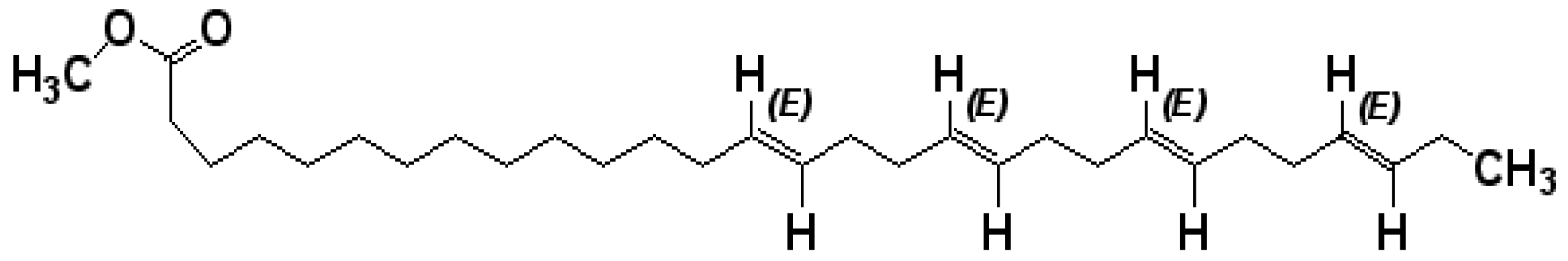
A Novel Tetraenoic Fatty Acid Isolated from Amaranthus spinosus Inhibits Proliferation and Induces Apoptosis of Human Liver Cancer Cells
Abstract
:1. Introduction
2. Results
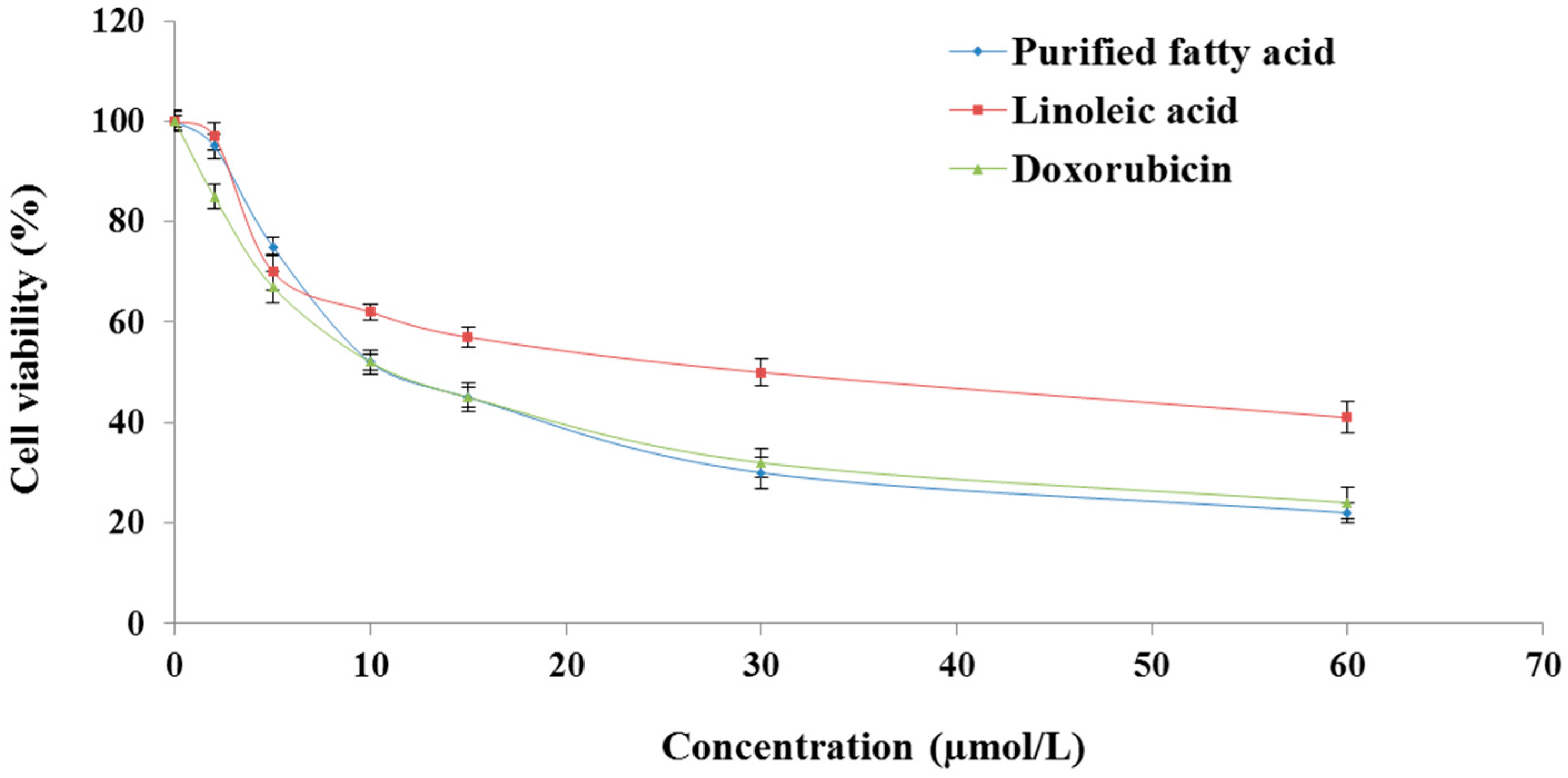
2.1. The Purified Fatty Acid Exerts an Antiproliferative Effect
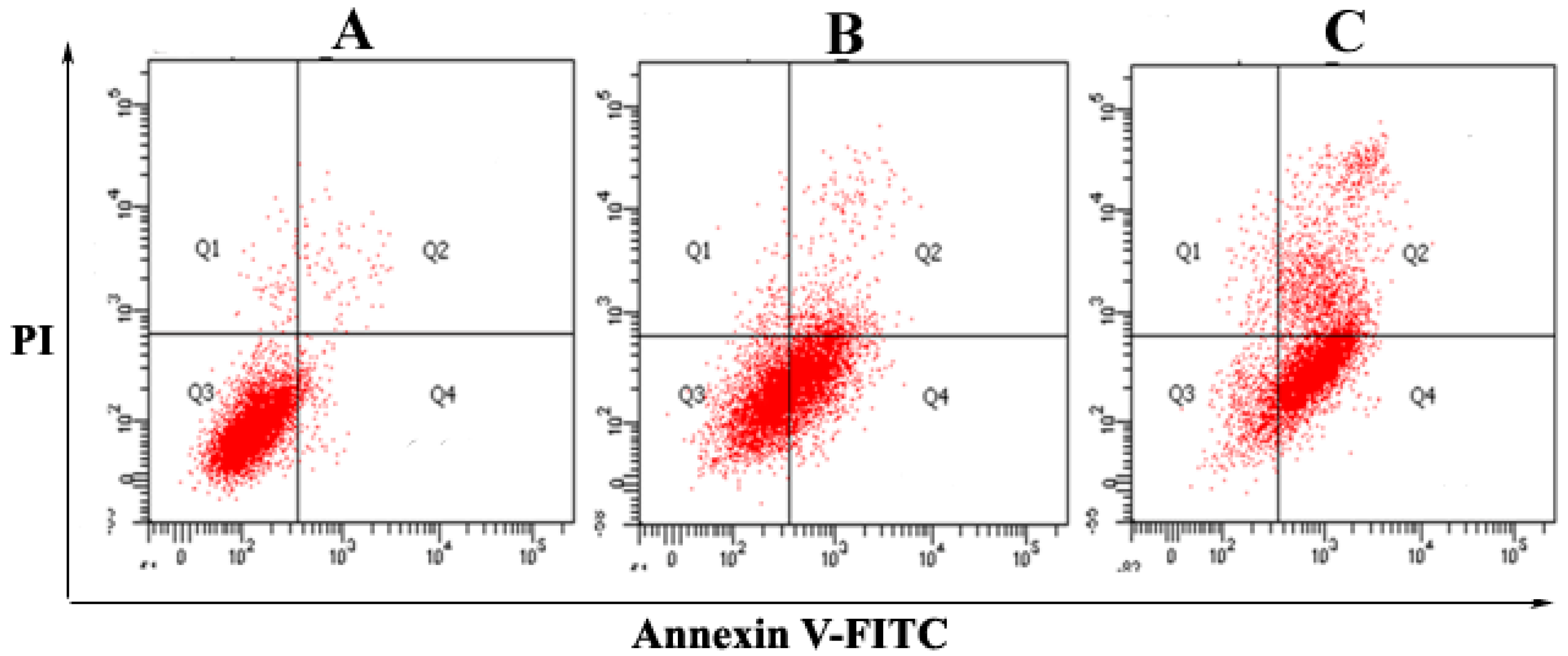
2.2. The Purified Fatty Acid Induces Apoptosis
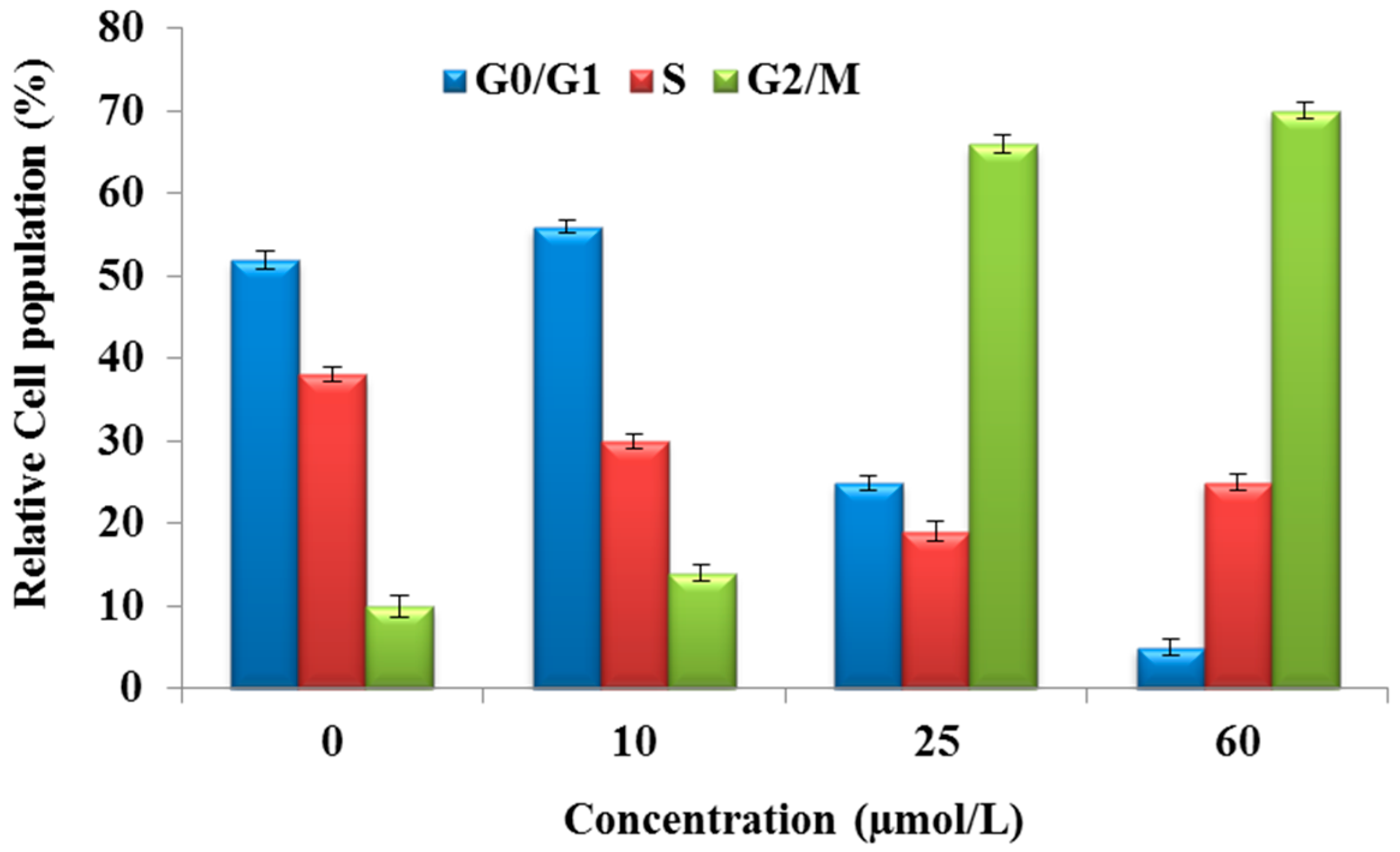
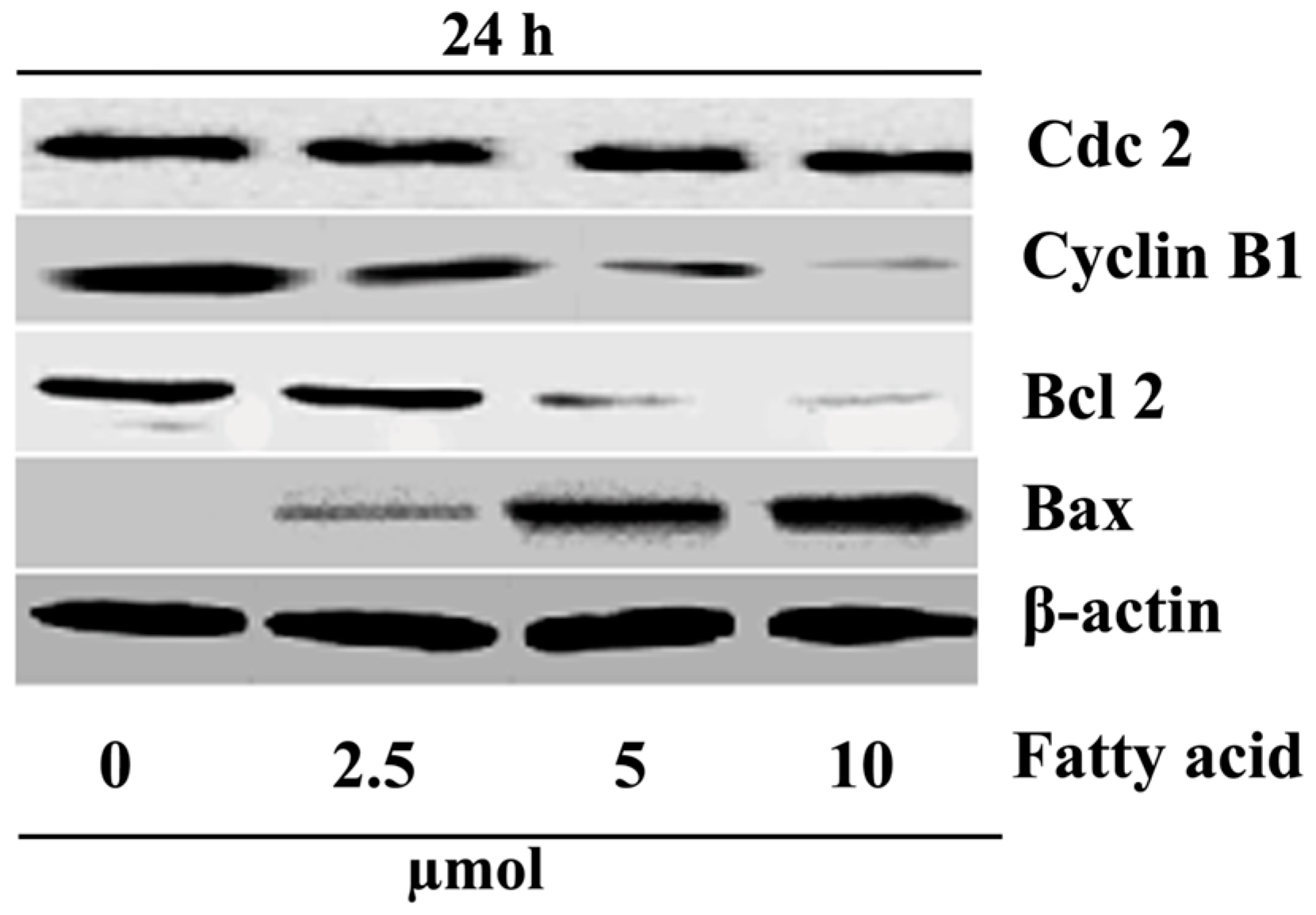
2.3. Purified Fatty Acid Exhibits Cell Cycle Arrest
2.4. Purified Fatty Acid Alters the Expression of Apoptosis-Associated Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material, Extraction, and Isolation
4.3. Cell Culture
4.4. Cell Viability Determination
4.5. Cell Cycle Arrest Analysis
4.6. Apoptosis Assay by Annexin V-FITC/PI
4.7. Western Blot Analysis
4.8. Statistical Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–105. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology 2004, 127, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts and Figures 2015; American Cancer Society: Atlanta, GA, USA, 2015; Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf (accessed on 8 September 2016).
