Golgi-Related Proteins GOLPH2 (GP73/GOLM1) and GOLPH3 (GOPP1/MIDAS) in Cutaneous Melanoma: Patterns of Expression and Prognostic Significance
Abstract
:1. Introduction
2. Results
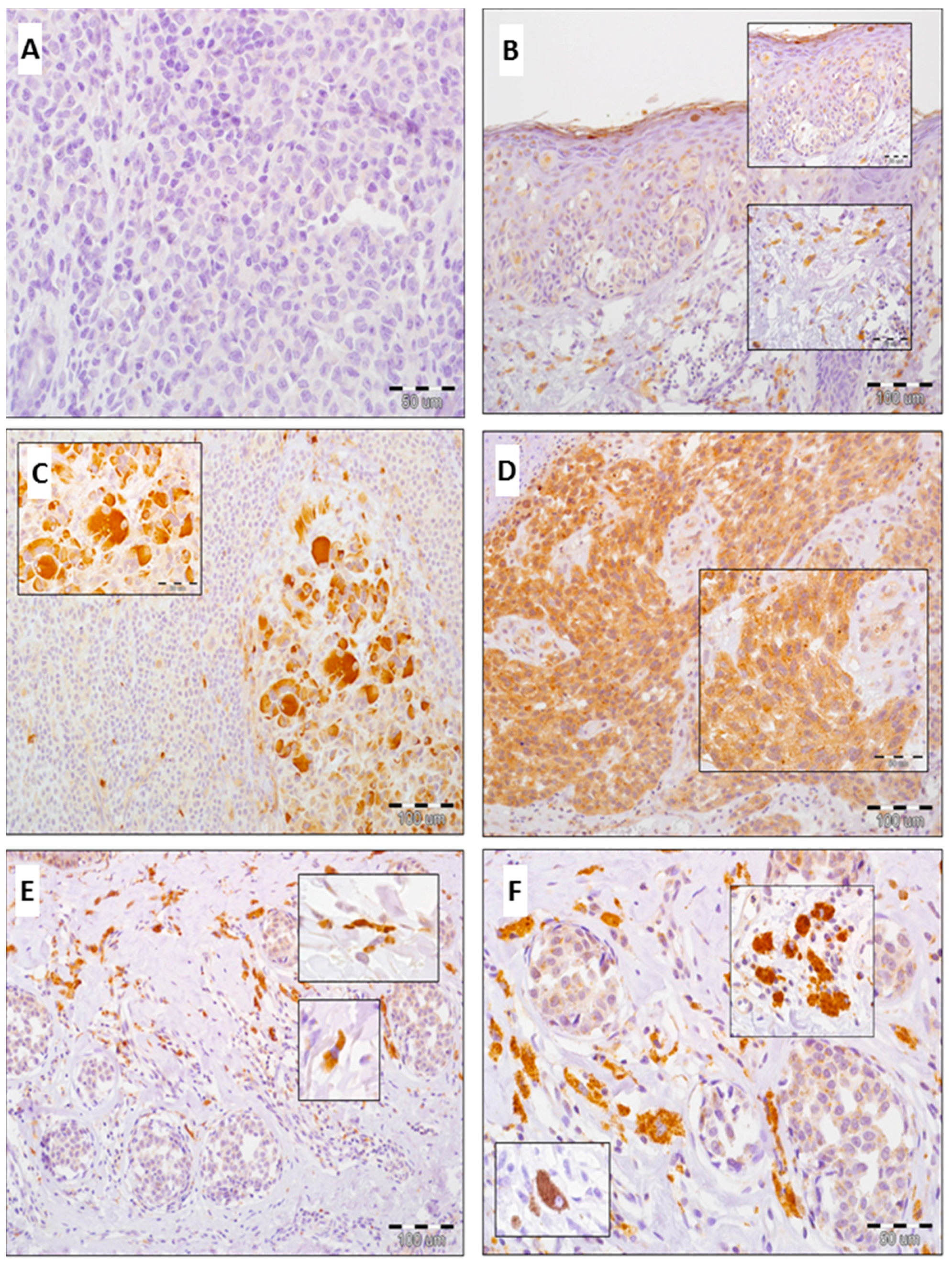
2.1. Expression of GOLPH2 and GOLPH3 in Normal Skin and Benign Nevi
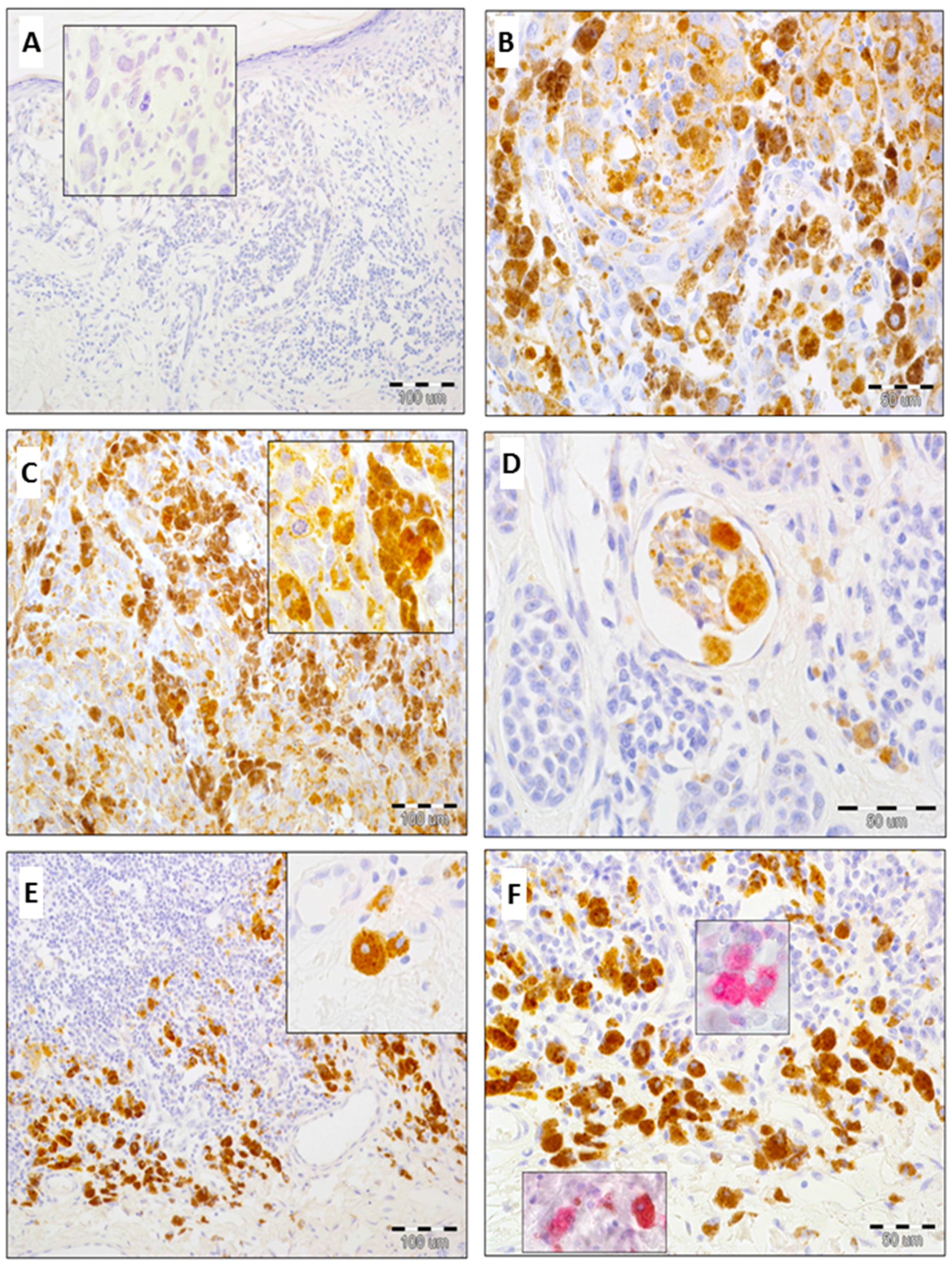
2.2. Expression of GOLPH2 in Melanoma Cells and Tumor-Associated Macrophages (TAMs)
2.3. Expression of GOLPH3 in Melanoma Cells, Tumor-Associated Macrophages (TAMs) and Cancer-Associated Fibroblasts (CAFs)
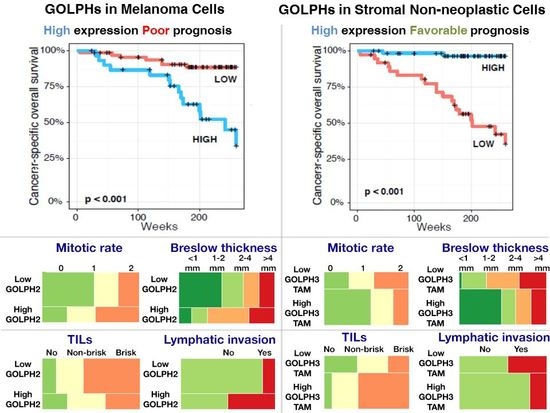
2.4. Analysis of Correlations between GOLPH2 and GOLPH3 Immunoreactivity in Melanoma Cells and Clinicopathological Parameters
2.5. Analysis of Correlations Between GOLPH2 and GOLPH3 Immunoreactivity in Stromal Compartment and Clinicopathological Parameters
2.6. Impact of Golgi-Related Protein Expression in Neoplastic Cells and Stromal Compartment on Melanoma Patient Survival—Kaplan-Meier Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Evaluation of Immunohistochemistry
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kladney, R.D.; Bulla, G.A.; Guo, L.; Mason, A.L.; Tollefson, A.E.; Simon, D.J.; Koutoubi, Z.; Fimmel, C.J. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene 2000, 249, 53–65. [Google Scholar] [CrossRef]
- Iftikhar, R.; Kladney, R.D.; Havlioglu, N.; Schmitt-Graff, A.; Gusmirovic, I.; Solomon, H.; Luxon, B.A.; Bacon, B.R.; Fimmel, C.J. Disease- and cell-specific expression of GP73 in human liver disease. Am. J. Gastroenterol. 2004, 99, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kladney, R.D.; Cui, X.; Bulla, G.A.; Brunt, E.M.; Fimmel, C.J. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology 2002, 35, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yang, H.; Xu, H.; Lu, X.; Sang, X.; Du, S.; Zhao, H.; Chen, W.; Xu, Y.; Chi, T.; et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010, 59, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Romano, P.R.; Nikolaeva, O.; Steel, L.; Mehta, A.; Fimmel, C.J.; Comunale, M.A.; D'Amelio, A.; Lok, A.S.; Block, T.M. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J. Hepatol. 2005, 43, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, H.; Mao, Y.; Xu, H.; Zhang, J.; Li, G.; Lu, X.; Sang, X.; Zhao, H.; Zhong, S.; et al. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival. J. Gastroenterol. Hepatol. 