Genome-Wide Identification, Characterization and Expression Analysis of the Solute Carrier 6 Gene Family in Silkworm (Bombyx mori)
Abstract
:1. Introduction
2. Results
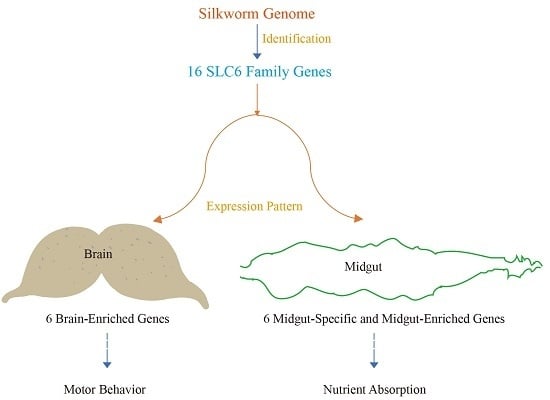
2.1. Identification of the Solute Carrier 6 (SLC6) Transporter Homologs in the Silkworm Genome
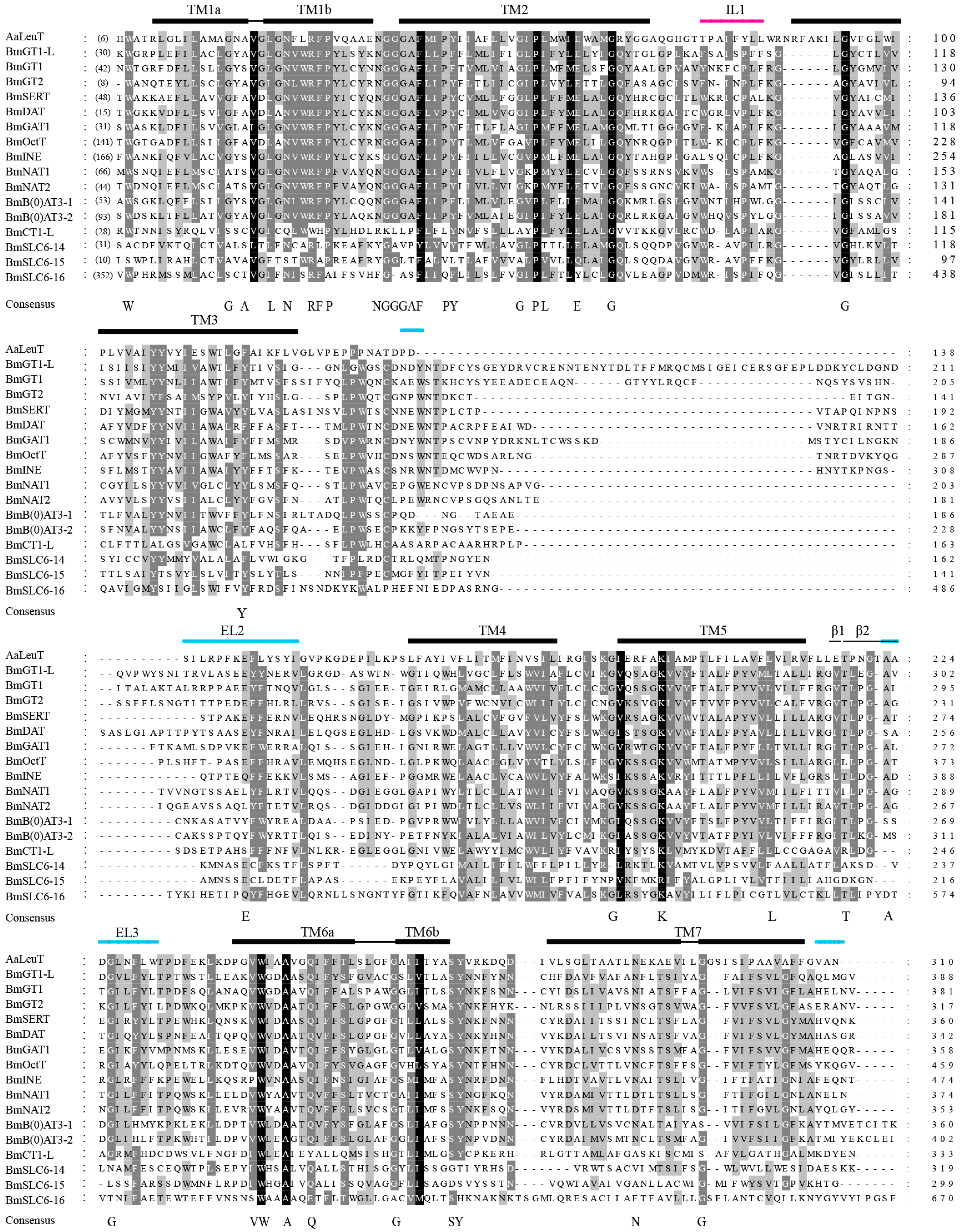
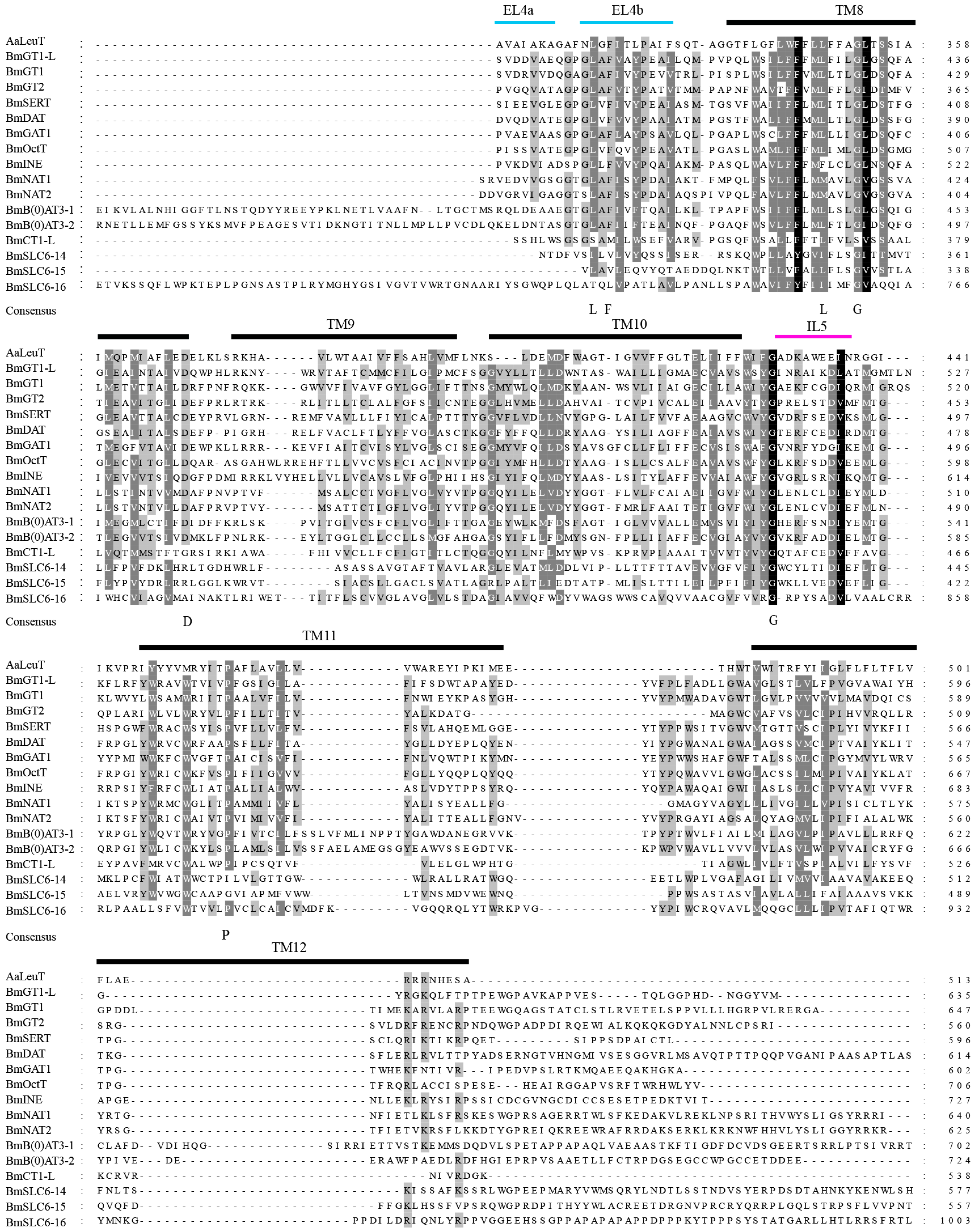
2.2. Multiple Sequence Alignment of the B. mori SLC6 Transporters
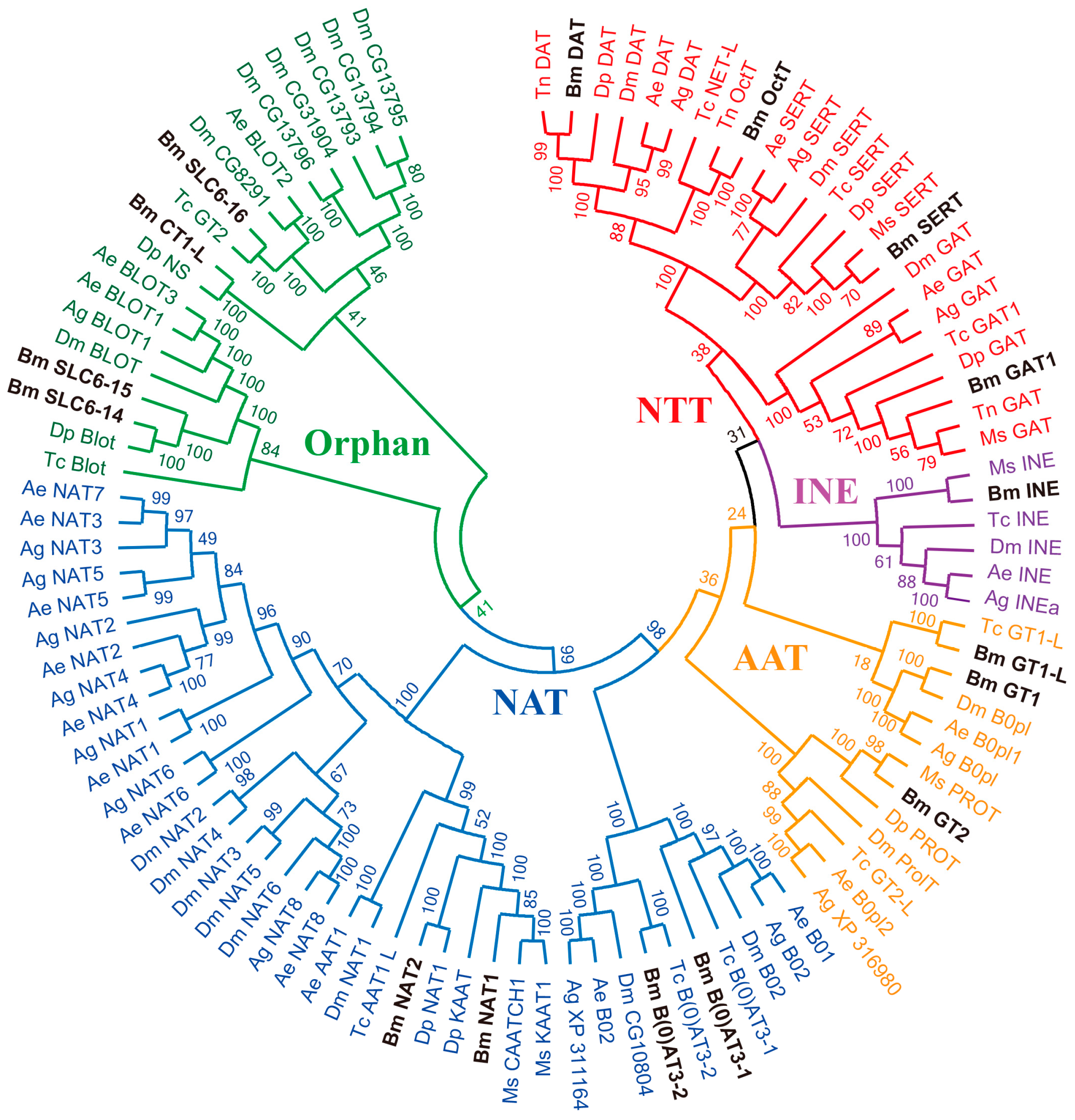
2.3. Phylogenetic Analysis of the Insect SLC6 Family Members
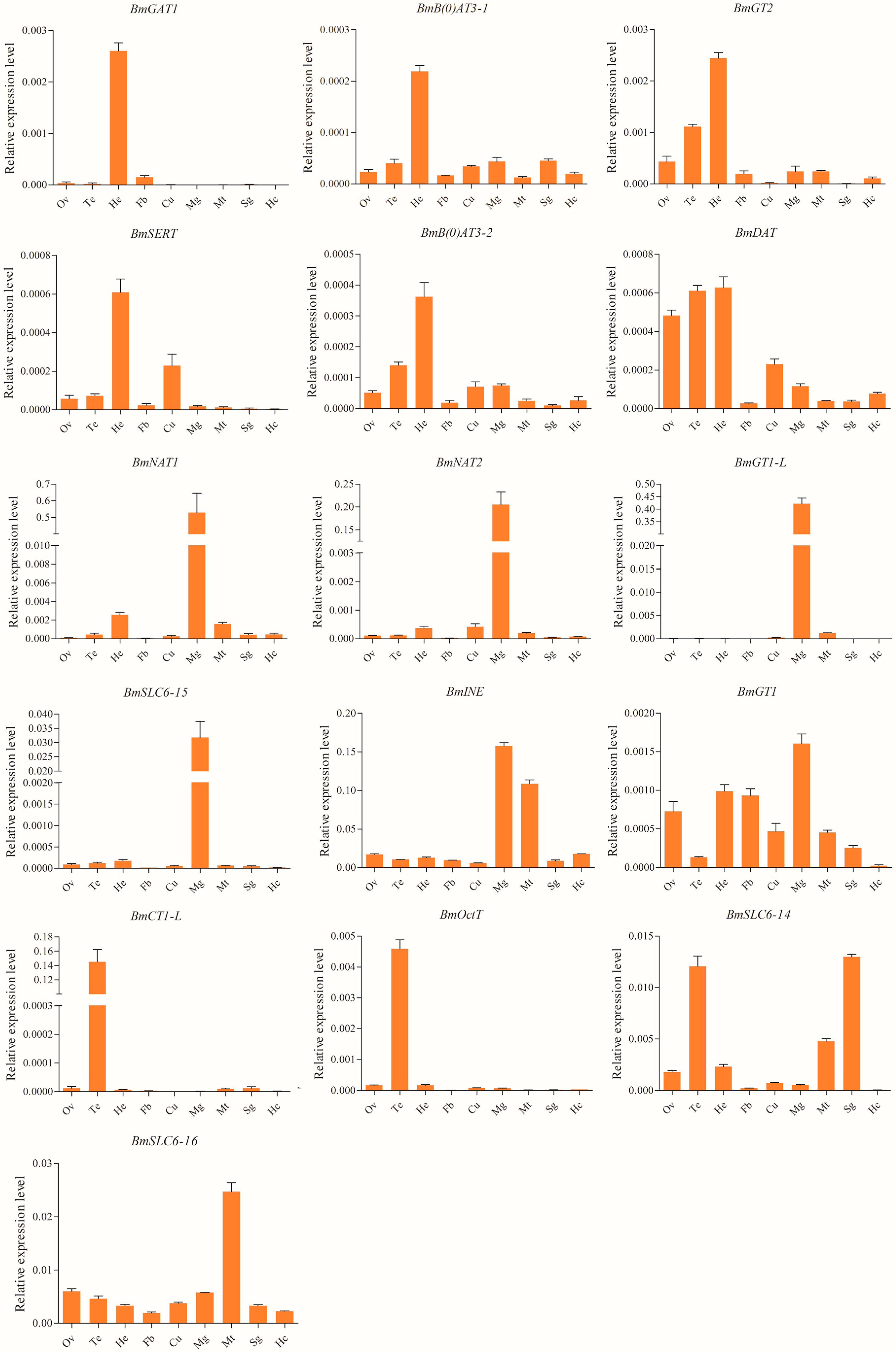
2.4. Spatial and Temporal Expression Profiles of the Silkworm SLC6 Genes
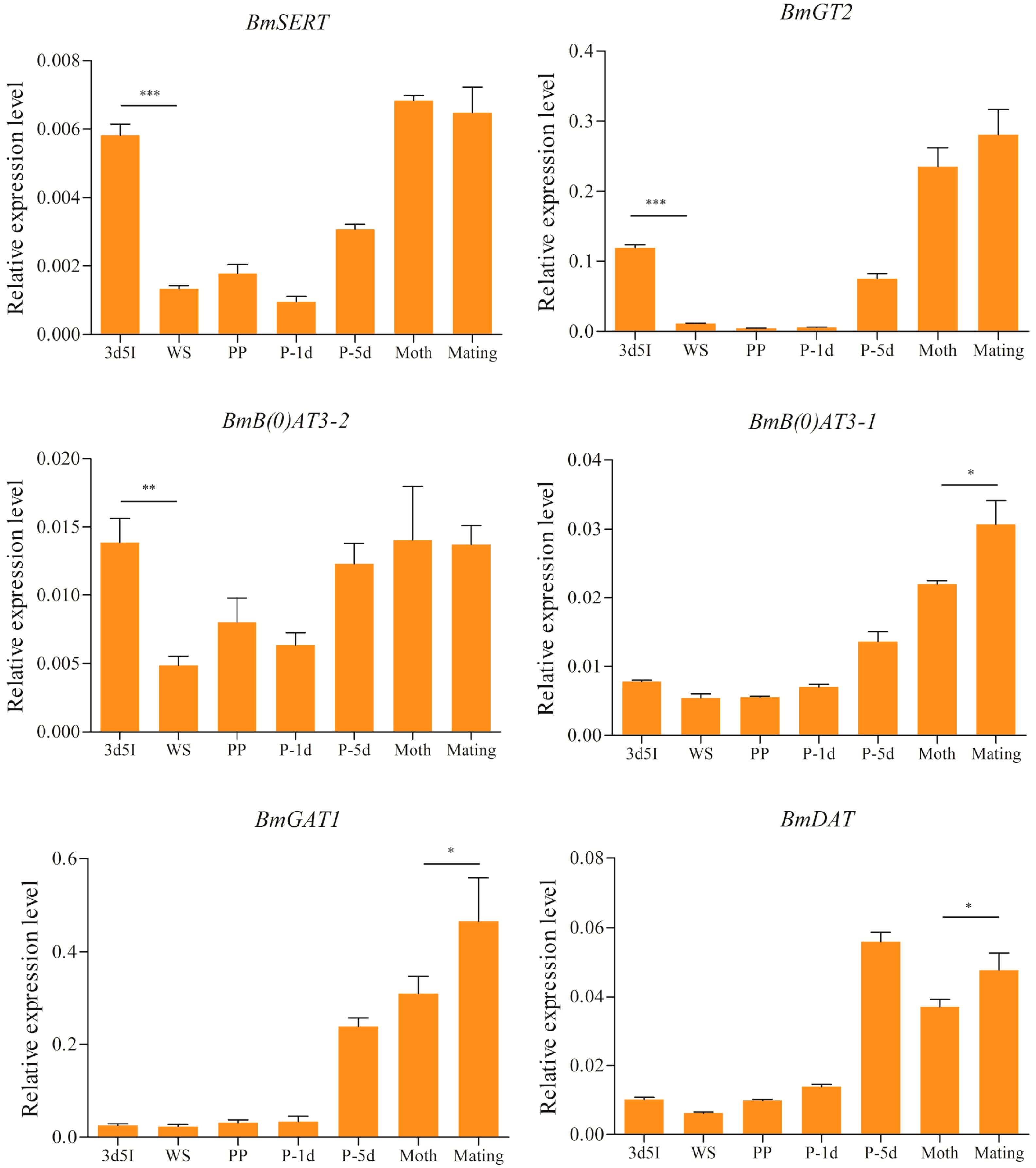
2.5. Expression Levels of Brain-Enriched Genes in Wandering and Mating Behaviors
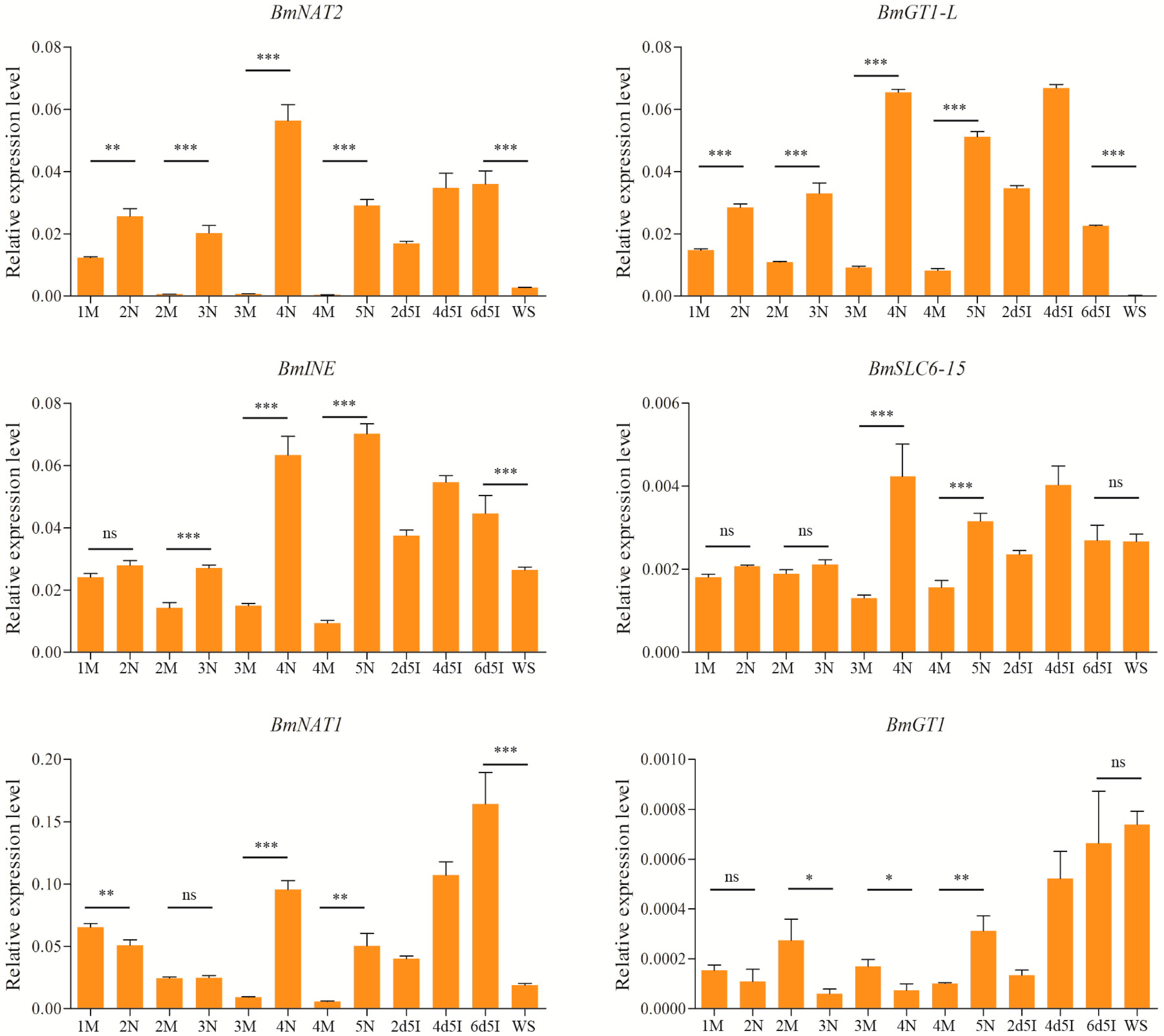
2.6. The Influences of Starvation on the Expression of the Silkworm SLC6 Genes
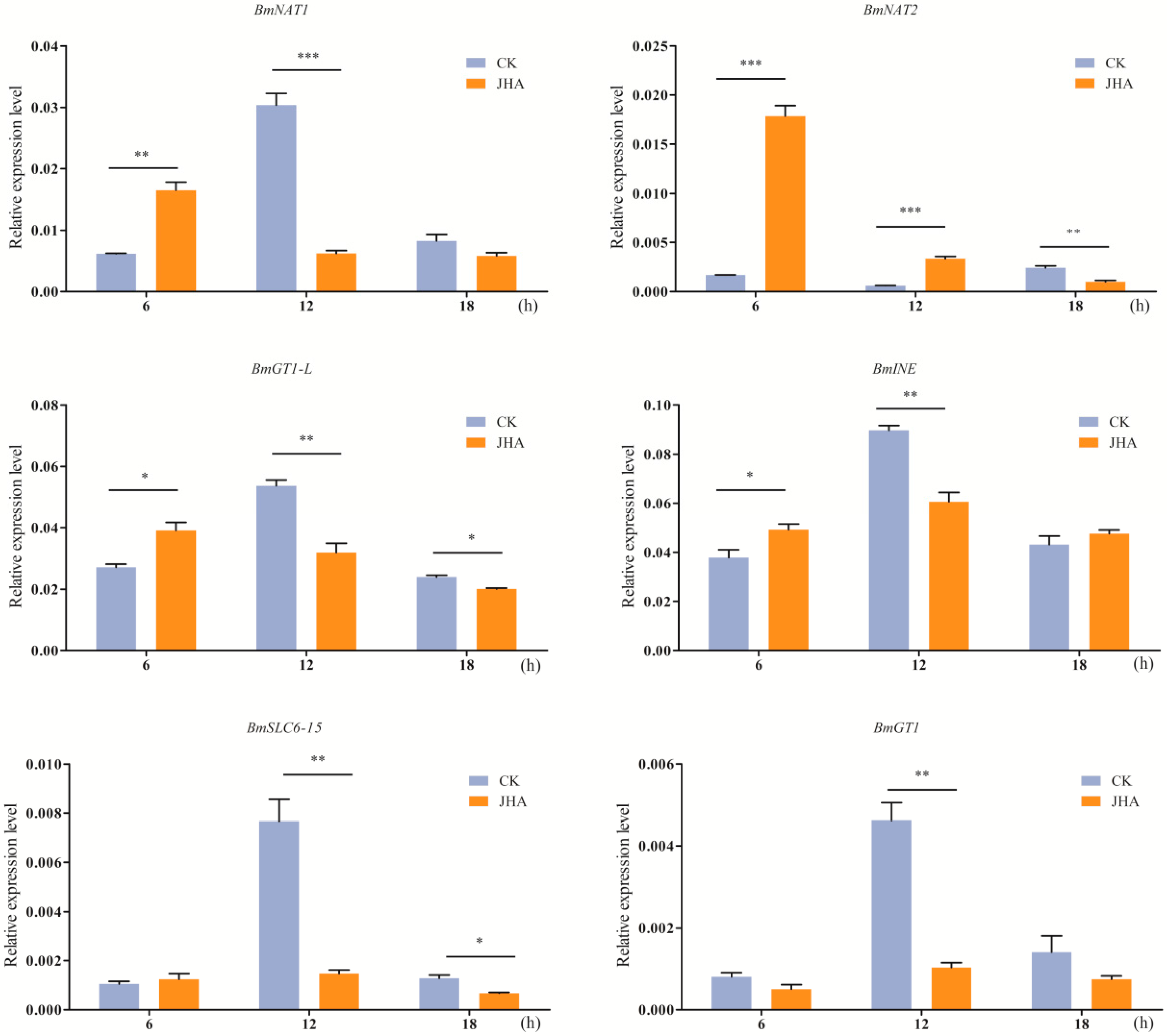
2.7. Induced Expression of SLC6 Genes by Juvenile Hormone Analog (JHA)
3. Discussion
4. Materials and Methods
4.1. Insect Rearing and Sample Collection
4.2. Identification and Multiple Sequence Alignment of the Silkworm SLC6 Family Transporters
4.3. Phylogenetic Tree Construction
4.4. Quantitative Real-Time PCR (qPCR) Analysis
4.5. Starvation Experiment and JHA (Juvenile Hormone Analog) Treatment
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nelson, N. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 1998, 71, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.G.; Arriza, J.L. Neurotransmitter transporters: Three distinct gene families. Curr. Opin. Neurobiol. 1993, 3, 337–344. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Rudnick, G. The cytoplasmic substrate permeation pathway of serotonin transporter. J. Biol. Chem. 2006, 281, 36213–36220. [Google Scholar] [CrossRef] [PubMed]