- Schütte, K.; Bornschein, J.; Malfertheiner, P. Hepatocellular carcinoma-epidemiological trends and risk factors. Dig. Dis. 2009, 27, 80–92. [Google Scholar] [PubMed]
- Bhattacharyya, G.S.; Babu, K.G.; Malhotra, H.; Ranade, A.B.A.; Murshed, S.; Datta, D. Hepatocellular carcinoma in India. Chin. Clin. Oncol. 2013, 2, 1–5. [Google Scholar]
- Paraskevi, A.; Ronald, A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 1–14. [Google Scholar]
- Yates, M.S.; Kensler, T.W. Keap1 eye on the target: Chemoprevention of liver cancer. Acta Pharmacol. Sin. 2007, 28, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Thoppil, R.J.; Waghray, A.; Kruse, J.A.; Novotny, N.A.; Darvesh, A.S. Dietary phytochemicals in the chemoprevention and treatment of hepatocellular carcinoma: In vivo evidence, molecular targets, and clinical relevance. Curr. Cancer Drug Targets 2012, 12, 1191–1232. [Google Scholar] [CrossRef] [PubMed]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; Oriental Enterprises: Dehradun, India, 2001; Volume 1, pp. 2832–2836. [Google Scholar]
- Girija, K.; Lakshman, K.; Udaya, C.; Sabhya, S.G.; Divya, T. Anti-diabetic and anticholesterolemic activity of methanol extracts of three species of Amaranthus. Asian Pac. J. Trop. Biomed. 2011, 1, 133–138. [Google Scholar] [CrossRef]
- Ashok Kumar, B.S.; Lakshman, K.; Velmurugan, C.; Sridhar, S.M.; Gopisetty, S. Antidepressant activity of methanolic extract of Amaranthus spinosus. Basic Clin. Neurosci. 2014, 5, 11–17. [Google Scholar] [PubMed]
- Olajide, O.A.; Ogunleye, B.R.; Erinle, T.O. Anti-inflammatory properties of Amaranthus spinosus leaf extract. Pharm. Biol. 2004, 42, 521–525. [Google Scholar] [CrossRef]
- Hilou, A.; Nacoulma, O.G.; Guiguemde, T.R. In vivo antimalarial activities of extract from Amaranthus spinosus L., and Boerhaaviaerecta L., in mice. J. Ethnopharmacol. 2006, 103, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Murgan, K.; Vanithakumari, G.; Sampathraj, R. Effects of combined extracts of Dolichosbiflorus seeds and Amaranthus spinosus roots on the accessory sex organs of male rats. Anc. Sci. Life 1993, 13, 351–357. [Google Scholar]
