Characterization of a β-Adrenergic-Like Octopamine Receptor in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel)
Abstract
:1. Introduction
2. Results
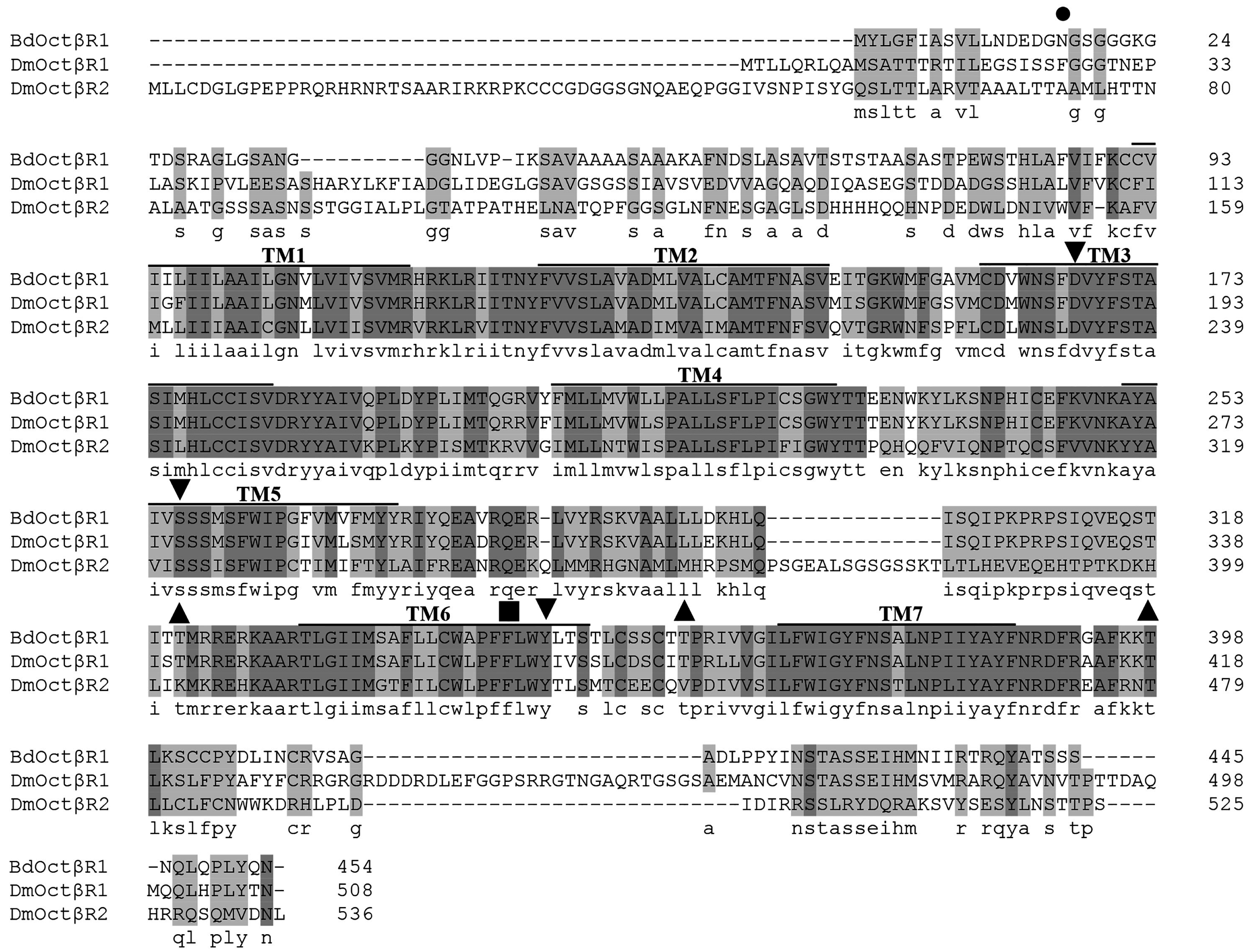
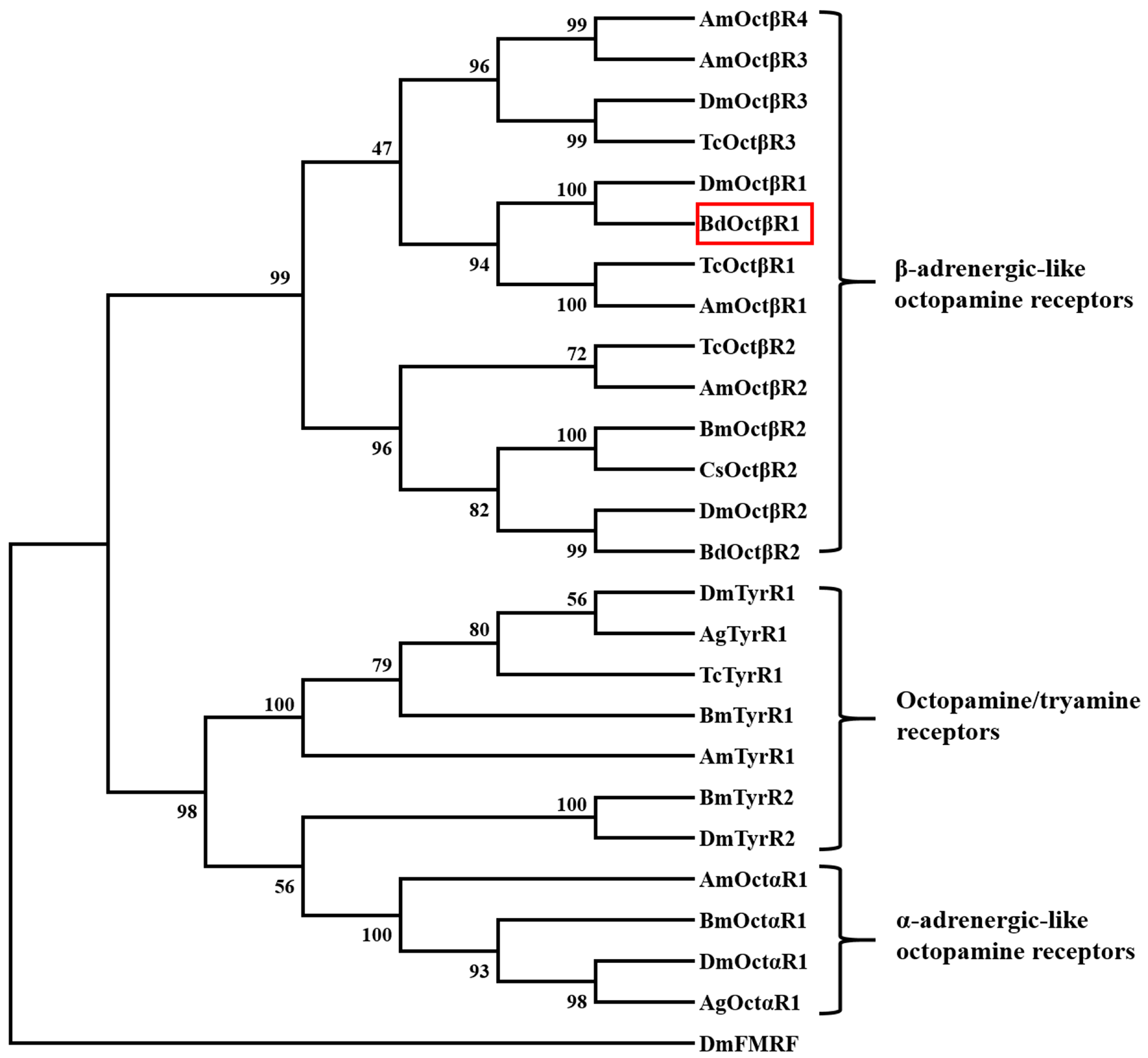
2.1. Cloning and Sequence Analysis of β-Adrenergic-Like Octopamine Receptor (BdOctβR1)
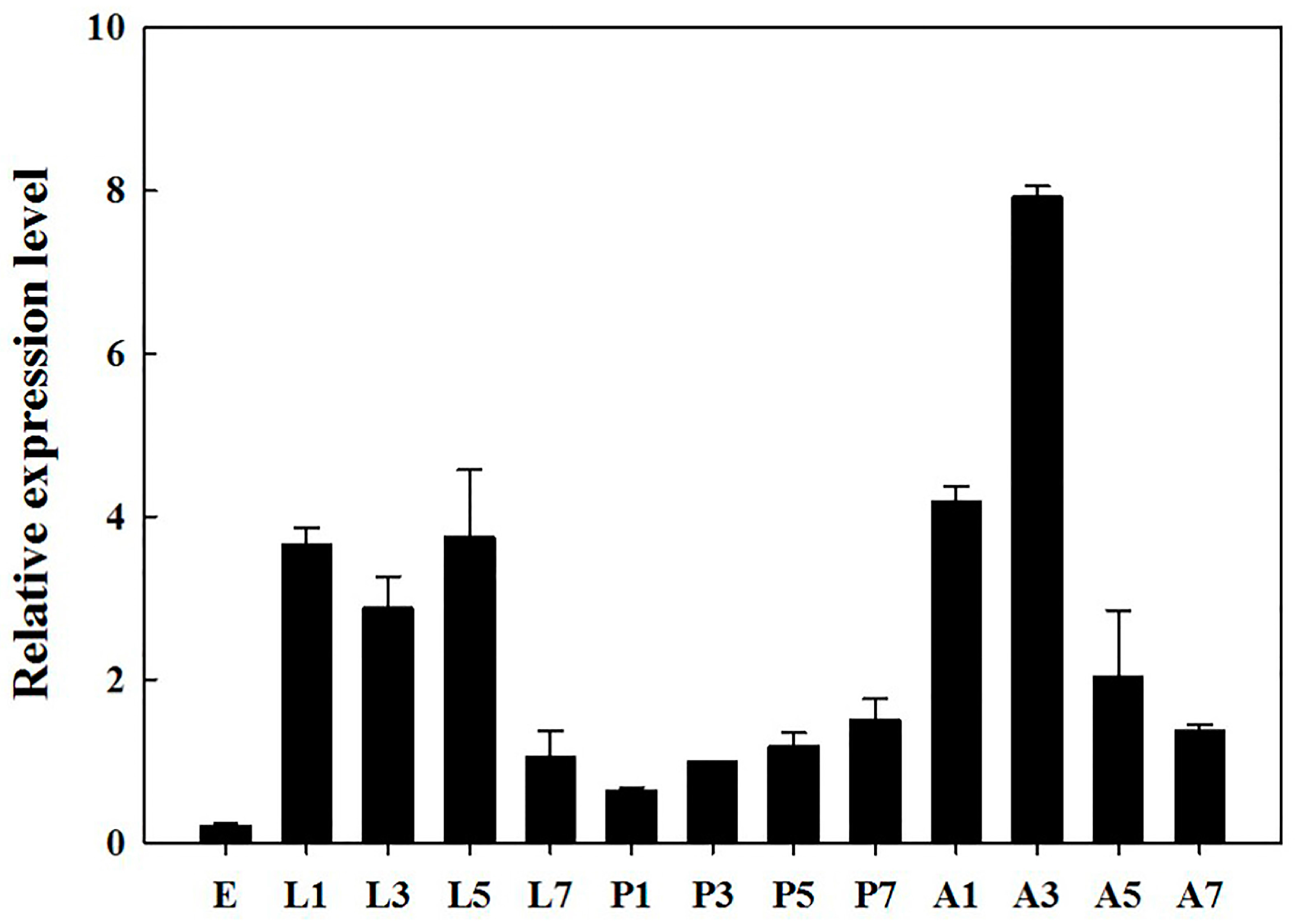
2.2. Developmental Stages and Tissue-Specific Expression Pattern
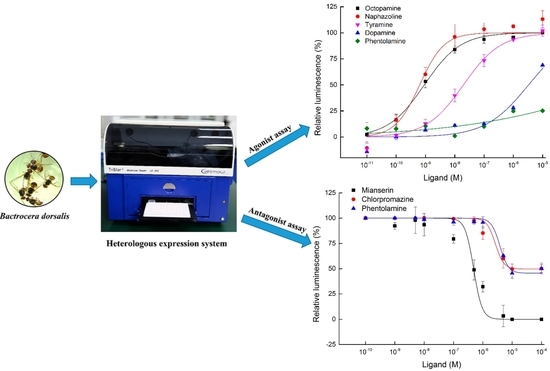
2.3. Functional Expression and Concentration Responses of BdOctβR1 to Typical Ligands
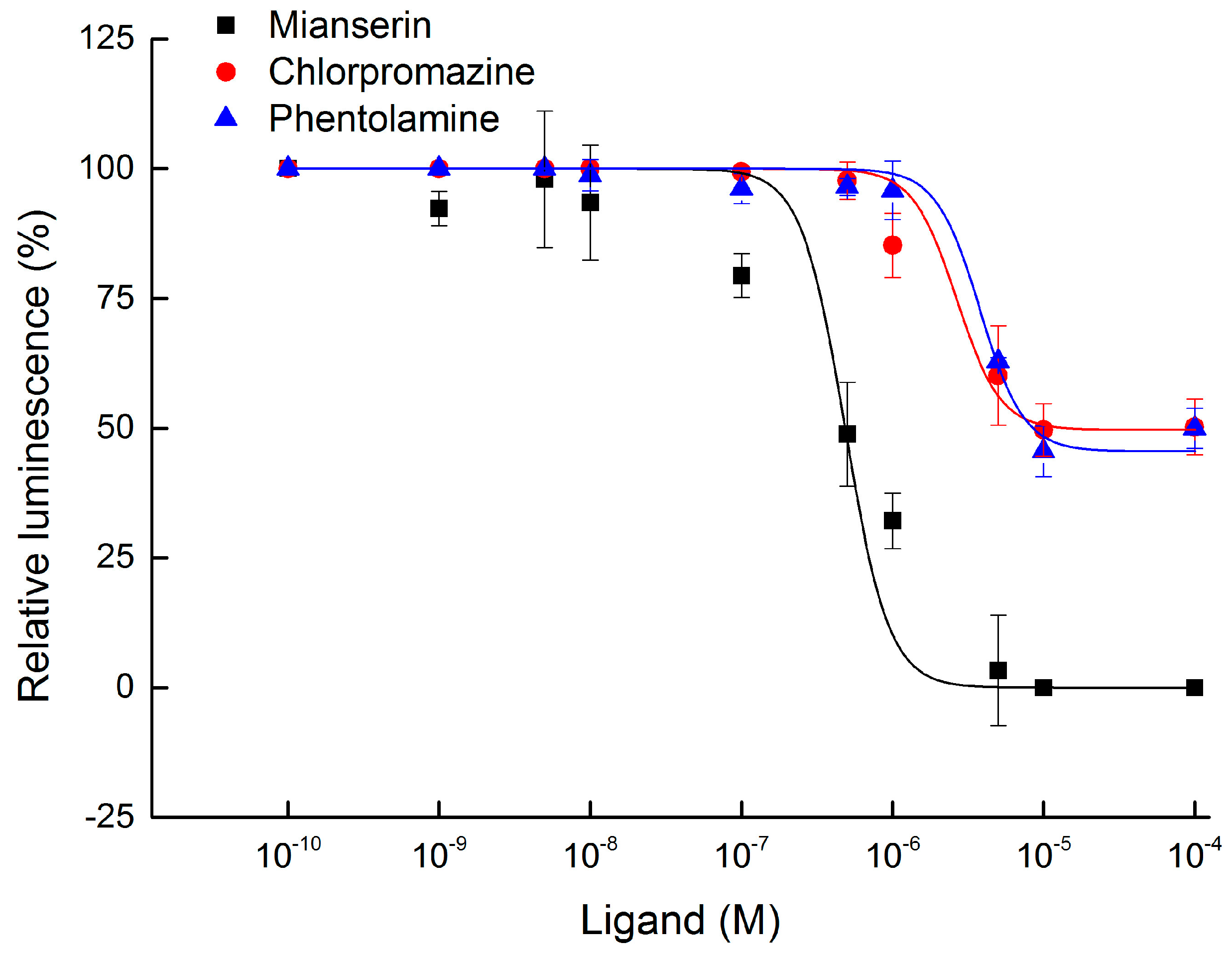
2.4. Antagonist Assay of BdOctβR1
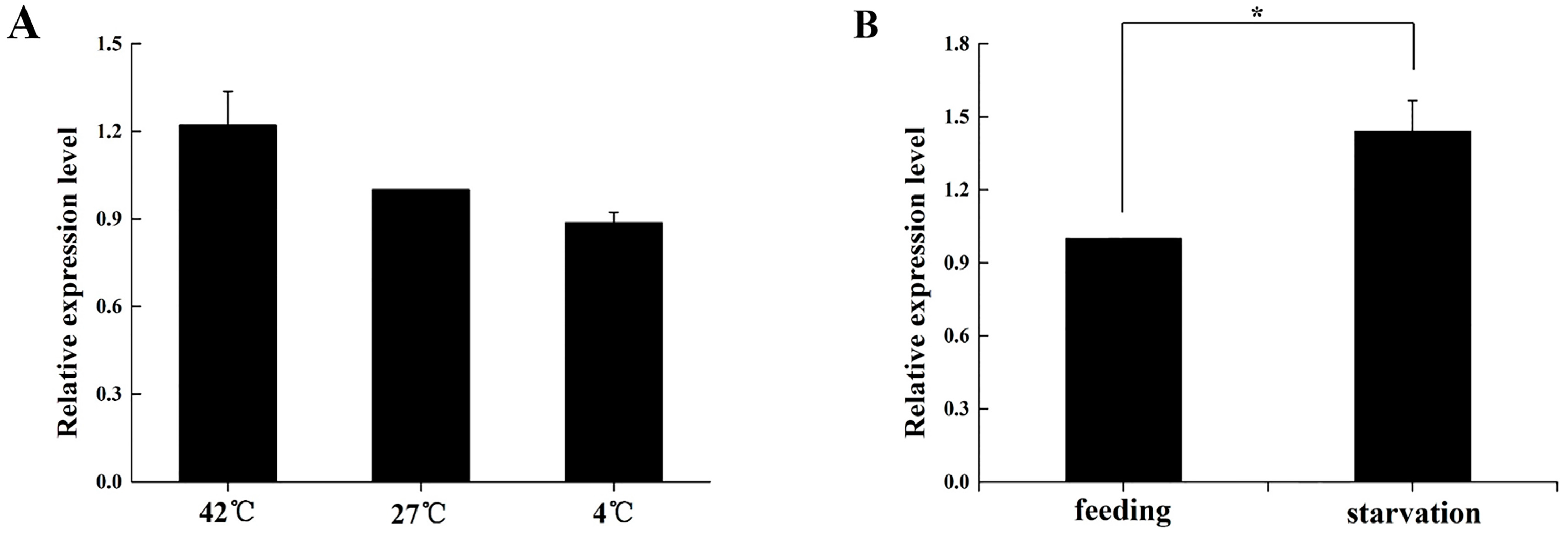
2.5. Expression Profile of BdOctβR1 When Adult Flies Are under Stress
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Reagents
4.3. Total RNA/DNA Extraction
4.4. Molecular Cloning
4.5. Bioinformatics Analysis
4.6. Quantitative Reverse Transcription-PCR (RT-qPCR)
4.7. Heterologous Expression and Functional Assay
4.8. Changes in BdOctβR1 Expression When Adult Flies Are under Stress
4.8.1. Thermo-Tolerance Assay
4.8.2. Food Deprivation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 1999, 59, 533–561. [Google Scholar] [CrossRef]
- Wu, S.F.; Yao, Y.; Huang, J.; Ye, G.Y. Characterization of a β-adrenergic-like octopamine receptor from the rice stem borer (Chilo suppressalis). J. Exp. Biol. 2012, 215, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Maqueira, B.; Chatwin, H.; Evans, P.D. Identification