- Castagna, M.; Shayakul, C.; Trotti, D.; Sacchi, V.F.; Harvey, W.R.; Hediger, M.A. Cloning and characterization of a potassium-coupled amino acid transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 5395–5400. [Google Scholar] [CrossRef] [PubMed]
- Meleshkevitch, E.A.; Assis-Nascimento, P.; Popova, L.B.; Miller, M.M.; Kohn, A.B.; Phung, E.N.; Mandal, A.; Harvey, W.R.; Boudko, D.Y. Molecular characterization of the first aromatic nutrient transporter from the sodium neurotransmitter symporter family. J. Exp. Biol. 2006, 209, 3183–3198. [Google Scholar] [CrossRef] [PubMed]
- Reizer, J.; Reizer, A.; Saier, M.H., Jr. A functional superfamily of sodium/solute symporters. Biochim. Biophys. Acta 1994, 1197, 133–166. [Google Scholar] [CrossRef]
- Yamashita, A.; Singh, S.K.; Kawate, T.; Jin, Y.; Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl−-Dependent neurotransmitter transporters. Nature 2005, 437, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem. Int. 2006, 48, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.S.; Andersen, J.; Jorgensen, T.N.; Sorensen, L.; Eriksen, J.; Loland, C.J.; Stromgaard, K.; Gether, U. SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef] [PubMed]
- Margheritis, E.; Terova, G.; Cinquetti, R.; Peres, A.; Bossi, E. Functional properties of a newly cloned fish ortholog of the neutral amino acid transporter B0AT1 (SLC6A19). Comp. Biochem. Physiol. A 2013, 166, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudko, D.Y. Molecular basis of essential amino acid transport from studies of insect nutrient amino acid transporters of the SLC6 family (NAT-SLC6). J. Insect Physiol. 2012, 58, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Broer, A.; Tietze, N.; Kowalczuk, S.; Chubb, S.; Munzinger, M.; Bak, L.K.; Broer, S. The orphan transporter v7–3 (slc6a15) is a Na+-dependent neutral amino acid transporter (B0AT2). Biochem. J. 2006, 393, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Assis, P.; Boudko, D.Y.; Meleshkevitch, E.A.; Phung, E. Molecular expression and electrochemical analysis of phenylalanine-tyrosine transporter from anopheles gambiae larvae. FASEB J. 2004, 18, A1269. [Google Scholar]