2011, 26, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Xu, H.; Lu, X.; Sang, X.; Zhong, S.; Huang, J.; Mao, Y. Golgi protein 73, not Glypican-3, may be a tumor marker complementary to alpha-Fetoprotein for hepatocellular carcinoma diagnosis. J. Gastroenterol. Hepatol. 2014, 29, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.M.; Huster, D.; Lutsenko, S.; Wrba, F.; Ferenci, P.; Fimmel, C.J. Hepatocyte GP73 expression in Wilson disease. J. Hepatol. 2009, 51, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, F.R.; Kristiansen, G.; Riener, M.O.; Dietel, M.; Oelrich, B. GOLPH2 expression may serve as diagnostic marker in seminomas. BMC Urol. 2010, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristiansen, G.; Fritzsche, F.R.; Wassermann, K.; Jager, C.; Tolls, A.; Lein, M.; Stephan, C.; Jung, K.; Pilarsky, C.; Dietel, M.; et al. GOLPH2 protein expression as a novel tissue biomarker for prostate cancer: Implications for tissue-based diagnostics. Br. J. Cancer 2008, 99, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, X.; Li, B.; Lu, J.; Chen, G. Diagnostic significance of overexpression of Golgi membrane protein 1 in prostate cancer. Urology 2012, 80, 952.e1–952.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; He, F.; Li, J.; Wei, X.; Li, Y.; Liao, X.; Sun, J.; Yi, W.; Niu, D. Expression of GOLPH2 is associated with the progression of and poor prognosis in gastric cancer. Oncol. Rep. 2014, 32, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Varambally, S.; Laxman, B.; Mehra, R.; Cao, Q.; Dhanasekaran, S.M.; Tomlins, S.A.; Granger, J.; Vellaichamy, A.; Sreekumar, A.; Yu, J.; et al. Golgi protein GOLM1 is a tissue and urine biomarker of prostate cancer. Neoplasia 2008, 10, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Dunn, T.A.; Isaacs, W.B.; de Marzo, A.M.; Luo, J. GOLPH2 and MYO6: Putative prostate cancer markers localized to the Golgi apparatus. Prostate 2008, 68, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gu, Y.; Li, X.; Wang, W.; He, J.; Peng, T. Up-regulated Golgi phosphoprotein 2 (GOLPH2) expression in lung adenocarcinoma tissue. Clin. Biochem. 2010, 43, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Frappaolo, A.; Belloni, G.; Colotti, G.; Giansanti, M.G. The multiple cellular functions of the oncoprotein Golgi phosphoprotein 3. Oncotarget 2015, 6, 3493–3506. [Google Scholar] [CrossRef] [PubMed]
- Bishe, B.; Syed, G.H.; Field, S.J.; Siddiqui, A. Role of phosphatidylinositol 4-phosphate (PI4P) and its binding protein GOLPH3 in hepatitis C virus secretion. J. Biol. Chem. 2012, 287, 27637–27647. [Google Scholar] [CrossRef] [PubMed]
