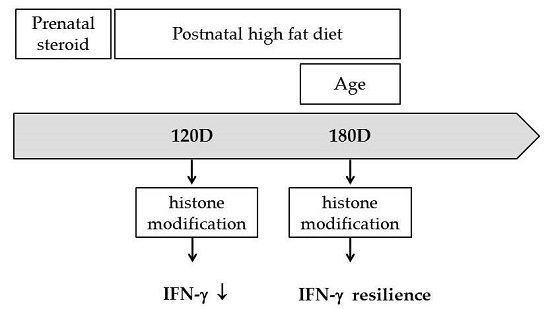
Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats
Abstract
:1. Background
2. Results
2.1. Postnatal High-Fat (HF) Diet Increased Body Weight (BW) but Decreased the Spleen:BW Ratio
2.2. Both Prenatal Dexamethasone Treatment and Postnatal HF Diet Changed the Leukocyte Subsets of the Rats at the Adolescent Stage
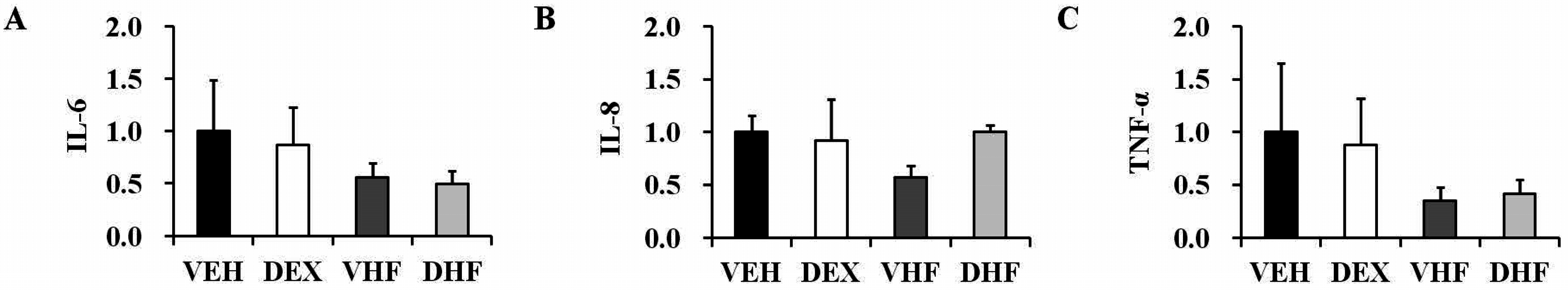
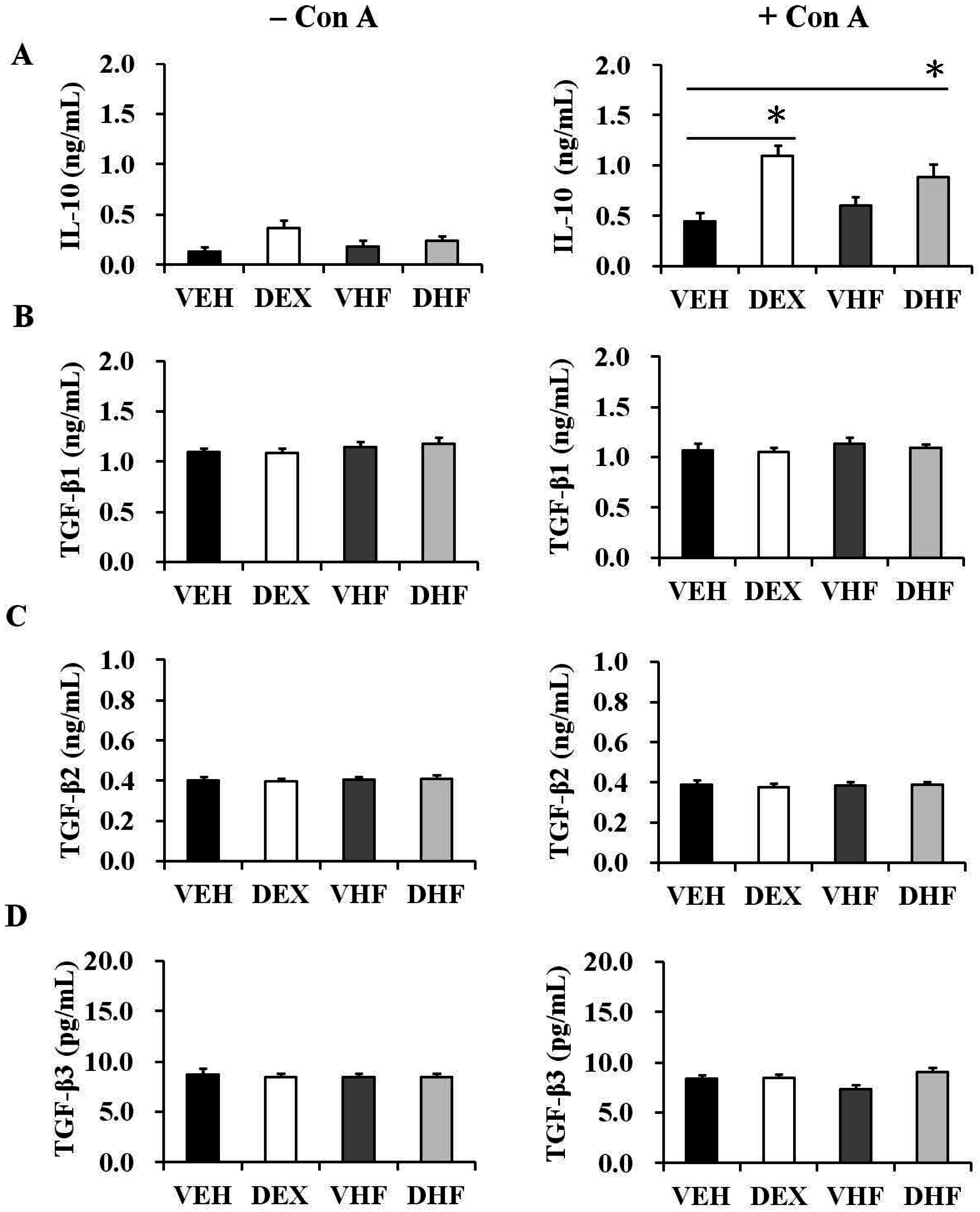
2.3. Prenatal Dexamethasone Treatment Plus a Postnatal HF Diet Changed Rat Spleen Innate Cytokine Production at D120
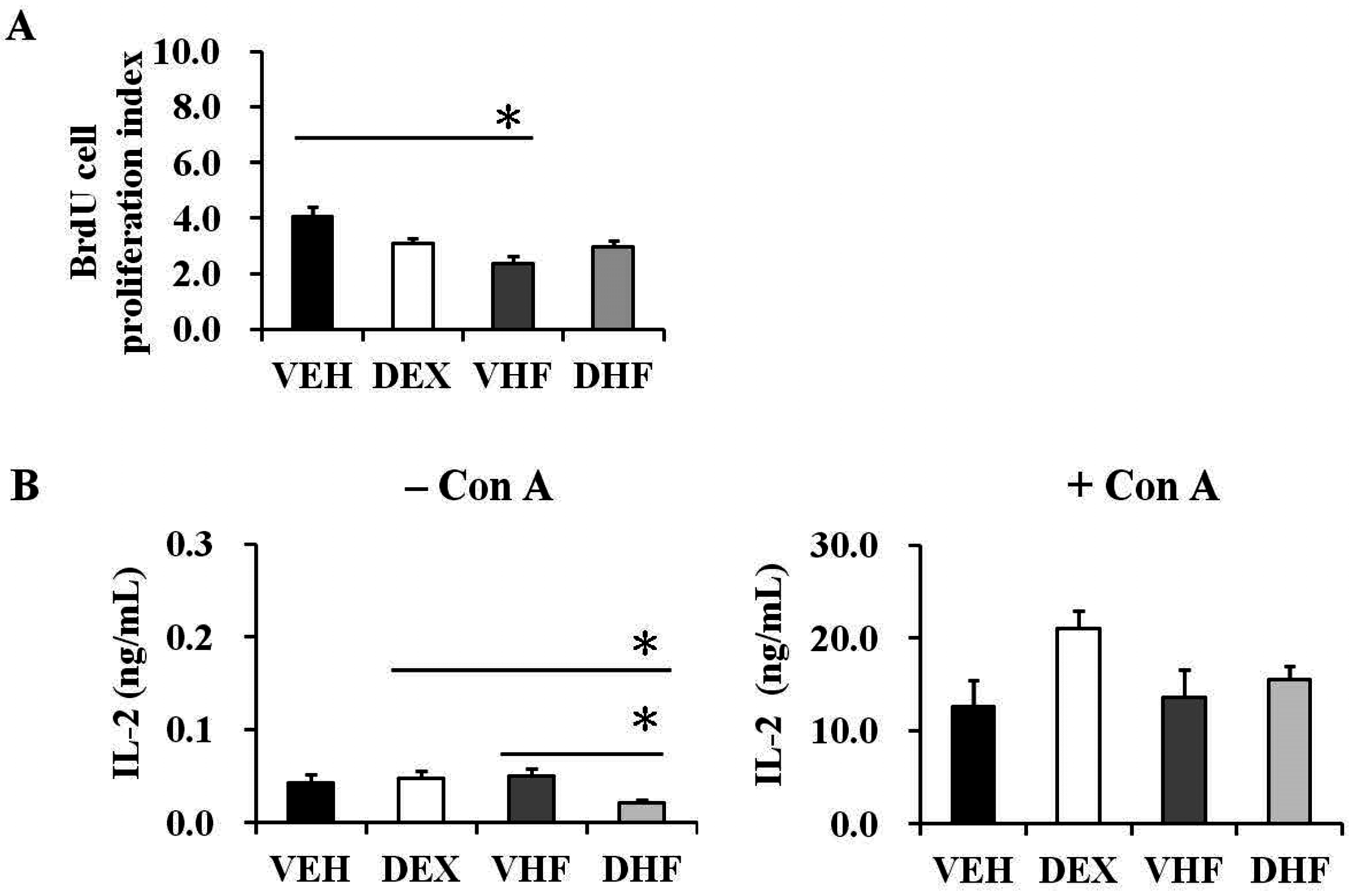
2.4. Postnatal HF Diet Decreased the Proliferation of Rat Splenocytes at the Adolescent Stage
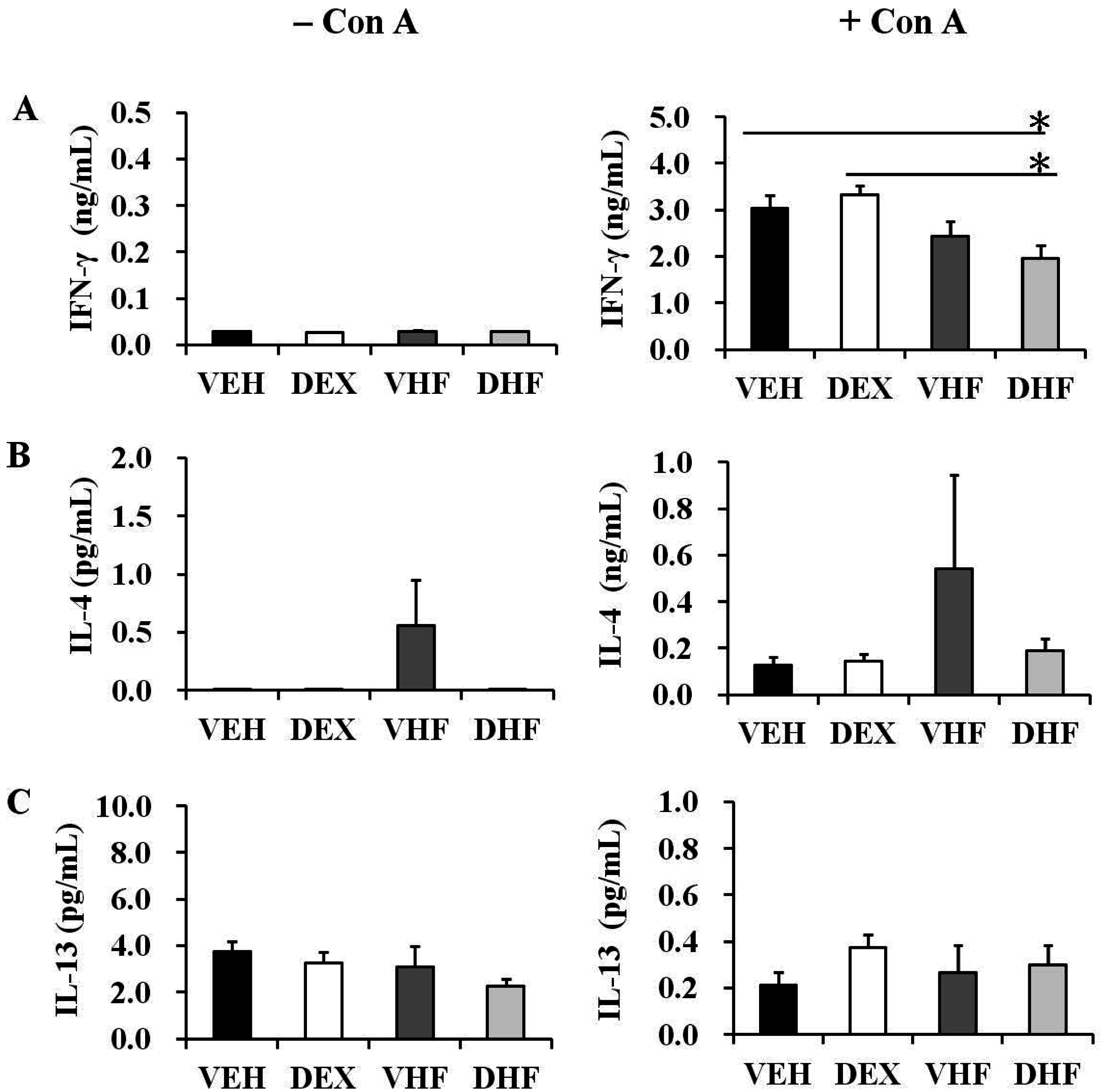
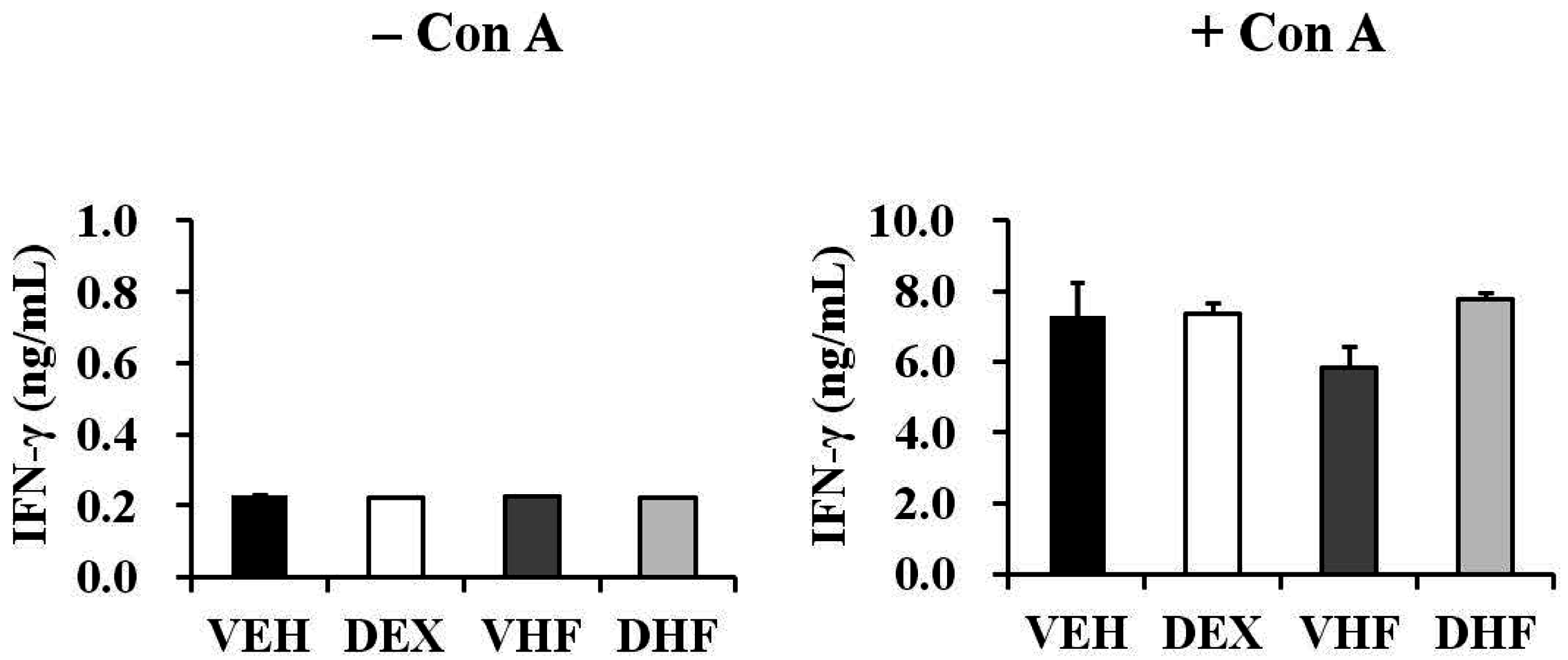
2.5. Prenatal Dexamethasone Exposure Plus a Postnatal HF Diet Suppressed Rat Splenocyte IFN-γ Production at D120
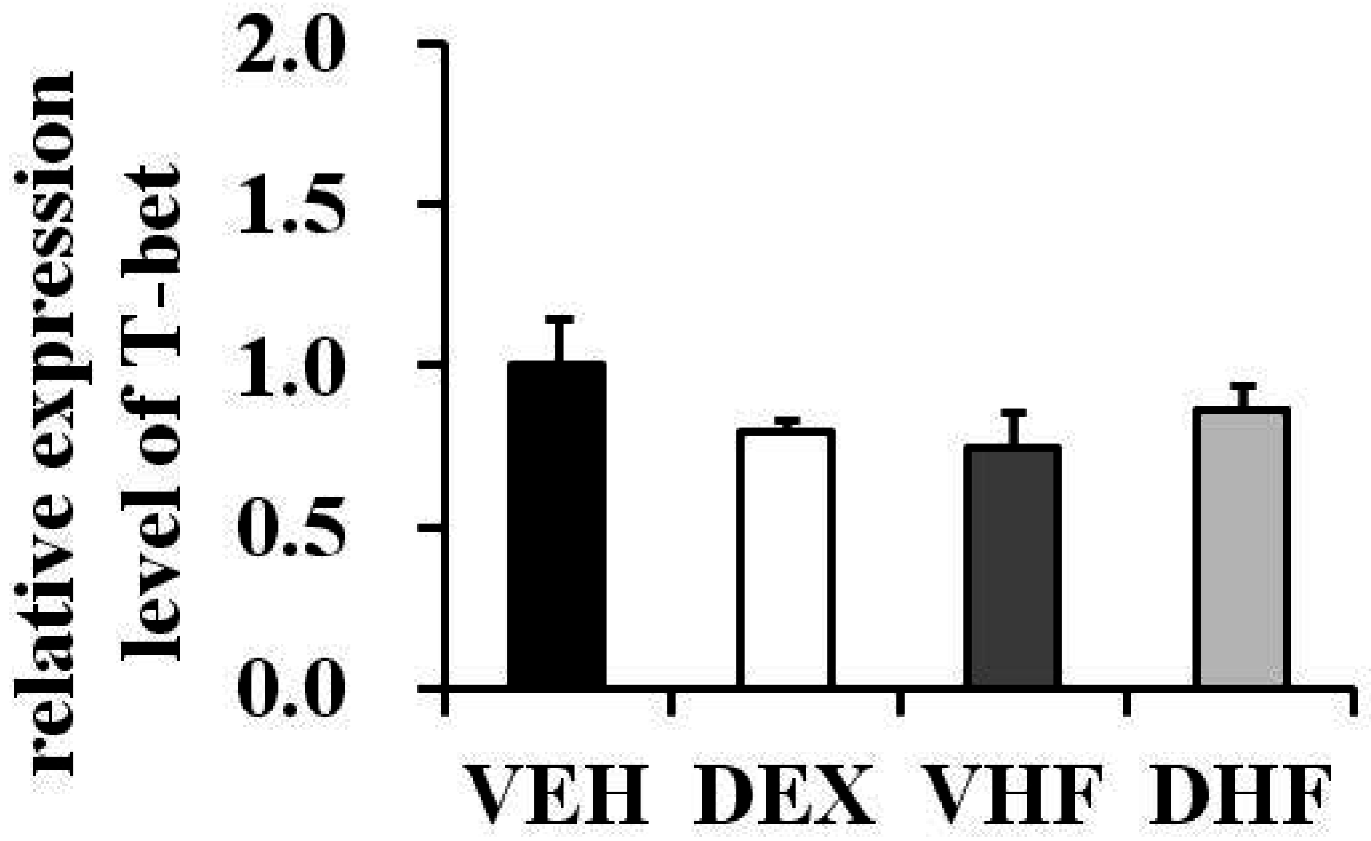
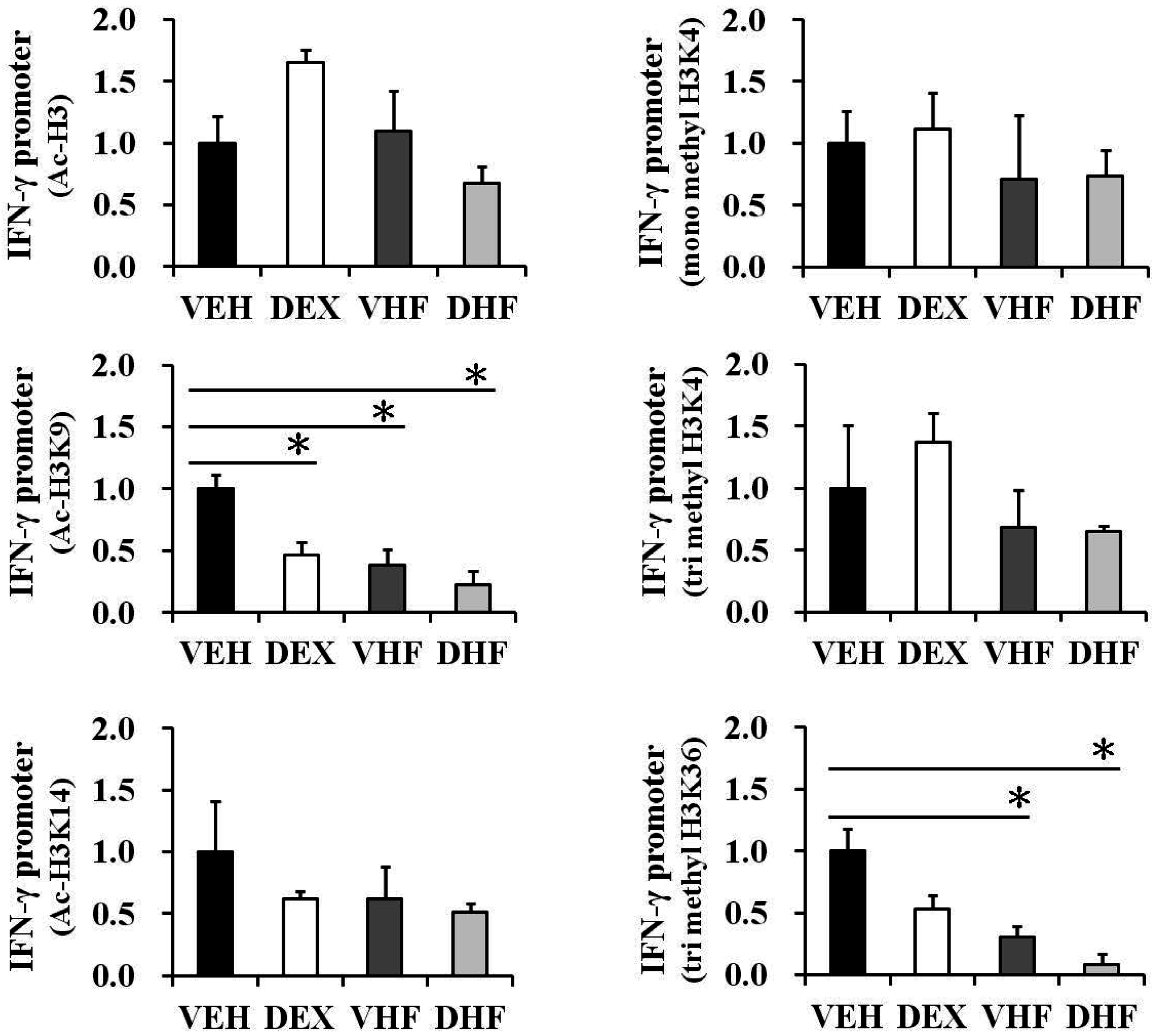
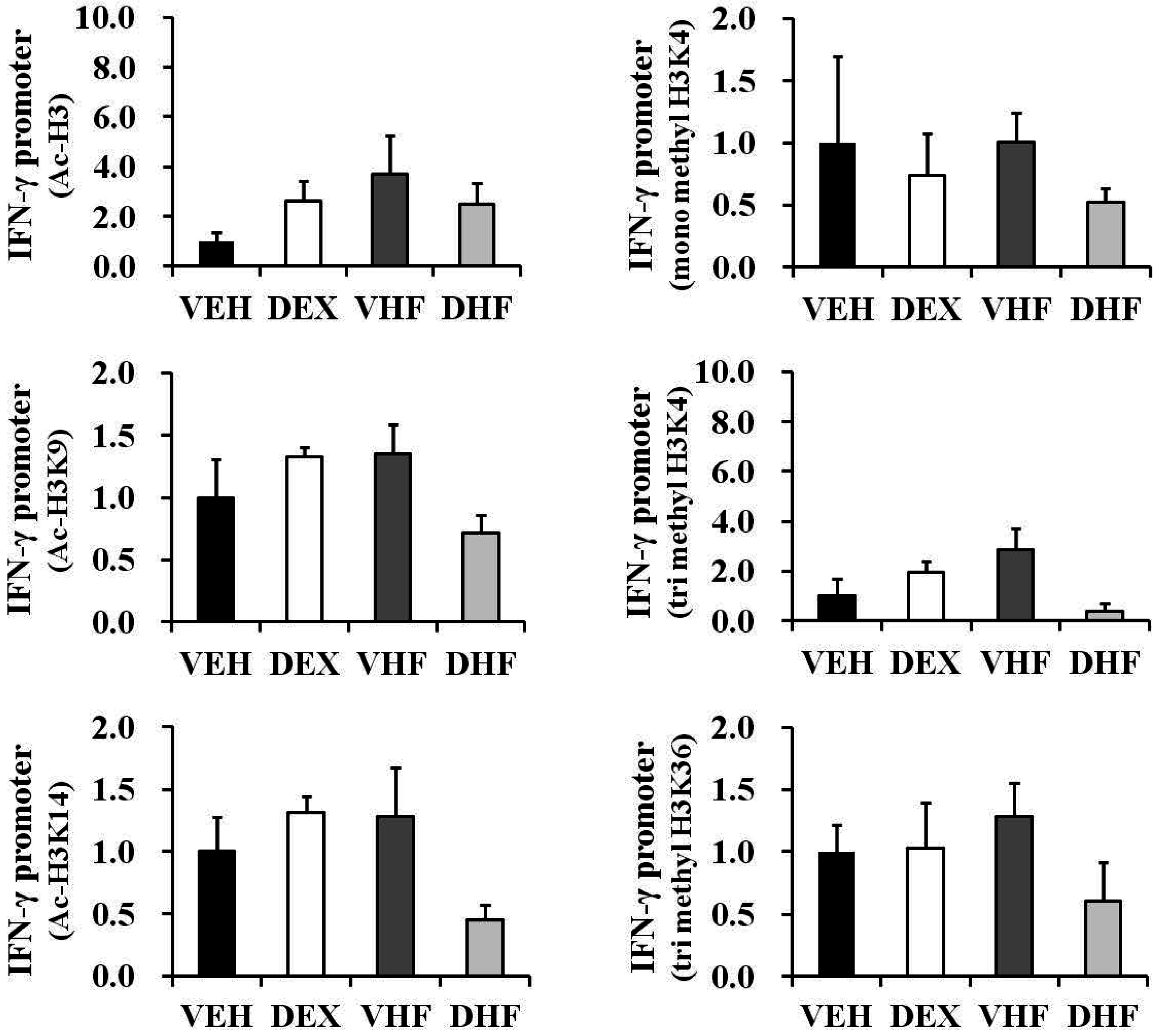
2.6. Prenatal Dexamethasone Exposure Plus Postnatal HF Diet-Induced IFN-γ Decrease at D120 Was Associated with Histone H3 Modification
2.7. The Impact of Prenatal Dexamethasone Exposure plus Postnatal HF Diet on IFN-γ Production by Rat Splenocytes at D120 Was Diminished at D180
2.8. Histone Modification of the IFN-γ Promoter Showed Age-Dependent Dynamic Changes Compatible with IFN-γ Production
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Experimental Procedures and Specimen Collection
4.3. Peripheral Blood Analysis and Plasma Immunoglobulin Detection
4.4. Splenocyte Cultures and Drug Treatment
4.5. 5-Bromo-2′-Deoxyuridine (BrdU) Cell Proliferation Assay
4.6. Cytokine Analysis
4.7. Reverse Transcription (RT)-Polymerase Chain Reaction (PCR)
4.8. Chromatin Immunoprecipitation (ChIP) Assay
4.9. Western Blotting
4.10. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brownfoot, F.C.; Gagliardi, D.I.; Bain, E.; Middleton, P.; Crowther, C.A. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2013, 8, CD006764. [Google Scholar] [PubMed]