- Gainetdinov, R.R.; Caron, M.G. Monoamine transporters: From genes to behavior. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn-Schmied. Arch. Pharmacol. 2008, 377, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Thoeringer, C.K.; Ripke, S.; Unschuld, P.G.; Lucae, S.; Ising, M.; Bettecken, T.; Uhr, M.; Keck, M.E.; Mueller-Myhsok, B.; Holsboer, F.; et al. The GABA transporter 1 (SLC6A1): A novel candidate gene for anxiety disorders. J. Neural Transm. 2009, 116, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Gainetdinov, R.R.; Wetsel, W.C.; Jones, S.R.; Bohn, L.M.; Miller, G.W.; Wang, Y.M.; Caron, M.G. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat. Neurosci. 2000, 3, 465–471. [Google Scholar] [PubMed]
- Ansorge, M.S.; Zhou, M.M.; Lira, A.; Hen, R.; Gingrich, J.A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 2004, 306, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Murphy, D.L.; Crawley, J.N. Abnormal behavioral phenotypes of serotonin transporter knockout mice: Parallels with human anxiety and depression. Biol. Psychiatry 2003, 54, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Jennings, K.A.; Loder, M.K.; Sheward, W.J.; Pei, Q.; Deacon, R.M.J.; Benson, M.A.; Olverman, H.J.; Hastie, N.D.; Harmar, A.J.; Shen, S.B.; et al. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J. Neurosci. 2006, 26, 8955–8964. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Thimgan, M.S.; Berg, J.S.; Stuart, A.E. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult drosophila melanogaster. J. Exp. Biol. 2006, 209, 3383–3404. [Google Scholar] [CrossRef] [PubMed]