- Dippold, H.C.; Ng, M.M.; Farber-Katz, S.E.; Lee, S.K.; Kerr, M.L.; Peterman, M.C.; Sim, R.; Wiharto, P.A.; Galbraith, K.A.; Madhavarapu, S.; et al. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell 2009, 139, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Nakashima-Kamimura, N.; Asoh, S.; Ishibashi, Y.; Mukai, Y.; Shidara, Y.; Oda, H.; Munakata, K.; Goto, Y.; Ohta, S. MIDAS/GPP34, a nuclear gene product, regulates total mitochondrial mass in response to mitochondrial dysfunction. J. Cell Sci. 2005, 118, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.F.; Whitaker-Menezes, D.; Lin, Z.; Martinez-Outschoorn, U.E.; Tanowitz, H.B.; Al-Zoubi, M.S.; Howell, A.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Two-compartment tumor metabolism: Autophagy in the tumor microenvironment and oxidative mitochondrial metabolism (OXPHOS) in cancer cells. Cell Cycle 2012, 11, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Isaji, T.; Im, S.; Gu, W.; Wang, Y.; Hang, Q.; Lu, J.; Fukuda, T.; Hashii, N.; Takakura, D.; Kawasaki, N.; et al. An oncogenic protein Golgi phosphoprotein 3 up-regulates cell migration via sialylation. J. Biol. Chem. 2014, 289, 20694–20705. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, E.; Itoh, T.; Hasegawa, J.; Ijuin, T.; Takeuchi, Y.; Irino, Y.; Fukumoto, M.; Takenawa, T. Phosphatidylinositol 4-phosphate in the Golgi apparatus regulates cell-cell adhesion and invasive cell migration in human breast cancer. Cancer Res. 2014, 74, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, Z.; Mo, J.; Sang, B.; Shi, Q.; Hu, J.; Xie, S.; Zhan, W.; Lu, D.; Yang, M.; et al. GOLPH3 promotes glioblastoma cell migration and invasion via the mTOR-YB1 pathway in vitro. Mol. Carcinog. 2015, 54, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Colotti, G.; Belloni, G.; Mattei, V.; Frappaolo, A.; Raffa, G.D.; Fuller, M.T.; Giansanti, M.G. GOLPH3 is essential for contractile ring formation and Rab11 localization to the cleavage site during cytokinesis in Drosophila melanogaster. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Farber-Katz, S.E.; Dippold, H.C.; Buschman, M.D.; Peterman, M.C.; Xing, M.; Noakes, C.J.; Tat, J.; Ng, M.M.; Rahajeng, J.; Cowan, D.M.; et al. DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3. Cell 2014, 156, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.L.; Kabbarah, O.; Liang, M.C.; Ivanova, E.; Anagnostou, V.; Wu, J.; Dhakal, S.; Wu, M.; Chen, S.; Feinberg, T.; et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature 2009, 459, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Su, Y.; Zhao, Y.; Pan, C.; Chen, L. Golgi phosphoprotein3 overexpression is associated with poor survival in patients with solid tumors: A meta-analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 10615–10624. [Google Scholar] [PubMed]