- Drake, A.J.; Raubenheimer, P.J.; Kerrigan, D.; McInnes, K.J.; Seckl, J.R.; Walker, B.R. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology 2010, 151, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.L.; Zuloaga, D.G.; Hiroi, R.; Foradori, C.D.; Legare, M.E.; Handa, R.J. Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology 2012, 153, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Venners, S.A.; Fu, L.; Pearson, C.; Ortiz, K.; Wang, X. Association of antenatal steroid use with cord blood immune biomarkers in preterm births. Early Hum. Dev. 2011, 87, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Biggioggero, M.; Borghi, M.O.; Gerosa, M.; Trespidi, L.; Cimaz, R.; Meroni, P.I. Immune function in children born to mothers with autoimmune diseases and exposed in utero to immunosuppressants. Lupus 2007, 16, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Rodriguez-Perez, C.; Tincani, A.; Lojacono, A.; Nacinovich, R.; Chirico, G. Neonates born from mothers with autoimmune disorders. Early Hum. Dev. 2009, 85, S67–S70. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.R.; Kuo, H.C.; Chen, C.C.; Sheen, J.M.; Tiao, M.M.; Chen, Y.C.; Chang, K.A.; Tain, Y.L.; Huang, L.T. Prenatal dexamethasone exposure in rats results in long-term epigenetic histone modifications and tumour necrosis factor-α production decrease. Immunology 2014, 143, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Ono, M.; Setoguchi, R.; Yagi, H.; Hori, S.; Fehervari, Z.; Shimizu, J.; Takahashi, T.; Nomura, T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006, 212, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R. Pregnancy: Success and failure within the Th1/Th2/Th3 paradigm. Semin. Immunol. 2001, 13, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, M. Th2 cells as targets for therapeutic intervention in allergic bronchial asthma. Expert Rev. Mol. Diagn. 2009, 9, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Nehlsen-Cannarella, S.L.; Ekkens, M.; Utter, A.C.; Butterworth, D.E.; Fagoaga, O.R. Influence of obesity on immune function. J. Am. Diet. Assoc. 1999, 99, 294–299. [Google Scholar] [CrossRef]
- Mito, N.; Hosoda, T.; Kato, C.; Sato, K. Change of cytokine balance in diet-induced obese mice. Metab. Clin. Exp. 2000, 49, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Acosta-Perez, E.; Brehm, J.M.; Han, Y.Y.; Alvarez, M.; Colon-Semidey, A.; Canino, G.; Celedon, J.C. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J. Allergy Clin. Immunol. 