- Meleshkevitch, E.A.; Robinson, M.; Popova, L.B.; Miller, M.M.; Harvey, W.R.; Boudko, D.Y. Cloning and functional expression of the first eukaryotic Na+-tryptophan symporter, agnat6. J. Exp. Biol. 2009, 212, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Castagna, M.; Bossi, E.; Sacchi, V.F. Molecular physiology of the insect K-activated amino acid transporter 1 (KAAT1) and cation-anion activated amino acid transporter/channel 1 (CAATCH1) in the light of the structure of the homologous protein LeuT. Insect Mol. Biol. 2009, 18, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.M.; Popova, L.B.; Meleshkevitch, E.A.; Tran, P.V.; Boudko, D.Y. The invertebrate B0 system transporter, d-melanogaster NAT1, has unique d-amino acid affinity and mediates gut and brain functions. Insect Biochem. Mol. Biol. 2008, 38, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, X.; Wei, H.; Han, D.D.; Gu, H.H. Molecular cloning and functional characterization of the dopamine transporter from Eloria noyesi, a caterpillar pest of cocaine-rich coca plants. Gene 2006, 366, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.H.; Wu, X.; Han, D.D. Conserved serine residues in serotonin transporter contribute to high-affinity cocaine binding. Biochem. Biophys. Res. Commun. 2006, 343, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Nene, V.; Wortman, J.R.; Lawson, D.; Haas, B.; Kodira, C.; Tu, Z.J.; Loftus, B.; Xi, Z.; Megy, K.; Grabherr, M.; et al. Genome sequence of aedes aegypti, a major arbovirus vector. Science 2007, 316, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.A.; Subramanian, G.M.; Halpern, A.; Sutton, G.G.; Charlab, R.; Nusskern, D.R.; Wincker, P.; Clark, A.G.; Ribeiro, J.M.; Wides, R.; et al. The genome sequence of the malaria mosquito anopheles gambiae. Science 2002, 298, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Cheng, D.; Duan, J.; Wang, G.; Cheng, T.; Zha, X.; Liu, C.; Zhao, P.; Dai, F.; Zhang, Z.; et al. Microarray-based gene expression profiles in multiple tissues of the domesticated silkworm, bombyx mori. Genome Biol. 2007, 8, R162. [Google Scholar] [CrossRef] [PubMed]