- Hussein, M.R. Tumour-associated macrophages and melanoma tumourigenesis: Integrating the complexity. Int. J. Exp. Pathol. 2006, 87, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Satyamoorthy, K.; Meier, F.; Berking, C.; Bogenrieder, T.; Herlyn, M. Function and regulation of melanoma-stromal fibroblast interactions: When seeds meet soil. Oncogene 2003, 22, 3162–3171. [Google Scholar] [CrossRef] [PubMed]
- Flach, E.H.; Rebecca, V.W.; Herlyn, M.; Smalley, K.S.; Anderson, A.R. Fibroblasts contribute to melanoma tumor growth and drug resistance. Mol. Pharm. 2011, 8, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xiao, M.; Ge, Y.; Krepler, C.; Belser, E.; Lopez-Coral, A.; Xu, X.; Zhang, G.; Azuma, R.; Liu, Q.; et al. BRAF Inhibition Stimulates Melanoma-Associated Macrophages to Drive Tumor Growth. Clin. Cancer Res. 2015, 21, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Tao, J.; Li, D.; Wang, Y.; Li, L.; Hu, Z.; Zhou, Z.; Chang, X.; Qu, C.; Zhang, H. Golgi protein 73 activation of MMP-13 promotes hepatocellular carcinoma cell invasion. Oncotarget 2015, 6, 33523–33533. [Google Scholar] [PubMed]
- Zigrino, P.; Kuhn, I.; Bauerle, T.; Zamek, J.; Fox, J.W.; Neumann, S.; Licht, A.; Schorpp-Kistner, M.; Angel, P.; Mauch, C. Stromal expression of MMP-13 is required for melanoma invasion and metastasis. J. Investig. Dermatol. 2009, 129, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Meierjohann, S.; Hufnagel, A.; Wende, E.; Kleinschmidt, M.A.; Wolf, K.; Friedl, P.; Gaubatz, S.; Schartl, M. MMP13 mediates cell cycle progression in melanocytes and melanoma cells: In vitro studies of migration and proliferation. Mol. Cancer 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Corte, M.D.; Gonzalez, L.O.; Corte, M.G.; Quintela, I.; Pidal, I.; Bongera, M.; Vizoso, F. Collagenase-3 (MMP-13) expression in cutaneous malignant melanoma. Int. J. Biol. Markers 2005, 20, 242–248. [Google Scholar] [PubMed]
- Tang, Q.F.; Ji, Q.; Tang, Y.; Hu, S.J.; Bao, Y.J.; Peng, W.; Yin, P.H. Golgi phosphoprotein 2 down-regulates the Th1 response in human gastric cancer cells by suppressing IL-12A. Asian Pac. J. Cancer Prev. 2013, 14, 5747–5751. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qi, K.; Wang, Z.; Gu, M.; Chen, G.; Guo, F. Golgi phosphoprotein 3 regulates metastasis of prostate cancer via matrix metalloproteinase 9. Int. J. Clin. Exp. Pathol. 2015, 8, 3691–3700. [Google Scholar] [PubMed]