2014, 133, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Kim, H.B.; Lee, S.Y.; Kim, B.S.; Kim, J.H.; Yu, J.H.; Kim, B.J.; Hong, S.J. Association between obesity and the prevalence of allergic diseases, atopy, and bronchial hyperresponsiveness in Korean adolescents. Int. Arch. Allergy Immunol. 2011, 154, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Colonna, M.; Klein, S. Impaired mononuclear cell immune function in extreme obesity is corrected by weight loss. Rejuvenation Res. 2007, 10, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, K.L.; Terrillion, C.E.; Hyun, J.; Koenig, J.I.; Moran, T.H. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 2009, 58, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.H.; Deng, S.; Kumpf, S.W.; Lee, P.A.; Zagouras, P.; Ryan, A.; Gallagher, D.S. Validation of rat reference genes for improved quantitative gene expression analysis using low density arrays. BioTechniques 2007, 42, 503–512. [Google Scholar] [PubMed]
- Andreollo, N.A.; Santos, E.F.; Araujo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef] [PubMed]
- De Araujo-Souza, P.S.; Hanschke, S.C.; Viola, J.P. Epigenetic control of interferon-γ expression in CD8 T cells. J. Immunol. Res. 2015, 2015, 849573. [Google Scholar] [CrossRef] [PubMed]
- Shnyreva, M.; Weaver, W.M.; Blanchette, M.; Taylor, S.L.; Tompa, M.; Fitzpatrick, D.R.; Wilson, C.B. Evolutionarily conserved sequence elements that positively regulate IFN-γ expression in T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 12622–12627. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Qui, H.Z.; Adler, A.J. In vitro and in vivo differentiated effector CD8 T cells display divergent histone acetylation patterns within the Ifng locus. Immunol. Lett. 2009, 122, 214–218. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Aste-Amezaga, M.; Valiante, N.M.; Ma, X.; Kubin, M.; Trinchieri, G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993, 178, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis—2012 Curt Richter Award Winner. Psychoneuroendocrinology 2013, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.G.; Winter, M.A.; Hunn, J.; Carnahan, J.; Pressman, E.K.; Glover, V.; Robertson-Blackmore, E.; Moynihan, J.A.; Lee, F.E.; Caserta, M.T. Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain Behav. Immun. 2013, 32, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; Lee, J.E.; Olsen, J.; Fitch, K.; Marsh, J.A. Developmental immunotoxicity of dexamethasone: Comparison of fetal versus adult exposures. Toxicology 2003, 194, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Cimaz, R.; Meregalli, E.; Biggioggero, M.; Borghi, O.; Tincani, A.; Motta, M.; Airo, P.; Meroni, P.L. Alterations in the immune system of children from mothers treated with immunosuppressive agents during pregnancy. Toxicol. Lett. 2004, 149, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Di Renzo, L.; Anania, C.; Osborn, J.F.; Ippoliti, F.; Schiavo, E.; Chiesa, C. Increased T-helper interferon-γ-secreting cells in obese children. Eur. J. Endocrinol. 2006, 154, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Iwabuchi, K.; Iwata, D.; Miyazaki, A.; Kon, Y.; Niino, M.; Kikuchi, S.; Yanagawa, Y.; Kaer, L.V.; Sasaki, H.; Onoe, K. Effect of high fat diet on NKT cell function and NKT cell-mediated regulation of Th1 responses. Scand. J. Immunol. 2008, 67, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Low birth weight and hypertension. Br. Med. J. 1988, 297, 134–135. [Google Scholar] [CrossRef]
- Mathers, J.C. Early nutrition: Impact on epigenetics. Forum Nutr. 2007, 60, 42–48. [Google Scholar] [PubMed]
- Crudo, A.; Petropoulos, S.; Moisiadis, V.G.; Iqbal, M.; Kostaki, A.; Machnes, Z.; Szyf, M.; Matthews, S.G. Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: Multigenerational effects. Endocrinology 2012, 153, 3269–3283. [Google Scholar] [CrossRef] [PubMed]
- Crudo, A.; Petropoulos, S.; Suderman, M.; Moisiadis, V.G.; Kostaki, A.; Hallett, M.; Szyf, M.; Matthews, S.G. Effects of antenatal synthetic glucocorticoid on glucocorticoid receptor binding, DNA methylation, and genome-wide mRNA levels in the fetal male hippocampus. Endocrinology 2013, 154, 4170–4181. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; de la Garza, A.L.; Magnet, U.; Aumueller, E.; Haslberger, A.G. Obesity: Epigenetic regulation-recent observations. Biomol. Concepts 2015, 6, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.M.; Gibney, E.R. Epigenetic regulation in obesity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Caramori, G.; Lim, S.; Oates, T.; Chung, K.F.; Barnes, P.J.; Adcock, I.M. Expression