- Pramod, A.B.; Foster, J.; Carvelli, L.; Henry, L.K. SLC6 transporters: Structure, function, regulation, disease association and therapeutics. Mol. Asp. Med. 2013, 34, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Furuta, K.; Ichikawa, A.; Murata, M.; Kuwano, E.; Shinoda, T.; Shiotsuki, T. Determination by LC–MS of juvenile hormone titers in hemolymph of the silkworm, bombyx mori. Biosci. Biotechnol. Biochem. 2013, 77, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Melikian, H.E. Neurotransmitter transporter trafficking: Endocytosis, recycling, and regulation. Pharmacol. Ther. 2004, 104, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zomot, E.; Kanner, B.I. The interaction of the γ-aminobutyric acid transporter GAT-1 with the neurotransmitter is selectively impaired by sulfhydryl modification of a conformationally sensitive cysteine residue engineered into extracellular loop IV. J. Biol. Chem. 2003, 278, 42950–42958. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sadamoto, H.; Aonuma, H. Molecular basis of the dopaminergic system in the cricket gryllus bimaculatus. Invertebr. Neurosci. 2013, 13, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Oland, L.A.; Gibson, N.J.; Tolbert, L.P. Localization of a GABA transporter to glial cells in the developing and adult olfactory pathway of the moth Manduca sexta. J. Comp. Neurol. 2010, 518, 815–838. [Google Scholar] [CrossRef] [PubMed]
- El Hassani, A.K.; Dupuis, J.P.; Gauthier, M.; Armengaud, C. Glutamatergic and GABAergic effects of fipronil on olfactory learning and memory in the honeybee. Invertebr. Neurosci. 2009, 9, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Hou, Y.; Zhou, X.; Chen, Q.; Guo, C.; Xia, Q.; Zhang, Y.; Zhao, P. Integrative proteomics and metabolomics analysis of insect larva brain: Novel insights into the molecular mechanism of insect wandering behavior. J. Proteome Res. 2016, 15, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, Y.; Chinnappan, R.; Bocchini, C.; Gustin, M.C.; Stern, M. The drosophila inebriated-encoded neurotransmitter/osmolyte transporter: Dual roles in the control of neuronal excitability and the osmotic stress response. Genetics 2002, 160, 561–569. [Google Scholar] [PubMed]
- Huang, Y.; Stern, M. In vivo properties of the drosophila inebriated-encoded neurotransmitter transporter. J. Neurosci. 2002, 22, 1698–1708. [Google Scholar] [PubMed]
- Luan, Z.; Quigley, C.; Li, H.S. The putative Na+/Cl−-dependent neurotransmitter/osmolyte transporter inebriated in the drosophila hindgut is essential for the maintenance of systemic water homeostasis. Sci. Rep. 2015, 5, 7993. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, R.I.; Sebastiao, A.M.; Valente, C.A. Glyt1 and Glyt2 in brain astrocytes: Expression, distribution and function. Brain Struct. Funct. 2014, 219, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Takanaga, H.; Mackenzie, B.; Suzuki, Y.; Hediger, M.A. Identification of mammalian proline transporter SIT1 (SLC6A20) with characteristics of classical system imino. J. Biol. Chem. 2005, 280, 8974–8984. [Google Scholar] [CrossRef] [PubMed]
- Boudko, D.Y.; Kohn, A.B.; Meleshkevitch, E.A.; Dasher, M.K.; Seron, T.J.; Stevens, B.R.; Harvey, W.R. Ancestry and progeny of nutrient amino acid transporters. Proc. Natl. Acad. Sci. USA 2005, 102, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Dominick, O.S.; Truman, J.W. The physiology of wandering behaviour in Manduca sexta. I. Temporal organization and the influence of the internal and external environments. J. Exp. Biol. 1984, 110, 35–51. [Google Scholar] [PubMed]
- Karube, F.; Kobayashi, M. Presence of eupyrene spermatozoa in vestibulum accelerates oviposition in the silkworm moth, bombyx mori. J. Insect Physiol. 1999, 45, 947–957. [Google Scholar] [CrossRef]