- Wang, R.; Ke, Z.F.; Wang, F.; Zhang, W.H.; Wang, Y.F.; Li, S.H.; Wang, L.T. GOLPH3 overexpression is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through upregulating MMP-2 and MMP-9. Cell. Physiol. Biochem. 2015, 35, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.T.; Qiu, C.Z.; Huang, Z.X.; Yu, W.S.; Yang, X.F.; Wang, M.Z. Correlational research of Golgi phosphorylation protein 3 expression in colorectal cancer. World J. Gastroenterol. 2015, 21, 13473–13479. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Tian, Y.; Yue, W.M.; Li, L.; Li, S.H.; Qi, L.; Hu, W.S.; Gao, C.; Si, L.B.; Tian, H. GOLPH3, a good prognostic indicator in early-stage NSCLC related to tumor angiogenesis. Asian Pac. J. Cancer Prev. 2014, 15, 5793–5798. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Zhang, D.; Cai, M.; Wang, C.; Wu, Z.; Ying, Z.; Wu, J.; Li, M.; Xie, D.; Li, J.; et al. Golgi phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma cell aggressiveness by activating the NF-κB pathway. J. Pathol. 2015, 235, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lin, H.; Zhao, X.; Liu, G.; Wang, X.; Xu, R.; Chen, K.; Li, J.; Song, L. Overexpression of GOLPH3 promotes proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor. Clin. Cancer Res. 2012, 18, 4059–4069. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.W.; Li, Z.J.; Tu, C. MiR-135 post-transcriptionally regulates FOXO1 expression and promotes cell proliferation in human malignant melanoma cells. Int. J. Clin. Exp. Pathol. 2015, 8, 6356–6366. [Google Scholar] [PubMed]
- Ueda, Y.; Richmond, A. NF-kappaB activation in melanoma. Pigment Cell Res. 2006, 19, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Nazimek, K.; Bryniarski, K. The biological activity of macrophages in health and disease. Postepy Hig. Med. Dosw. 2012, 66, 507–520. [Google Scholar] [CrossRef]
- Fairweather, D.; Cihakova, D. Alternatively activated macrophages in infection and autoimmunity. J. Autoimm. 2009, 33, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Labrousse, A.L.; Ntayi, C.; Hornebeck, W.; Bernard, P. Stromal reaction in cutaneous melanoma. Crit. Rev. Oncol. Hematol. 2004, 49, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, X.; Houghton, J. Tumor microenvironment: The role of the tumor stroma in cancer. J. Cell. Biochem. 2007, 101, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Kohn, E.C. The microenvironment of the tumour-host interface. Nature 2001, 411, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. 2010, 15, 166–179. [Google Scholar] [CrossRef]



