Radiological Patterns of Brain Metastases in Breast Cancer Patients: A Subproject of the German Brain Metastases in Breast Cancer (BMBC) Registry
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
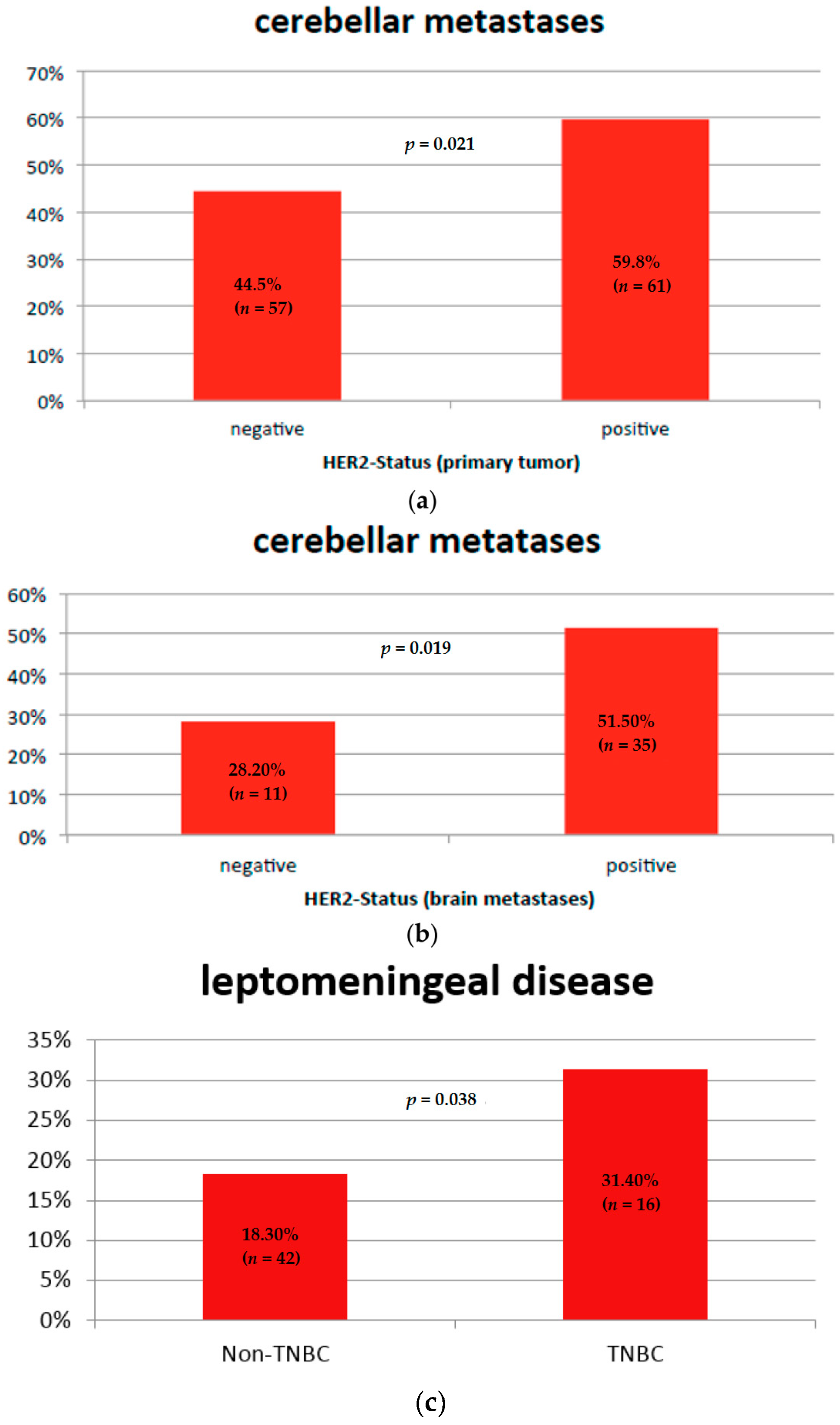
2.2. Brain Metastases Patterns and Tumor Subtype
Leptomeningeal Disease and Tumor Subtype
2.3. Brain Metastases Patterns and Systemic Therapy
Leptomeningeal Disease and Systemic Therapy
2.4. Brain Metastases Patterns and Patient Outcome
Leptomeningeal Disease and Patient Outcome
3. Discussion
4. Materials and Methods
Author Contributions
Conflicts of Interest
References
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Muller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M.; Mayer, M.; Rugo, H.S.; Kaufman, P.A.; Tan-Chiu, E.; Tripathy, D.; Tudor, I.C.; Wang, L.I.; Brammer, M.G.; Shing, M.; et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: Incidence, treatment, and survival in patients from registHER. Clin. Cancer Res. 2011, 17, 4834–4843. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.H.; Kim, S.Y.; Han, H.S.; Kwon, Y.; Lee, K.S.; Kim, T.H.; Ro, J. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008, 10, R20. [Google Scholar] [CrossRef] [PubMed]
- Church, D.N.; Modgil, R.; Guglani, S.; Bahl, A.; Hopkins, K.; Braybrooke, J.P.; Blair, P.; Price, C.G. Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am. J. Clin. Oncol. 2008, 31, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Lekanidi, K.; Evans, A.L.; Shah, J.; Jaspan, T.; Baker, L.; Evans, A.J. Pattern of brain metastatic disease according to HER-2 and ER receptor status in breast cancer patients. Clin. Radiol. 2013, 68, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Niwinska, A.; Rudnicka, H.; Murawska, M. Breast cancer leptomeningeal metastasis: Propensity of breast cancer subtypes for leptomeninges and the analysis of factors influencing survival. Med. Oncol. 2013, 30, 408. [Google Scholar] [CrossRef] [PubMed]
- Tomasevic, Z.; Tomasevic, Z.M.; Kovac, Z.; Milovanovic, Z. Breast cancer metastases to cerebellum. J. Clin. Oncol. 2014, 32, e11600. [Google Scholar]
- Duchnowska, R.; Jassem, J.; Goswami, C.P.; Dundar, M.; Gokmen-Polar, Y.; Li, L.; Woditschka, S.; Biernat, W.; Sosinska-Mielcarek, K.; Czartoryska-Arlukowicz, B.; et al. Predicting early brain metastases based on clinicopathological factors and gene expression analysis in advanced HER2-positive breast cancer patients. J. Neurooncol. 