and characterization of a novel family of Drosophilaβ-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 2005, 94, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Balfanz, S.; Strünker, T.; Frings, S.; Baumann, A. A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 2005, 93, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T. Octopamine-mediated neuromodulation of insect senses. Neurochem. Res. 2007, 32, 1511–1529. [Google Scholar] [CrossRef] [PubMed]
- Yuya, O.; Yuko, S.N.; Ryusuke, N.; Yasunari, K.; Yoshiki, H.; Kazutaka, A.; Hitoshi, U.; Kimiko, Y.K.; Satoru, K. Autocrine regulation of ecdysone synthesis by β3-octopamine receptor in the prothoracic gland is essential for Drosophila metamorphosis. Proc. Natl. Acad. Sci. USA 2015, 112, 1452–1457. [Google Scholar]
- Li, Y.; Fink, C.; El-Kholy, S.; Roeder, T. The octopamine receptor octβ2R is essential for ovulation and fertilization in the fruit fly Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2015, 88, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Sabandal, P.R.; Fernandez, A.; Sabandal, J.M.; Lee, H.G.; Evans, P.; Han, K.A. The octopamine receptor Octβ2R regulates ovulation in Drosophila melanogaster. PLoS ONE 2014, 9, e104441. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ohta, H.; Ozoe, F.; Miyazawa, K.; Huang, J.; Ozoe, Y. Functional and pharmacological characterization of a β-adrenergic-like octopamine receptor from