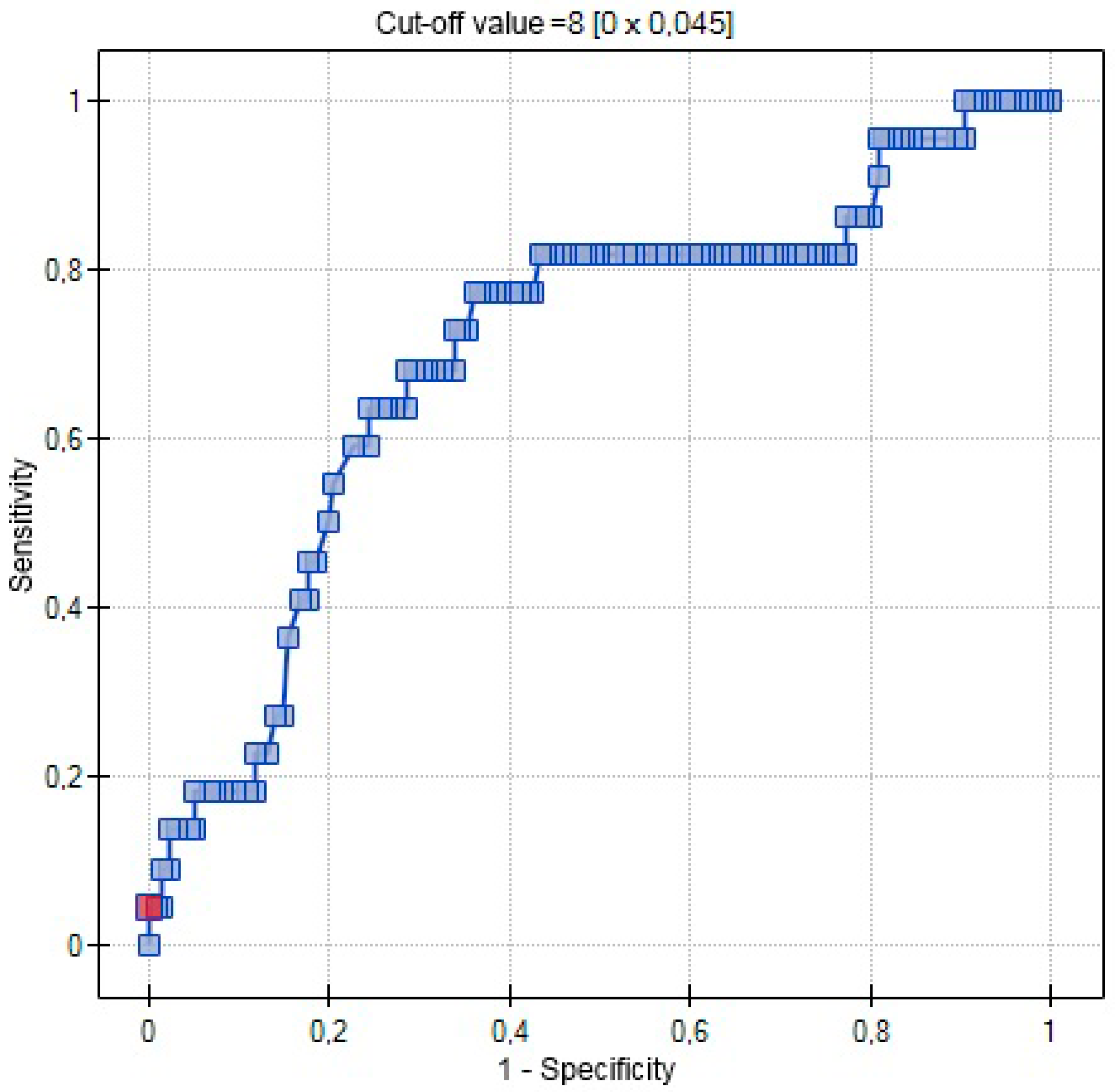
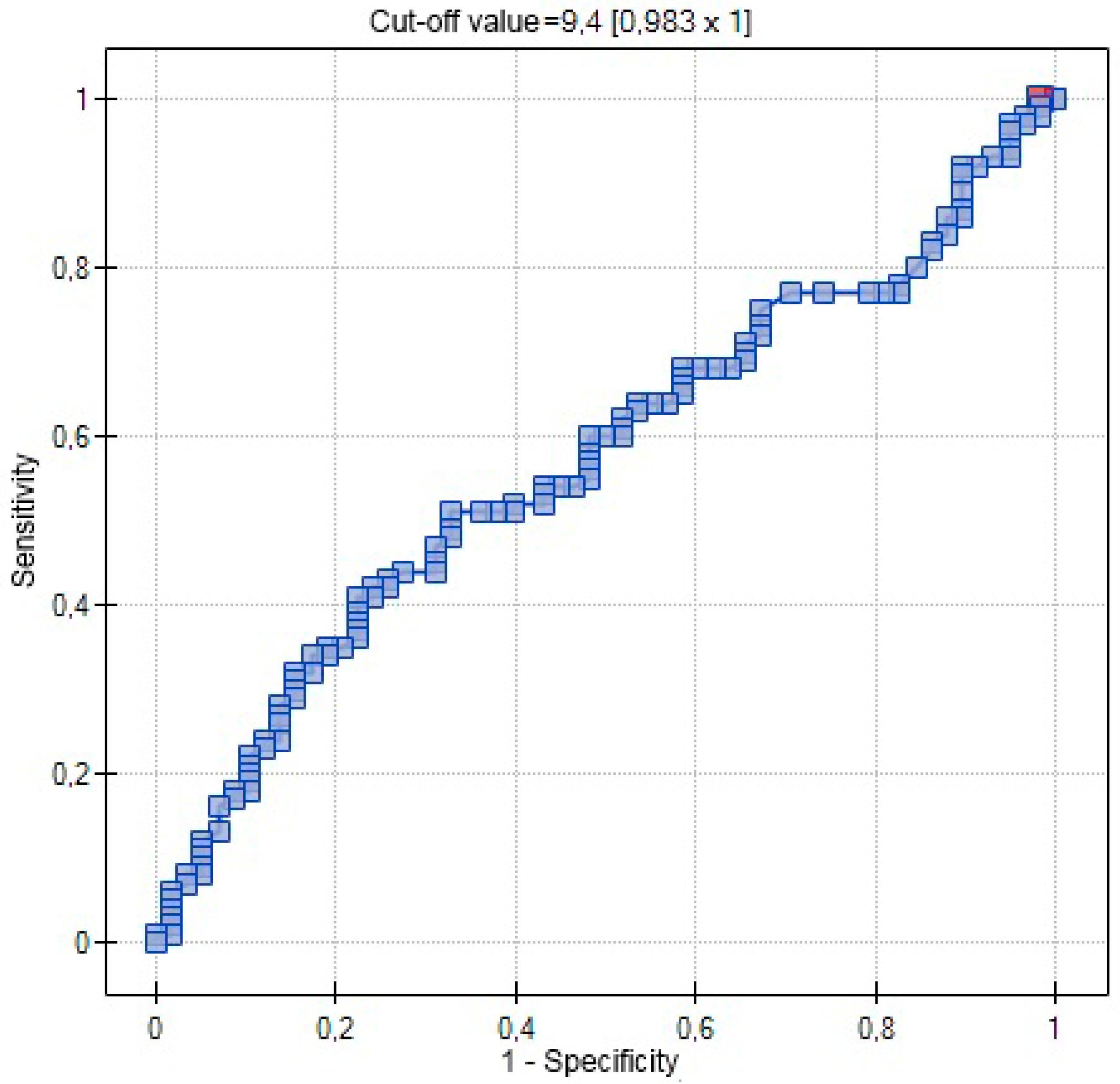
| AUC | 0.5767 | 0.7029 | 0.5721 |
| SE (AUC) | 0.0509 | 0.0631 | 0.0462 |
| −95% CI | 0.4769 | 0.5792 | 0.4817 |
| +95% CI | 0.6765 | 0.8265 | 0.6626 |
| Z-statistic | 1.4806 | 3.0486 | 1.5096 |
| p | 0.1387 | 0.0023 | 0.1311 |
| Cut-off value | 0.3541 | 8 | 9.4 |
| Parameter | b-Coefficient | p-Value | Odd Ratio | +95% CI | +95% CI |
|---|---|---|---|---|---|
| Intercept | 0.4965 | 0.5113 | 1.6429 | 0.3735 | 7.2268 |
| 25(OH)D (ng/mL) | −0.1208 | 0.0033 | 0.8862 | 0.8177 | 0.9605 |
| Intercept | 0.0467 | 0.9703 | 1.0478 | 0.0899 | 12.2172 |
| Age > 35 years old | −0.0644 | 0.1298 | 0.9377 | 0.8627 | 1.0191 |
| Intercept | −2.6509 | <0.0001 | 0.0706 | 0.0308 | 0.1615 |
| Primiparity | 1.3410 | 0.0077 | 3.8228 | 1.4260 | 10.2478 |
| Intercept | 0.3649 | 0.8328 | 1.4403 | 0.0487 | 42.5836 |
