Age-Related Modulations of AQP4 and Caveolin-1 in the Hippocampus Predispose the Toxic Effect of Phoneutria nigriventer Spider Venom
Abstract
:1. Introduction
2. Results
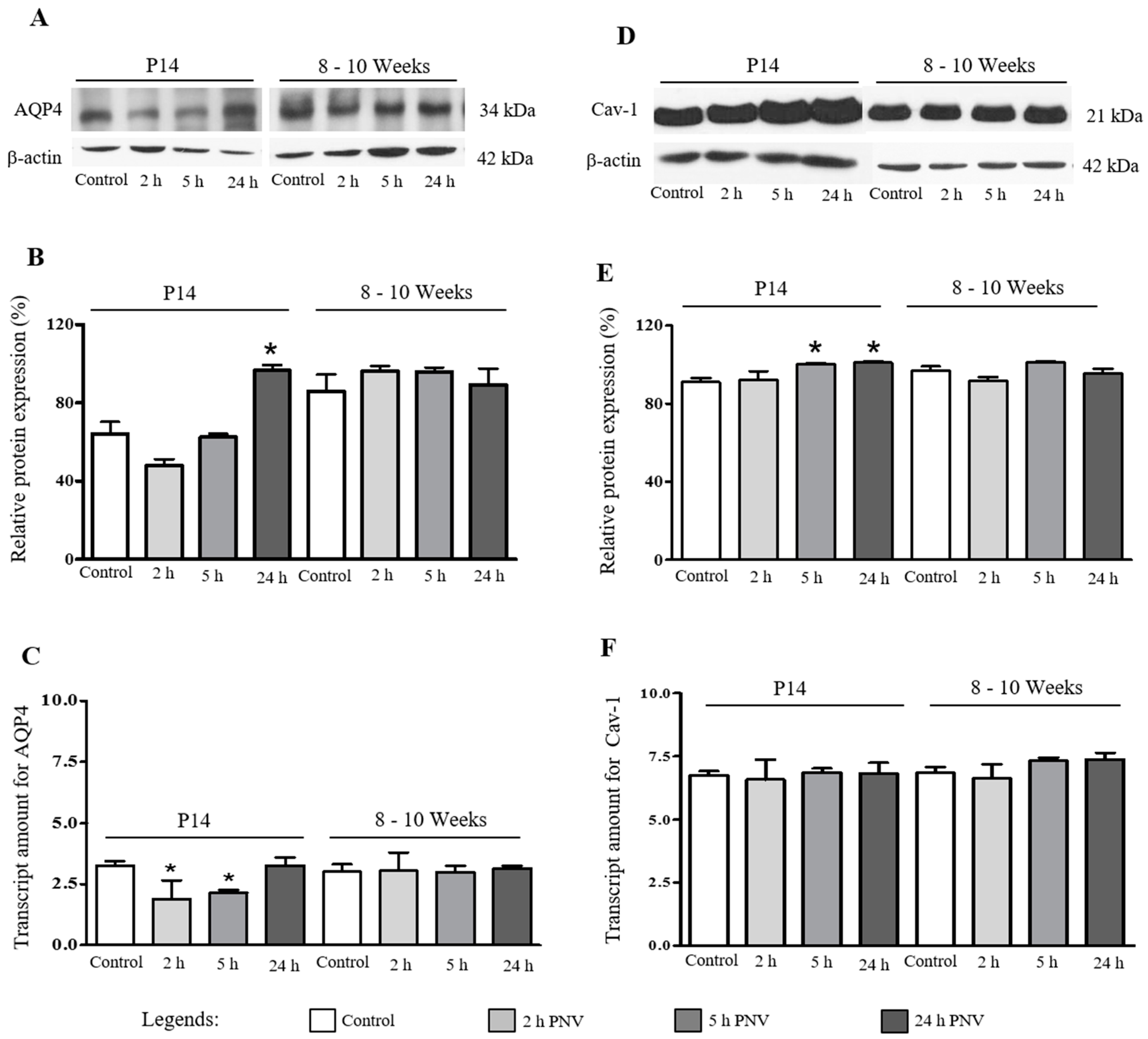
2.1. Western Blotting (WB) and Real Time-Polymerase Chain Reaction (qPCR)
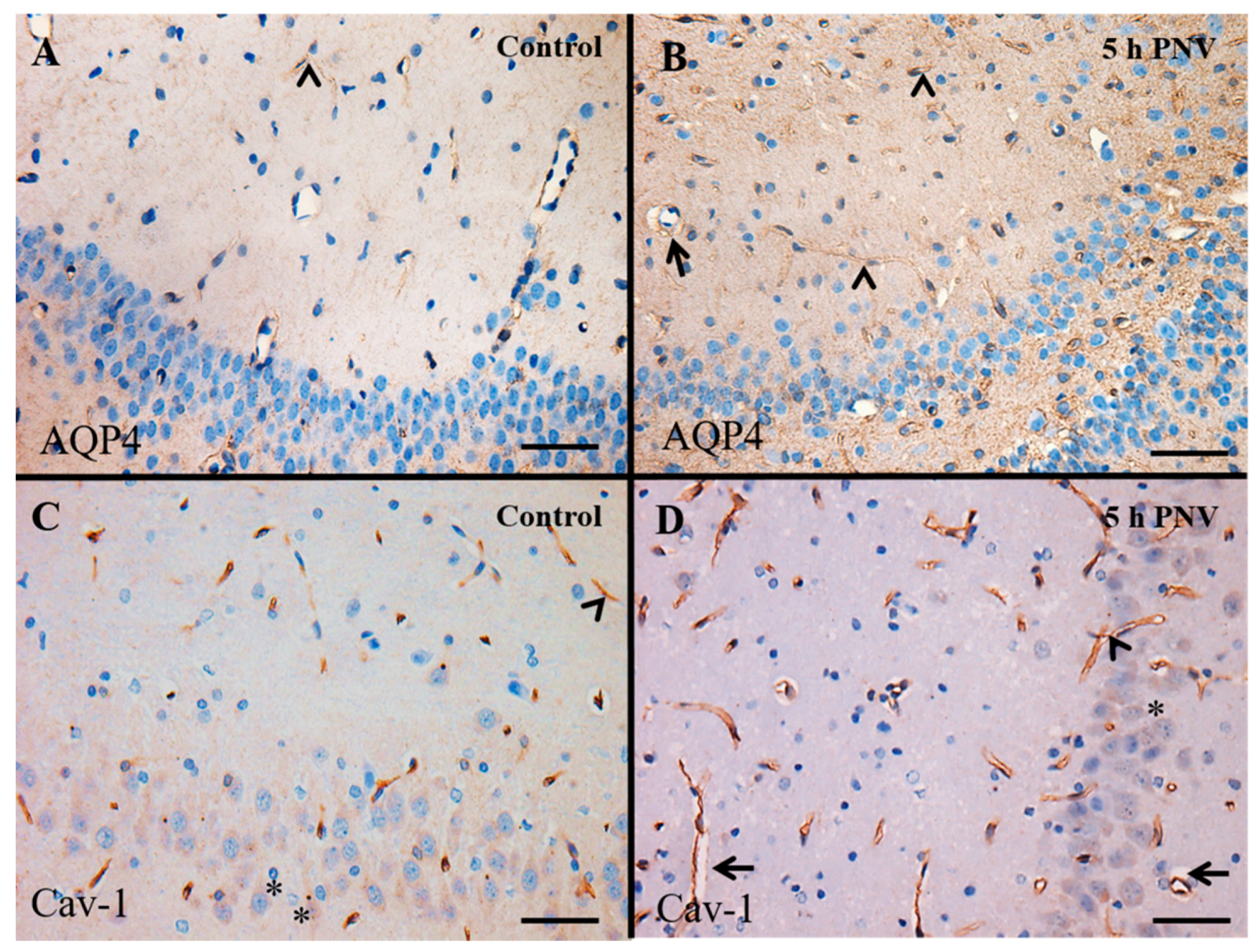
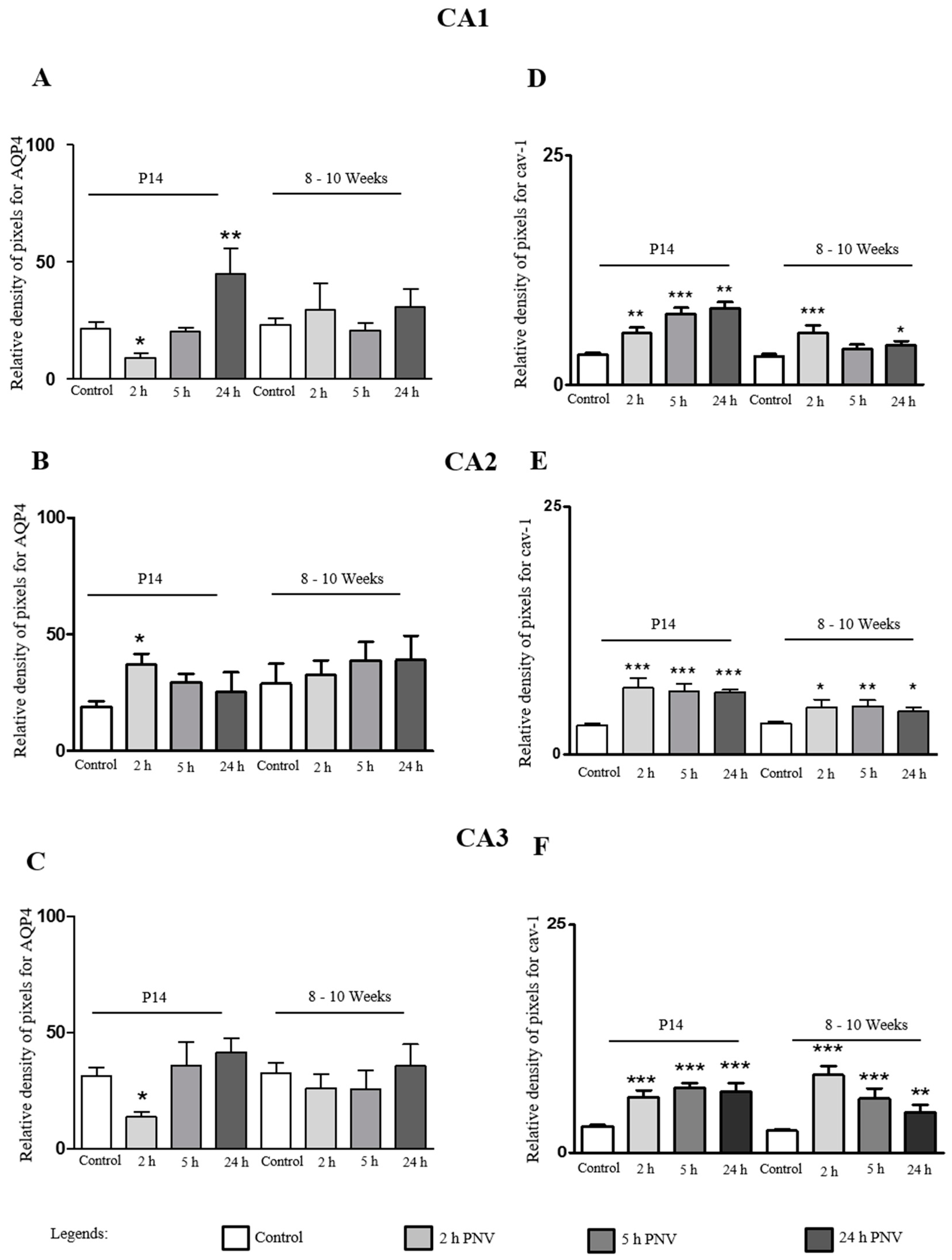
2.2. Immunohistochemistry (IHC)
2.3. AQP4
2.4. Cav-1
3. Discussion
3.1. AQP4
3.2. Cav-1
4. Materials and Methods
4.1. Venom and Animals
4.2. Envenoming Procedure
4.3. IHC
IHC Analysis
4.4. qPCR
4.5. Western Blotting (WB)
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bucaretchi, F.; Deus, R.C.R.; Hyslop, S.; Madureira, P.R.; de Capitani, E.M.; Vieira, R.J. A clinico-epidemiological study of bites by spiders of the genus Phoneutria. Rev. Inst. Med. Trop. São Paulo 2000, 42, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.R. Separation of proteins and characterization of active substances in the venom of Brazilian spiders. Anais Acad. Bras. Cienc. 1963, 35, 283–291. [Google Scholar]