and activity of histone deacetylases in human asthmatic airways. Am. J. Respir. Crit. Care Med. 2002, 166, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Histone deacetylase-2 and airway disease. Ther. Adv. Respir. Dis. 2009, 3, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Wang, L.; Fu, J.J.; Fan, T.; Wang, Z.L.; Wang, G. Association between histone hyperacetylation status in memory T lymphocytes and allergen-induced eosinophilic airway inflammation. Respirology 2016, 21, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Eddy, J.L.; Krukowski, K.; Janusek, L.; Mathews, H.L. Glucocorticoids regulate natural killer cell function epigenetically. Cell. Immunol. 2014, 290, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Trac, C.; Du, J.; Natarajan, R.; Schones, D.E. Persistent chromatin modifications induced by high fat diet. J. Biol. Chem. 2016, 291, 10446–10455. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wang, C.; Xia, L.; Fan, C.; Dong, H.; Deckelbaum, R.J.; Qi, K. Epigenetic modification of the leptin promoter in diet-induced obese mice and the effects of N-3 polyunsaturated fatty acids. Sci. Rep. 2014, 4, 5282. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.R.; Bale, T.L.; Epperson, C.N. Prenatal programming of mental illness: Current understanding of relationship and mechanisms. Curr. Psychiatry Rep. 2015, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Lopez, G.; Giovanoli, S.; Langhans, W.; Meyer, U. Priming of metabolic dysfunctions by prenatal immune activation in mice: Relevance to schizophrenia. Schizophr. Bull. 2013, 39, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Alisch, R.S.; Barwick, B.G.; Chopra, P.; Myrick, L.K.; Satten, G.A.; Conneely, K.N.; Warren, S.T. Age-associated DNA methylation in pediatric populations. Genome Res. 2012, 22, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Nakamura, A.; Ishigami, A.; Goto, S.; Takahashi, R. Age-related difference of site-specific histone modifications in rat liver. Biogerontology 2009, 10, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.; Maugeri, U.; Flak, E.; Mroz, E.; Bianchi, I. Predisposition to acute respiratory infections among overweight preadolescent children: An epidemiologic study in Poland. Public Health 1998, 112, 189–195. [Google Scholar] [CrossRef]