the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Tobback, J.; Pflüger, H.J.; Blenau, W.; Broeck, J.V. The cloning, phylogenetic relationship and distribution pattern of two new putative GPCR-type octopamine receptors in the desert locust (Schistocerca gregaria). J. Insect Physiol. 2010, 56, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.B.; Douthit, M.K.; Moore, A.J. Expression of octopaminergic receptor genes in 4 nonneural tissues in female Nicrophorus vespilloides beetles. Insect Sci. 2014, 22, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Huang, L.H.; Feng, H.T.; Su, W.Y. Do organophosphate-based traps reduce control efficiency of resistant tephritid flies? J. Pest Sci. 2014, 88, 1–10. [Google Scholar] [CrossRef]
- Wei, D.; Li, H.M.; Yang, W.J.; Wei, D.D.; Dou, W.; Huang, Y.; Wang, J.J. Transcriptome profiling of the testis reveals genes involved in spermatogenesis and marker discovery in the oriental fruit fly, Bactrocera dorsalis. Insect Mol. Biol. 2015, 24, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Huang, Y.; Lu, X.P.; Jiang, X.Z.; Smagghe, G.; Feng, Z.J.; Yuan, G.R.; Wei, D.; Wang, J.J. Overexpression of two α-esterase genes mediates metabolic resistance to malathion in the oriental fruit fly, Bactrocera dorsalis (Hendel). Insect Mol. Biol. 2015, 24, 467–479. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, S.; Stephano, F.; Li, Y.; Bhandari, A.; Fink, C.; Roeder, T. Expression analysis of octopamine and tyramine receptors in Drosophila. Cell Tissue Res. 2015, 361, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Tomancak, P.; Beaton, A.; Weiszmann, R.; Kwan, E.; Shu, S.Q.; Lewis, S.E.; Richards, S.; Ashburner, M.; Hartenstein, V.; Celniker, S.E. Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 2002, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Balfanz, S.; Jordan, N.; Langenstück, T.; Breuer, J.; Bergmeier, V.; Baumann, A. Molecular, pharmacological, and signaling properties of octopamine receptors from honeybee (Apis mellifera) brain. J. Neurochem. 2014, 129, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.; Mcneil, J.N.; Donly, C. Octopamine receptor gene expression in three lepidopteran species of insect. Peptides 2013, 41, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.D.; Maqueira, B. Insect octopamine receptors: A new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invertebr. Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Roeder, T.; Seifert, M.; Kähler, C.; Gewecke, M. Tyramine and octopamine: Antagonistic modulators of behavior and metabolism. Arch. Insect Biochem. Physiol. 2003, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, A.; Arai, Y.; Ozoe, F.; Ohta, H.; Narusuye, K.; Huang, J.; Enomoto, K.; Kataoka, H.; Hirota, A.; Ozoe, Y. Molecular cloning and heterologous expression of an α-adrenergic-like octopamine receptor from the silkworm Bombyx mori. Insect Mol. Biol. 2006, 15, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hamasaki, T.; Ozoe, F.; Ohta, H.; Enomoto, K.I.; Kataoka, H.; Sawa, Y.; Hirota, A.; Ozoe, Y. Identification of critical structural determinants responsible for octopamine binding to the α-adrenergic-like Bombyx mori octopamine receptor. Biochemistry 2007, 46, 5896–5903. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, L.; Blenau, W.; Erber, J.; Ebert, P.R.; Strunker, T.; Baumann, A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J. Neurochem. 2003, 86, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hamasaki, T.; Ozoe, Y. Pharmacological characterization of a Bombyx mori α-adrenergic-like octopamine receptor stably expressed in a mammalian cell line. Arch. Insect Biochem. Physiol. 2009, 73, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ohta, H.; Inoue, N.; Takao, H.; Kita, T.; Ozoe, F.; Ozoe, Y. Molecular cloning and pharmacological characterization of a Bombyx mori tyramine receptor selectively coupled to intracellular calcium mobilization. Insect Biochem. Mol. Biol. 2009, 39, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.I.; Vogel, C.F.A. Synergistic action of octopamine receptor agonists on the activity of selected novel insecticides for control of dengue vector Aedes aegypti (Diptera: Culicidae) mosquito. Pestic. Biochem. Physiol. 2015, 120, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yellman, C.; Tao, H.; He, B.; Hirsh, J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc. Natl. Acad. Sci. USA 1997, 94, 4131–4136. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, K.M.C. The inactive mutation leads to abnormal experience-dependent courtship modification in male Drosophila melanogaster. Behav. Genet. 1994, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Schwaerzel, M.; Monastirioti, M.; Scholz, H.; Friggi-Grelin, F.; Birman, S.; Heisenberg, M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 2003, 23, 10495–10502. [Google Scholar] [PubMed]
- Long, T.F.; Murdock, L.L. Stimulation of blowfly feeding behavior by octopaminergic drugs. Proc. Natl. Acad. Sci. USA 1983, 80, 4159–4163. [Google Scholar] [CrossRef] [PubMed]
- Mercer, A.R.; Menzel, R. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. J. Comp. Physiol. 1982, 145, 363–368. [Google Scholar] [CrossRef]
- Hammer, M.; Menzel, R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 1998, 5, 146–156. [Google Scholar] [PubMed]
- Menzel, R.; Heyne, A.; Kinzel, C.; Gerber, B.; Fiala, A. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav. Neurosci. 1999, 113, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Evans, P.D. Changes in haemolymph octopamine levels associated with food deprivation in the locust, Schistocerca gregaria. Physiol. Entomol. 2008, 9, 269–274. [Google Scholar] [CrossRef]
- Hirashima, A.; Nagano, T.; Eto, M. Stress-induced changes in the biogenic amine levels and larval growth of Tribolium castaneum Herbst. Biosci. Biotechnol. Biochem. 2014, 57, 2085–2089. [Google Scholar] [CrossRef]