- Schenberg, S.; Pereira Lima, F.A. Phoneutria nigriventer venom. In Venomous Animals and Their Venom; Bücherl, W., Buckley, E.E., Eds.; Academic Press: New York, NY, USA, 1971; Volume 3, pp. 279–285. [Google Scholar]
- Cruz-Höfling, M.A.; Love, S.; Brook, G.; Duchen, L.W. Effects of Phoneutria nigriventer spider venom on mouse peripheral nerve. Q. J. Exp. Physiol. 1985, 70, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.D.; Vital Brazil, O. Mode of action of Phoneutria nigriventer spider venom at the isolated phrenic nerve-diaphragm of the rat. Braz. J. Med. Biol. Res. 1985, 18, 557–565. [Google Scholar] [PubMed]
- De Lima, M.E.; Figueired, S.G.; Matavel, A.; Nunes, K.P.; Silva, C.N.; Almeida, F.M.; Diniz, M.R.V.; Cordeiro, M.N.; Stankiewicz, M.; Beirão, P.S.L. Phoneutria nigriventer Venom and Toxins: A Review. In Spider Venoms; Gopalakrishnakone, P., Corzo, G., de Lima, M.E., Diego-García, E., Eds.; Springer Netherlands (Toxinology): Dordrecht, The Netherlands, 2015; pp. 1–24. [Google Scholar]