- Gabbert, C.; Donohue, M.; Arnold, J.; Schwimmer, J.B. Adenovirus 36 and obesity in children and adolescents. Pediatrics 2010, 126, 721–726. [Google Scholar] [CrossRef] [PubMed]
- D’Acquisto, F.; Crompton, T. CD3+CD4−CD8− (double negative) T cells: Saviours or villains of the immune response? Biochem. Pharmacol. 2011, 82, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.S.; Zhang, Z.X.; Chen, W.; Zhang, L. Double-negative T regulatory cells can develop outside the thymus and do not mature from CD8+ T cell precursors. J. Immunol. 2006, 177, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.; Sacedon, R.; Vicente, A.; Hernandez-Lopez, C.; Zapata, A.G.; Varas, A. Rat peripheral CD4+CD8+ T lymphocytes are partially immunocompetent thymus-derived cells that undergo post-thymic maturation to become functionally mature CD4+ T lymphocytes. J. Immunol. 2002, 168, 5005–5013. [Google Scholar] [CrossRef] [PubMed]
- Gulvady, A.A.; Ciolino, H.P.; Cabrera, R.M.; Jolly, C.A. Resveratrol inhibits the deleterious effects of diet-induced obesity on thymic function. J. Nutr. Biochem. 2013, 24, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodero, S.; Fernandez-Morera, J.L.; Fernandez, A.F.; Menendez-Torre, E.; Fraga, M.F. Epigenetic regulation of aging. Discov. Med. 2010, 10, 225–233. [Google Scholar] [PubMed]
- Rink, L.; Cakman, I.; Kirchner, H. Altered cytokine production in the elderly. Mech. Ageing Dev. 1998, 102, 199–209. [Google Scholar] [CrossRef]
- Yu, H.R.; Kuo, H.C.; Huang, L.T.; Chen, C.C.; Tain, Y.L.; Sheen, J.M.; Tiao, M.M.; Huang, H.C.; Yang, K.D.; Ou, C.Y.; Hsu, T.Y. l-Arginine modulates neonatal lymphocyte proliferation through an interleukin-2 independent pathway. Immunology 2014, 143, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.S.; Zueva, E.; Cammas, F.; Schreiber, H.A.; Masson, V.; Belz, G.T.; Roche, D.; Maison, C.; Quivy, J.P.; Almouzni, G.; Amigorena, S. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature 2012, 487, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Huang, L.-T.; Tain, Y.-L.; Chen, C.-C.; Sheen, J.-M.; Tiao, M.-M.; Tsai, C.-M.; Kuo, H.-C.; Huang, C.-C.; Chang, K.-A.; et al. Prenatal glucocorticoid contributed to rat lung dysplasia is related to asymmetric dimethylarginine/nitric oxide pathway. Sci. Bull. 2015, 60, 1416–1425. [Google Scholar] [CrossRef]