2015, 122, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duchnowska, R.; Szczylik, C. Central nervous system metastases in breast cancer patients administered trastuzumab. Cancer Treat. Rev. 2005, 31, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, H.J.; Schmitt, M.; Willems, A.; Bernhard, H.; Harbeck, N.; Heinemann, V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anti-Cancer Drugs 2007, 18, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.M.; Suo, C.; Valenzuela, M.; Haydu, L.E.; Jacobsen, K.D.; Reisse, C.H.; Fogarty, G. Low incidence of melanoma brain metastasis in the hippocampus. Radiother. Oncol. 2014, 111, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.C.; Kang, M.J.; Kim, J.E.; Ahn, J.H.; Jung, K.H.; Gong, G.; Kim, H.H.; Ahn, S.D.; Kim, S.S.; Son, B.H.; et al. Clinical features and outcome of leptomeningeal metastasis in patients with breast cancer: A single center experience. Cancer Chemother. Pharmacol. 2013, 72, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.W.; Jeong, I.H.; Joung, A.; Cho, H.J.; Kim, S.H.; Kim, H.J. Leptomeningeal metastasis: Clinical experience of 519 cases. Eur. J. Cancer 2016, 56, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Deng, H.; Jia, W.; Zeng, Y.; Rao, N.; Li, S.; Jin, L.; Wu, J.; Song, E.; Su, F. Comparison of ER/PR and HER2 statuses in primary and paired liver metastatic sites of breast carcinoma in patients with or without treatment. J. Cancer Res. Clin. Oncol. 2012, 138, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Jordan, L.B.; Quinlan, P.; Anderson, E.; Skene, A.; Dewar, J.A.; Purdie, C.A. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: The Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 2010, 12, R92. [Google Scholar] [CrossRef] [PubMed]
- Mueller, V.; Laakmann, E.; Fehm, T.; Moebus, V.; Von Minckwitz, G.; Loibl, S.; Witzel, I. Brain Metastases in Breast Cancer Network Germany (BMBC; GBG 79): Multicentric, retro- and prospective collection of patient data and biomaterial from patients with brain metastases. J. Clin. Oncol. 2015, 33, TPS639. [Google Scholar]

| Patient’s Characteristic | Median or Number | Range or Percent | |
|---|---|---|---|
| Age at diagnosis of breast cancer in years | 51 (median) | Range 23–89 | |
| Age at diagnosis of BMs in years | 56 (median) | Range 27–89 | |
| Time to brain metastases from first diagnosis of BC in months | 39 (median) | Range 0–415 | |
| Survival after BM diagnosis in months | 4 (median) | Range 0–91 | |
| Estrogen-receptor (n = 261, n = 39 missing data) | positive | 141 | 54.0% |
| negative | 120 | 46.0% | |
| Progesterone-receptor (n = 260, n = 40 missing data) | positive | 125 | 48.1% |