- Wang, J.J.; Wei, D.; Dou, W.; Hu, F.; Liu, W.F.; Wang, J.J. Toxicities and synergistic effects of several insecticides against the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.M.; Jiang, H.B.; Wang, X.N.; Wang, J.J. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol. Biol. 2010, 11, 1471–2199. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, Z.; Nachman, R.J.; Adams, M.E.; Park, Y. Functional phylogenetics reveals contributions of pleiotropic peptide action to ligand-receptor coevolution. Sci. Rep. 2014, 4, 6800. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, Z.; Nachman, R.J.; Kaczmarek, K.; Zabrocki, J.; Park, Y. Functional characterization of five different PRXamide receptors of the red flour beetle Tribolium castaneum with peptidomimetics and identification of agonists and antagonists. Peptides 2014, 68, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, Z.; Nachman, R.J.; Park, Y. Molecular cloning and functional characterization of the diapause hormone receptor in the corn earworm Helicoverpa zea. Peptides 2014, 53, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Kim, D.; Dobesh, S.; Evans, J.D.; Nachman, R.J.; Kaczmarek, K.; Zabrocki, J.; Park, Y. Ligand selectivity in tachykinin and natalisin neuropeptidergic systems of the honey bee parasitic mite Varroa destructor. Sci. Rep. 2015, 6, 19547. [Google Scholar] [CrossRef] [PubMed]
- Šimo, L.; Juraj, K.; Žitňan, D.; Park, Y. Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS ONE 2011, 6, e16158. [Google Scholar] [CrossRef] [PubMed]