| BW/Immune Organ Weight at D120 | ||||
|---|---|---|---|---|
| Group | VEH | DEX | VHF | DHF |
| BW (g) | 497.60 ± 40.40 | 500.17 ± 19.93 | 646.00 ± 19.93 (p = 0.006) | 568.50 ± 34.45 |
| Thymus (g) | 0.34 ± 0.03 | 0.40 ± 0.04 (p = 0.046) | 0.39 ± 0.04 | 0.36 ± 0.04 |
| Spleen (g) | 0.79 ± 0.10 | 0.91 ± 0.03 (p = 0.002) | 0.92 ± 0.03 (p = 0.016) | 0.88 ± 0.05 |
| Thymus/BW | (4.99 ± 0.07)× 10−4 | (8.98 ± 0.60) × 10−4 (p = 0.033) | (6.14 ± 0.81) × 10−4 | (6.39 ± 0.08) × 10−4 |
| Spleen/BW | (1.52 ± 0.08) × 10−3 | (1.74 ± 0.07) × 10−3 (p = 0.045) | (1.43 ± 0.05) × 10−3 (p = 0.039) | (1.55 ± 0.04) × 10−3 (p = 0.049) |
| BW/Immune Organ Weight at D180 | ||||
| Group | VEH | DEX | VHF | DHF |
| BW (g) | 649.71 ± 22.93 | 691.11 ± 26.03 | 751.67 ± 29.90 (p = 0.046) | 943.38 ± 49.43 (p < 0.001) |
| Thymus (g) | 0.27 ± 0.02 | 0.20 ± 0.02 | 0.34 ± 0.04 (p = 0.044) | 0.31 ± 0.04 |
| Spleen (g) | 1.01 ± 0.05 | 1.06 ± 0.07 | 1.01 ± 0.05 | 1.24 ± 0.06 (p = 0.014) |
| Thymus/BW | (4.11 ± 0.31) × 10−4 | (2.91 ± 0.17) × 10−4 (p = 0.049) | (4.56 ± 0.56) × 10−4 | (3.34 ± 0.38) × 10−4 |
| Spleen/BW | (1.55 ± 0.06) × 10−3 | (1.56 ± 0.09) × 10−3 | (1.35 ± 0.07) × 10−3 | (1.33 ± 0.08) × 10−3 |
| D120 | ||||
|---|---|---|---|---|
| Group | VEH | DEX | VHF | DHF |
| WBC (103/μL) | 12.16 ± 2.49 | 10.57 ± 1.56 | 10.57 ± 0.94 | 8.58 ± 0.89 |
| RBC (106/μL) | 9.26 ± 0.24 | 9.45 ± 0.26 | 9.14 ± 0.21 | 9.07 ± 0.17 |
| HGB (g/dL) | 16.38 ± 0.44 | 15.44 ± 0.45 | 15.98 ± 0.32 | 15.33 ± 0.23 |
| PLT (103/μL) | 1036.50 ± 51.86 | 1075.60 ± 191.95 | 1100.67 ± 70.79 | 935.00 ± 51.41 |
| Neutrophil (%) | 12.00 ± 0.24 | 17.34 ± 1.67 (p = 0.014) | 22.33 ± 3.45 (p = 0.018) | 19.08 ± 2.74 (p = 0.033) |
| Lymphocyte (%) | 84.05 ± 0.35 | 78.66 ± 1.82 (p = 0.014) | 73.78 ± 3.57 (p = 0.028) | 76.32 ± 3.14 |
| Monocyte (%) | 2.33 ± 0.30 | 2.68 ± 0.47 | 2.85 ± 0.37 | 3.65 ± 0.47 (p = 0.049) |
| Eosinophil (%) | 1.58 ± 0.41 | 1.28 ± 0.45 | 1.00 ± 0.18 | 0.92 ± 0.18 |
| Basophil (%) | 0.05 ± 0.03 | 0.04 ± 0.02 | 0.03 ± 0.02 | 0.03 ± 0.03 |
| CD4+ (%) | 62.08 ± 3.77 | 60.02 ± 1.99 | 58.97 ± 2.54 | 55.72 ± 1.71 |