- Sangameswaran, B.; Jayakar, B. Anti-diabetic, anti-hyperlipidemic and spermatogenic effects of Amaranthus spinosus Linn., on streptozotocin-induced diabetic rats. J. Nat. Med. 2008, 62, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Olufemi, B.E.; Assiak, I.E.; Ayoade, G.O.; Onigemo, M.A. Studies on the effect of Amaranthus spinosus leaf extract on the hematology of growing pigs. Afr. J. Biomed. Res. 2003, 6, 149–150. [Google Scholar]
- Murgan, K.; Vanithakumari, G.; Sampathraj, R. Biochemical changes in epididymis following treatment with combined extracts of Amaranthusspinosus roots and Dolichosbiflorus seeds. Anc. Sci. Life 1993, 13, 154–159. [Google Scholar]
- Samuel, J.L.; Pal, V.C.; Senthil Kumar, K.L.; Sahu, R.K.; Roy, A. Antitumor activity of the ethanol extract of Amaranthusspinosus leaves against EAC bearing Swiss albino mice. Der Pharm. Lett. 2010, 2, 10–15. [Google Scholar]
- Bulbul, I.J.; Nahar, L.; Alam, R.F.; Haque, O. Antibacterial, cytotoxic and antioxidant activity of chloroform, n-hexane and ethyl acetate extracts of plant Amaranthus spinosus. Int. J. PharmTech. Res. 2011, 3, 1675–1680. [Google Scholar]