| Clinical Parameters | GOLPH2 Immunoreactivity | |||||
|---|---|---|---|---|---|---|
| Melanoma Cells | Tumor-Associated Macrophages (TAMs) | |||||
| Low | High | p Value | Low | High | p Value | |
| Age in years (21–79) a | ||||||
| mean, 56 ± 15.4; median, 58 | 1.000 | 0.555 | ||||
| Gender b | ||||||
| Female | 44 | 15 | 0.220 | 26 | 33 | 0.122 |
| Male | 25 | 16 | 11 | 30 | ||
| Primary tumor location c | ||||||
| Head/neck | 10 | 4 | 0.911 | 9 | 5 | 0.145 |
| Extremities | 31 | 12 | 13 | 30 | ||
| Hand/foot | 2 | 1 | 1 | 2 | ||
| Trunk | 26 | 14 | 14 | 26 | ||
| Primary tumor (pT) a | ||||||
| pT1 | 30 | 4 | 0.002 | 5 | 29 | 0.005 |
| pT2 | 16 | 5 | 10 | 11 | ||
| pT3 | 10 | 13 | 9 | 14 | ||
| pT4 | 13 | 9 | 13 | 9 | ||
| Regional lymph nodes status (pN) b | ||||||
| No metastases (pN−) | 64 | 21 | 0.003 | 29 | 56 | 0.258 |
| Metastases present (pN+) | 5 | 10 | 8 | 7 | ||
| Distant metastases (pM) b | ||||||
| No metastases (pM−) | 68 | 27 | 0.053 | 32 | 63 | 0.012 |
| Metastases present (pM+) | 1 | 4 | 5 | 0 | ||
| Sentinel lymph node biopsy status (SNLB) b (57 patients) | ||||||
| No metastases (SNLB−) | 37 | 10 | 0.007 | 10 | 37 | 0.398 |
| Metastases present (SNLB+) | 3 | 7 | 4 | 6 | ||
| Recurrence b | ||||||
| No | 63 | 21 | 0.007 | 27 | 57 | 0.043 |
| Yes | 6 | 10 | 10 | 6 | ||
| Histopathological Parameters | GOLPH2 Immunoreactivity | |||||
|---|---|---|---|---|---|---|
| Melanoma Cells | Tumor-Associated Macrophages (TAMs) | |||||
| Low | High | p Value | Low | High | p Value | |
| Breslow thickness a | ||||||
| ≤1 mm | 30 | 4 | 0.004 | 5 | 29 | 0.004 |
| 1.01–2.00 mm | 15 | 5 | 10 | 10 | ||
| 2.01–4.00 mm | 11 | 13 | 9 | 15 | ||
| >4 mm | 13 | 9 | 13 | 9 | ||
| Clark level a | ||||||
| I | - | - | 0.004 | - | - | 0.015 |
| II | 17 | 1 | 3 | 15 | ||
| III | 35 | 13 | 16 | 32 | ||
| IV | 14 | 11 | 11 | 14 | ||
| V | 3 | 6 | 7 | 2 | ||
| Histologic type b | ||||||
| Superficial spreading melanoma (SSM) | 47 | 20 | 0.939 | 23 | 44 | 0.691 |
| Nodular malignant melanoma (NMM) | 20 | 10 | 13 | 17 | ||
| Acral-lentiginous melanoma (ALM) | 2 | 1 | 1 | 2 | ||
| Mitotic rate a | ||||||
| 0 | 37 | 8 | 0.019 | 12 | 33 | 0.138 |
| ≥1 | 32 | 23 | 25 | 30 | ||
| Ulceration c | ||||||