| negative | 135 | 51.9% | |
| HER2-receptor (n = 230, n = 70 missing data) | positive | 102 | 44.3% |
| negative | 128 | 55.7% | |
| Triple-negative primary tumor (n = 281, n = 19 missing data) | yes | 51 | 18.1% |
| no | 230 | 81.9% | |
| Chemotherapy before the diagnosis of BMs (n = 267, missing data n = 33) | yes | 238 | 89.1% |
| no | 29 | 10.9% | |
| Endocrine therapy before the diagnosis of BMs (n = 136, missing data n = 5) | yes | 116 | 85.3% |
| no | 20 | 14.7% | |
| HER2-targeted therapy before the diagnosis of BMs in the subgroup of patients with a HER2-positive BC (n = 99, missing data n = 3) | yes | 85 | 85.9% |
| no | 14 | 14.1% | |
| Receptor Status | BM Number (Mean, SD) | p-Value | |
|---|---|---|---|
| Estrogen receptor status | positive | 7.19 (0.24) | <0.001 |
| negative | 15.26 (0.37) | ||
| Progesterone receptor status | positive | 6.95 (0.25) | <0.001 |
| negative | 14.56 (0.34) | ||
| HER2 status | positive | 8.24 (0.29) | <0.001 |
| negative | 15.44 (0.38) | ||
| Variable | Hazard Ratio | p-Value | 95% Confidence Interval |
|---|---|---|---|
| HER2-negativity | 1.685 | 0.008 | 1.144–2.480 |
| Older age at diagnosis of BMs | 1.016 | 0.037 | 1.001–1.031 |
| No radiotherapy of the brain | 3.297 | <0.001 | 2.042–5.322 |
| No neurosurgery | 1.981 | 0.002 | 1.297–3.026 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laakmann, E.; Witzel, I.; Scriba, V.; Grzyska, U.; Zu Eulenburg, C.; Burchardi, N.; Hesse, T.; Würschmidt, F.; Fehm, T.; Möbus, V.; et al. Radiological Patterns of Brain Metastases in Breast Cancer Patients: A Subproject of the German Brain Metastases in Breast Cancer (BMBC) Registry. Int. J. Mol. Sci. 2016, 17, 1615. https://doi.org/10.3390/ijms17101615
Laakmann E, Witzel I, Scriba V, Grzyska U, Zu Eulenburg C, Burchardi N, Hesse T, Würschmidt F, Fehm T, Möbus V, et al. Radiological Patterns of Brain Metastases in Breast Cancer Patients: A Subproject of the German Brain Metastases in Breast Cancer (BMBC) Registry. International Journal of Molecular Sciences. 2016; 17(10):1615. https://doi.org/10.3390/ijms17101615
Chicago/Turabian StyleLaakmann, Elena, Isabell Witzel, Verena Scriba, Ulrich Grzyska, Christine Zu Eulenburg, Nicole Burchardi, Tobias Hesse, Florian Würschmidt, Tanja Fehm, Volker Möbus, and et al. 2016. "Radiological Patterns of Brain Metastases in Breast Cancer Patients: A Subproject of the German Brain Metastases in Breast Cancer (BMBC) Registry" International Journal of Molecular Sciences 17, no. 10: 1615. https://doi.org/10.3390/ijms17101615