- Ge, L.Q.; Cheng, Y.; Wu, J.C.; Jahn, G.C. Proteomic analysis of insecticide triazophos-induced mating-responsive proteins of Nilaparvata lugens stal (Hemiptera: Delphacidae). J. Proteome Res. 2011, 10, 4597–4612. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.; Heisenberg, M. A higher control center of locomotor behavior in the drosophila brain. J. Neurosci. 1993, 13, 1852–1861. [Google Scholar] [PubMed]
- Eulenburg, V.; Armsen, W.; Betz, H.; Gomeza, J. Glycine transporters: Essential regulators of neurotransmission. Trends Biochem. Sci. 2005, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Chen, X.Y.; Zhang, C.D.; Tang, X.F.; Wang, L.; Liu, T.H.; Pan, M.H.; Lu, C. Effects of starvation and hormones on DNA synthesis in silk gland cells of the silkworm Bombyx mori. Insect Sci. 2015. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Ma, L.; Tian, L.; Wang, S.; Sheng, Z.; Jiang, R.J.; Bendena, W.G.; Li, S. Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the bombyx fat body. J. Insect Physiol. 2010, 56, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, E.B.; Dubrovskaya, V.A.; Berger, E.M. Juvenile hormone signaling during oogenesis in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2002, 32, 1555–1565. [Google Scholar] [CrossRef]
- Fu, K.Y.; Guo, W.C.; Ahmat, T.; Li, G.Q. Knockdown of a nutrient amino acid transporter gene LdNAT1 reduces free neutral amino acid contents and impairs Leptinotarsa decemlineata pupation. Sci. Rep. 2015, 5, 18124. [Google Scholar] [CrossRef] [PubMed]

| SilkDB ID | Gene Name | Scaffold | Chr | Length (aa) | Exon | GenBank Accession Number | SilkDB Probe |
|---|---|---|---|---|---|---|---|
| BGIBMGA004216 | BmB(0)AT3-1 | nscaf2780 | 20 | 704 | 14 | XP_004927289.1 | sw06236 |
| BGIBMGA004312 | BmOctT | nscaf2789 | 20 | 706 | 19 | XP_012545126.1 | sw19499 |
| BGIBMGA004570 | BmGAT1 | nscaf2800 | 27 | 602 | 12 | XP_004923491.1 | sw07510 |
| BGIBMGA006164 | BmGT2 | nscaf2847 | 4 | 560 | 12 | XP_004929221.1 | sw03144 |