| 25(OH)D (ng/mL) | −0.1170 | 0.0053 | 0.8896 | 0.8195 | 0.9658 |
| Age > 35 years old | −0.0267 | 0,5818 | 0.9737 | 0.8856 | 1.0706 |
| Primiparity | 1.3980 | 0.0166 | 4.0469 | 1.2893 | 12.7030 |
| Parameter | Study Group N = 171 | Control Group N = 36 | P |
|---|---|---|---|
| Age (years) | 29.6 ± 5.2 | 29.4 ± 4.9 | NS |
| BMI (kg/m2) | 27.8 ± 2.2 | 26.9 ± 2.4 | NS |
| Parity | 1.9 ± 1.1 | 1.8 ± 1.0 | NS |
| pH of umbilical artery | 7.35 ± 0.09 | 7.35 ± 0.07 | NS |
| BMI (kg/m2) | 24.8 ± 2.0 | 25.2 ± 2.5 | NS |
| Pregnancy (weeks) | 38 ± 2.96 | 40 ± 1.08 | NS |
| Caesarian sections % | 43.3 | 37.6 | NS |
| Weight of newborn (g) | 3340 ± 680 | 3590 ± 430 | NS |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domaracki, P.; Sadlecki, P.; Odrowaz-Sypniewska, G.; Dzikowska, E.; Walentowicz, P.; Siodmiak, J.; Grabiec, M.; Walentowicz-Sadlecka, M. Serum 25(OH) Vitamin D Levels in Polish Women during Pregnancies Complicated by Hypertensive Disorders and Gestational Diabetes. Int. J. Mol. Sci. 2016, 17, 1574. https://doi.org/10.3390/ijms17101574
Domaracki P, Sadlecki P, Odrowaz-Sypniewska G, Dzikowska E, Walentowicz P, Siodmiak J, Grabiec M, Walentowicz-Sadlecka M. Serum 25(OH) Vitamin D Levels in Polish Women during Pregnancies Complicated by Hypertensive Disorders and Gestational Diabetes. International Journal of Molecular Sciences. 2016; 17(10):1574. https://doi.org/10.3390/ijms17101574
Chicago/Turabian StyleDomaracki, Piotr, Pawel Sadlecki, Grazyna Odrowaz-Sypniewska, Ewa Dzikowska, Pawel Walentowicz, Joanna Siodmiak, Marek Grabiec, and Malgorzata Walentowicz-Sadlecka. 2016. "Serum 25(OH) Vitamin D Levels in Polish Women during Pregnancies Complicated by Hypertensive Disorders and Gestational Diabetes" International Journal of Molecular Sciences 17, no. 10: 1574. https://doi.org/10.3390/ijms17101574