- Mondal, A.; Guria, T.; Maity, T.K. A new ester of fatty acid from a methanol extract of the whole plant of Amaranthusspinosus and its α-glucosidase inhibitory activity. Pharm. Biol. 2015, 53, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Tang, Q.; Li, Y.; Hu, B.R.; Ming, Z.Y.; Fu, Q.; Qian, J.Q.; Xiang, J.Z. Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol. Sin. 2006, 27, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M. Cyclin ubiquitination: The destructive end of mitosis. Cell 1995, 81, 149–152. [Google Scholar]
- Reed, J.C. Double identity for proteins of the Bcl-2 family. Nature 1997, 387, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, B.; Matinou, J.C. The Bcl-2 protein family. Exp. Cell Res. 2000, 256, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mauceri, H.J.; Hanna, N.N.; Beckett, M.A.; Gorski, D.H.; Staba, M.J.; Stellato, K.A.; Bigelow, K.; Heimann, R.; Gately, S.; Dhanabal, M.; et al. Combined effects of angiostatin and ionizing radiation in anti-tumour therapy. Nature 1998, 394, 287–291. [Google Scholar] [PubMed]
- Wang, G.; Chen, H.; Huang, M.; Wang, N.; Zhang, J.; Zhang, Y.; Bai, G.; Fong, W.F.; Yang, M.; Yao, X. Methyl protodioscin induces G2/M cell cycle arrest and apoptosis in HepG2 liver cancer cells. Cancer Lett. 2006, 24, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; John, J.K. Anticancer therapy targeting the apoptotic pathway. Oncology 2003, 4, 721–729. [Google Scholar]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, A.; Guria, T.; Maity, T.K.; Bishayee, A. A Novel Tetraenoic Fatty Acid Isolated from Amaranthus spinosus Inhibits Proliferation and Induces Apoptosis of Human Liver Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1604. https://doi.org/10.3390/ijms17101604
Mondal A, Guria T, Maity TK, Bishayee A. A Novel Tetraenoic Fatty Acid Isolated from Amaranthus spinosus Inhibits Proliferation and Induces Apoptosis of Human Liver Cancer Cells. International Journal of Molecular Sciences. 2016; 17(10):1604. https://doi.org/10.3390/ijms17101604
Chicago/Turabian StyleMondal, Arijit, Tanmoy Guria, Tapan Kumar Maity, and Anupam Bishayee. 2016. "A Novel Tetraenoic Fatty Acid Isolated from Amaranthus spinosus Inhibits Proliferation and Induces Apoptosis of Human Liver Cancer Cells" International Journal of Molecular Sciences 17, no. 10: 1604. https://doi.org/10.3390/ijms17101604