| BGIBMGA006618-9 | BmDAT | nscaf2855 | 10 | 619 | UN | NP_001037362.1 | sw00946 + sw06578 |
| BGIBMGA006857 | BmINE | nscaf2859 | 10 | 727 | 9 | XP_004924838.1 | sw12282 |
| BGIBMGA007228 | BmNAT1 | nscaf2874 | UN | 640 | 14 | XP_004927040.1 | sw19553 |
| BGIBMGA007228 | BmNAT2 | nscaf2874 | UN | 625 | 14 | NP_001124343.1 | sw19553 |
| BGIBMGA008026-7 | BmGT1-L | nscaf2889 | 9 | 635 | 12 | XP_004922886.1 | sw19800 |
| BGIBMGA000927 | BmGT1 | nscaf1898 | 13 | 647 | 13 | XP_012547426.1 | sw08765 |
| BGIBMGA010528 | BmSLC6-14 | nscaf2993 | 12 | 663 | 10 | XP_004929448.1 | sw14009 |
| BGIBMGA011081 | BmSLC6-16 | nscaf3015 | 23 | 1145 | 15 | XP_004921736.1 | sw18195 |
| BGIBMGA011849 | BmCT1-L | nscaf3031 | 11 | 538 | 13 | XP_012550400.1 | sw16320 |
| BGIBMGA011949-50 | BmB(0)AT3-2 | nscaf3032 | 11 | 724 | 5 | XP_004924205.1 | sw11049 + sw05356 |
| BGIBMGA012340 | BmSLC6-15 | nscaf3040 | 1 | 637 | 10 | XP_012551814.1 | sw10873 |
| BGIBMGA014231 | BmSERT | nscaf98 | 8 | 596 | 13 | NP_001037436.1 | sw05387 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Liu, H.; Chen, Q.; Wang, X.; Xiong, Y.; Zhao, P. Genome-Wide Identification, Characterization and Expression Analysis of the Solute Carrier 6 Gene Family in Silkworm (Bombyx mori). Int. J. Mol. Sci. 2016, 17, 1675. https://doi.org/10.3390/ijms17101675
Tang X, Liu H, Chen Q, Wang X, Xiong Y, Zhao P. Genome-Wide Identification, Characterization and Expression Analysis of the Solute Carrier 6 Gene Family in Silkworm (Bombyx mori). International Journal of Molecular Sciences. 2016; 17(10):1675. https://doi.org/10.3390/ijms17101675
Chicago/Turabian StyleTang, Xin, Huawei Liu, Quanmei Chen, Xin Wang, Ying Xiong, and Ping Zhao. 2016. "Genome-Wide Identification, Characterization and Expression Analysis of the Solute Carrier 6 Gene Family in Silkworm (Bombyx mori)" International Journal of Molecular Sciences 17, no. 10: 1675. https://doi.org/10.3390/ijms17101675