| Application of Primers | Primer Names | Sequence (5′–3′) | Amplicon Length (bp) |
|---|---|---|---|
| ORF cloning | BdOctβR1-F1 | AGCCGATGTATTTAGGTTTC | 1377 |
| BdOctβR1-R1 | TTTATTTTTAGTTCTGGTAGAGC | ||
| BdOctβR1-F2 | ATGTATTTAGGTTTCATTGCG | 1372 | |
| BdOctβR1-R2 | TTTTATTTTTAGTTCTGGTAGAGC | ||
| RT-qPCR | BdOctβR1-qF | GCGCCATTTTTCCTATGGTA | 150 |
| BdOctβR1-qR | TCCACGAAAATCACGATTGA | ||
| α-Tubulin-qF | CGCATTCATGGTTGATAACG | 184 | |
| α-Tubulin-qR | GGGCACCAAGTTAGTCTGGA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-M.; Jiang, H.-B.; Gui, S.-H.; Liu, X.-Q.; Liu, H.; Lu, X.-P.; Smagghe, G.; Wang, J.-J. Characterization of a β-Adrenergic-Like Octopamine Receptor in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). Int. J. Mol. Sci. 2016, 17, 1577. https://doi.org/10.3390/ijms17101577
Li H-M, Jiang H-B, Gui S-H, Liu X-Q, Liu H, Lu X-P, Smagghe G, Wang J-J. Characterization of a β-Adrenergic-Like Octopamine Receptor in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). International Journal of Molecular Sciences. 2016; 17(10):1577. https://doi.org/10.3390/ijms17101577
Chicago/Turabian StyleLi, Hui-Min, Hong-Bo Jiang, Shun-Hua Gui, Xiao-Qiang Liu, Hong Liu, Xue-Ping Lu, Guy Smagghe, and Jin-Jun Wang. 2016. "Characterization of a β-Adrenergic-Like Octopamine Receptor in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel)" International Journal of Molecular Sciences 17, no. 10: 1577. https://doi.org/10.3390/ijms17101577