| No | 45 | 10 | 0.005 | 15 | 40 | 0.043 |
| Yes | 24 | 21 | 22 | 23 | ||
| TILs c | ||||||
| No | 10 | 8 | 0.105 | 11 | 7 | 0.038 |
| Non-brisk | 19 | 12 | 12 | 19 | ||
| Brisk | 40 | 11 | 14 | 37 | ||
| Microsatellitosis c | ||||||
| No | 65 | 30 | 0.960 | 34 | 61 | 0.537 |
| Yes | 4 | 1 | 3 | 2 | ||
| Lymphatic invasion c | ||||||
| No | 58 | 15 | <0.001 | 19 | 54 | <0.001 |
| Yes | 11 | 16 | 18 | 9 | ||
| Tumor regression c | ||||||
| No | 64 | 28 | 0.987 | 35 | 57 | 0.725 |
| Yes | 5 | 3 | 2 | 6 | ||
| Clinical Parameters | GOLPH3 Immunoreactivity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Melanoma Cells | Tumor-Associated Macrophages (TAMs) | Cancer-Associated Fibroblasts (CAFs) | |||||||
| Low | High | p Value | Low | High | p Value | Low | High | p Value | |
| Age in years (21–79) a | |||||||||
| mean, 56 ± 15.4; median, 58 | 1.000 | 0.910 | 0.984 | ||||||
| Gender b | |||||||||
| Female | 31 | 28 | 0.518 | 18 | 41 | 0.868 | 16 | 43 | 0.216 |
| Male | 18 | 23 | 14 | 27 | 6 | 35 | |||
| Primary tumor location c | |||||||||
| Head/neck | 8 | 6 | 0.338 | 9 | 5 | 0.017 | 5 | 9 | 0.160 |
| Extremities | 22 | 21 | 10 | 33 | 5 | 38 | |||
| Hand/foot | 0 | 3 | 2 | 1 | 1 | 2 | |||
| Trunk | 19 | 21 | 11 | 29 | 11 | 29 | |||
| Primary tumor (pT) a | |||||||||
| pT1 | 25 | 9 | 0.001 | 1 | 33 | <0.001 | 1 | 33 | 0.005 |
| pT2 | 11 | 10 | 10 | 11 | 5 | 16 | |||
| pT3 | 8 | 15 | 11 | 12 | 7 | 16 | |||
| pT4 | 5 | 17 | 10 | 12 | 9 | 13 | |||
| Regional lymph nodes status (pN) b | |||||||||
| No metastases (pN−) | 45 | 40 | 0.110 | 25 | 60 | 0.307 | 15 | 70 | 0.031 |
| Metastases present (pN+) | 4 | 11 | 7 | 8 | 7 | 8 | |||
| Distant metastases (pM) b | |||||||||
| No metastases (pM−) | 48 | 47 | 0.383 | 28 | 67 | 0.062 | 18 | 77 | 0.008 |
| Metastases present (pM+) | 1 | 4 | 4 | 1 | 4 | 1 | |||
| Sentinel lymph node biopsy status (SNLB) b (57 patients) | |||||||||
| No metastases (SNLB−) | 27 | 20 | 0.018 | 10 | 37 | 0.398 | 4 | 43 | 0.177 |
| Metastases present (SNLB+) | 1 | 9 | 4 | 6 | 3 | 7 | |||
| Recurrence b | |||||||||
| No | 47 | 37 | 0.004 | 22 | 62 | 0.010 | 19 | 65 | 0.989 |
| Yes | 2 | 14 | 10 | 6 | 3 | 13 | |||
| Histopathological Parameters | GOLPH3 Immunoreactivity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Melanoma Cells | Tumor-Associated Macrophages (TAMs) | Cancer-Associated Fibroblasts (CAFs) | |||||||
| Low | High | p Value | Low | High | p Value | Low | High | p Value | |
| Breslow thickness a | |||||||||
| ≤1 mm | 25 | 9 | <0.001 | 1 | 33 | <0.001 | 1 | 33 | 0.006 |