| CD8a+ (%) | 36.06 ± 3.72 | 37.37 ± 2.53 | 36.68 ± 2.54 | 40.93 ± 1.74 |
| CD4+CD8a+ (%) | 1.50 ± 0.21 | 2.20 ± 0.67 | 3.65 ± 0.85 (p = 0.042) | 2.72 ± 0.69 |
| CD4−CD8a− (%) | 0.32 ± 0.04 | 0.40 ± 0.08 | 0.67 ± 0.13 (p = 0.014) | 0.62 ± 0.06 (p = 0.032) |
| CD45RA (%) | 2.40 ± 0.65 | 1.15 ± 0.40 | 1.28 ± 0.39 | 1.68 ± 0.70 |
| D180 | ||||
| Group | VEH | DEX | VHF | DHF |
| WBC (103/μL) | 11.55 ± 1.98 | 11.79 ± 1.33 | 7.82 ± 0.86 | 7.06 ± 0.76 (p = 0.030) |
| RBC (106/μL) | 9.16 ± 0.31 | 9.52 ± 0.33 | 9.04 ± 0.11 | 8.92 ± 0.17 |
| HGB (g/dL) | 15.35 ± 0.51 | 16.68 ± 0.63 | 15.07 ± 0.18 | 16.30 ± 0.30 |
| PLT (103/μL) | 1070.75 ± 158.53 | 1215.40 ± 112.81 | 1137.33 ± 51.84 | 1217.50 ± 20.33 |
| Neutrophil (%) | 19.35 ± 3.44 | 18.43 ± 0.77 | 20.75 ± 2.92 | 19.27 ± 2.22 |
| Lymphocyte (%) | 75.88 ± 3.54 | 77.83 ± 1.31 | 61.00 ± 10.85 | 76.70 ± 2.33 |
| Monocyte (%) | 3.30 ± 0.46 | 2.83 ± 0.34 | 4.67 ± 0.54 | 3.25 ± 0.38 |
| Eosinophil (%) | 1.45 ± 0.44 | 0.90 ± 0.23 | 1.12 ± 0.12 | 0.78 ± 0.23 |
| Basophil (%) | 0.03 ± 0.03 | 0.04 ± 0.02 | 0.02 ± 0.02 | 0.00 ± 0.00 |
| CD4+ (%) | 66.03 ± 2.25 | 66.06 ± 2.04 | 58.75 ± 2.71 | 62.83 ± 1.50 |
| CD8a+ (%) | 31.80 ± 1.98 | 32.60 ± 2.08 | 39.78 ± 2.58 | 35.60 ± 1.58 |
| CD4+CD8a+ (%) | 0.73 ± 0.07 | 0.64 ± 0.07 | 0.60 ± 0.09 | 0.57 ± 0.08 |
| CD4−CD8a− (%) | 1.43 ± 0.42 | 0.74 ± 0.05 | 0.88 ± 0.07 | 0.98 ± 0.19 |
| CD45RA (%) | 1.28 ± 0.23 | 2.22 ± 0.67 | 1.03 ± 0.18 | 1.22 ± 0.28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-R.; Tain, Y.-L.; Sheen, J.-M.; Tiao, M.-M.; Chen, C.-C.; Kuo, H.-C.; Hung, P.-L.; Hsieh, K.-S.; Huang, L.-T. Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats. Int. J. Mol. Sci. 2016, 17, 1610. https://doi.org/10.3390/ijms17101610
Yu H-R, Tain Y-L, Sheen J-M, Tiao M-M, Chen C-C, Kuo H-C, Hung P-L, Hsieh K-S, Huang L-T. Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats. International Journal of Molecular Sciences. 2016; 17(10):1610. https://doi.org/10.3390/ijms17101610
Chicago/Turabian StyleYu, Hong-Ren, You-Lin Tain, Jiunn-Ming Sheen, Mao-Meng Tiao, Chih-Cheng Chen, Ho-Chang Kuo, Pi-Lien Hung, Kai-Sheng Hsieh, and Li-Tung Huang. 2016. "Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats" International Journal of Molecular Sciences 17, no. 10: 1610. https://doi.org/10.3390/ijms17101610