| 1.01−2.00 mm | 11 | 9 | 9 | 11 | 5 | 15 | |||
| 2.01−4.00 mm | 8 | 16 | 12 | 12 | 7 | 17 | |||
| >4 mm | 5 | 17 | 10 | 12 | 9 | 13 | |||
| Clark level a | |||||||||
| I | - | - | 0.001 | - | - | 0.021 | - | - | 0.012 |
| II | 16 | 2 | 1 | 17 | 1 | 17 | |||
| III | 21 | 27 | 15 | 33 | 8 | 40 | |||
| IV | 10 | 15 | 11 | 14 | 8 | 17 | |||
| V | 2 | 7 | 5 | 4 | 5 | 4 | |||
| Histologic type b | 0.042 | 0.036 | |||||||
| Superficial spreading melanoma (SSM) | 38 | 29 | 16 | 51 | 12 | 55 | 0.369 | ||
| Nodular malignant melanoma (NMM) | 11 | 19 | 14 | 16 | 9 | 21 | |||
| Acral-lentiginous melanoma (ALM) | 0 | 3 | 2 | 1 | 1 | 2 | |||
| Mitotic rate a | |||||||||
| 0 | 31 | 14 | 0.002 | 9 | 36 | 0.037 | 4 | 41 | 0.010 |
| ≥1 | 18 | 37 | 23 | 32 | 18 | 37 | |||
| Ulceration c | |||||||||
| No | 35 | 20 | 0.002 | 10 | 45 | 0.002 | 6 | 49 | 0.007 |
| Yes | 14 | 31 | 22 | 23 | 16 | 29 | |||
| TILs c | |||||||||
| No | 4 | 14 | 0.017 | 12 | 6 | <0.001 | 7 | 11 | 0.071 |
| Non-brisk | 14 | 17 | 11 | 20 | 8 | 23 | |||
| Brisk | 31 | 20 | 9 | 42 | 7 | 44 | |||
| Microsatellitosis c | |||||||||
| No | 47 | 48 | 1.000 | 29 | 66 | 0.376 | 20 | 75 | 0.658 |
| Yes | 2 | 3 | 3 | 2 | 2 | 3 | |||
| Lymphatic invasion c | |||||||||
| No | 44 | 29 | <0.001 | 18 | 55 | 0.019 | 10 | 63 | 0.003 |
| Yes | 5 | 22 | 14 | 13 | 12 | 15 | |||
| Tumor regression c | |||||||||
| No | 46 | 46 | 0.757 | 28 | 64 | 0.458 | 20 | 72 | 1.000 |
| Yes | 3 | 5 | 4 | 4 | 2 | 6 | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donizy, P.; Kaczorowski, M.; Biecek, P.; Halon, A.; Szkudlarek, T.; Matkowski, R. Golgi-Related Proteins GOLPH2 (GP73/GOLM1) and GOLPH3 (GOPP1/MIDAS) in Cutaneous Melanoma: Patterns of Expression and Prognostic Significance. Int. J. Mol. Sci. 2016, 17, 1619. https://doi.org/10.3390/ijms17101619
Donizy P, Kaczorowski M, Biecek P, Halon A, Szkudlarek T, Matkowski R. Golgi-Related Proteins GOLPH2 (GP73/GOLM1) and GOLPH3 (GOPP1/MIDAS) in Cutaneous Melanoma: Patterns of Expression and Prognostic Significance. International Journal of Molecular Sciences. 2016; 17(10):1619. https://doi.org/10.3390/ijms17101619
Chicago/Turabian StyleDonizy, Piotr, Maciej Kaczorowski, Przemyslaw Biecek, Agnieszka Halon, Teresa Szkudlarek, and Rafal Matkowski. 2016. "Golgi-Related Proteins GOLPH2 (GP73/GOLM1) and GOLPH3 (GOPP1/MIDAS) in Cutaneous Melanoma: Patterns of Expression and Prognostic Significance" International Journal of Molecular Sciences 17, no. 10: 1619. https://doi.org/10.3390/ijms17101619