- Cruz-Höfling, M.A.; Tavares, J.C.; Rapôso, C. Phoneutria nigriventer Venom. Action in the Central Nervous System. In Spider Venoms; Gopalakrishnakone, P., Corzo, G.A., de Lima, M.E., Diego-García, E., Eds.; Springer Netherlands (Toxinology): Dordrecht, The Netherlands, 2015; pp. 175–202. [Google Scholar]
- Le Sueur, L.; Kalapothakis, E.; Cruz-Höfling, M.A. Breakdown of the blood-brain barrier and neuropathological changes induced by Phoneutria nigriventer spider venom. Acta Neuropathol. 2003, 2, 125–134. [Google Scholar]
- Le Sueur, L.P.; Collares-Buzato, C.B.; Cruz-Höfling, M.A. Mechanisms involved in the blood-brain barrier increased permeability induced by Phoneutria nigriventer spider venom in rats. Brain Res. 2004, 1027, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rapôso, C.; Zago, G.M.; Silva, G.H.; Cruz-Höfling, M.A. Acute blood-brain barrier permeabilization in rats after systemic Phoneutria nigriventer venom. Brain Res. 2007, 1149, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.; Soares, E.S.; Stávale, L.M.; Irazusta, S.P.; Cruz-Höfling, M.A. Upregulation of the vascular endothelial growth factor, Flt-1, in rat hippocampal neurons after envenoming by Phoneutria nigriventer; age-related modulation. Toxicon 2012, 60, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Stávale, L.M.; Soares, E.S.; Mendonça, M.C.; Irazusta, S.P.; da Cruz Höfling, M.A. Temporal relationship between aquaporin-4 and glial fibrillary acidic protein in cerebellum of neonate and adult rats administered a BBB disrupting spider venom. Toxicon 2013, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.S.; Mendonça, M.C.; Irazusta, S.P.; Coope, A.; Stávale, L.M.; da Cruz-Höfling, M.A. Evidences of endocytosis via caveolae following blood-brain barrier breakdown by Phoneutria nigriventer spider venom. Toxicol. Lett. 2014, 229, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.S.; Mendonça, M.C.; da Cruz-Höfling, M.A. Caveolae as a target for Phoneutria nigriventer spider venom. Neurotoxicology 2016, 54, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Ashwal, S.; Obenaus, A. Aquaporins in cerebrovascular disease: A target for treatment of brain edema? Cerebrovasc. Dis. 2011, 31, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [PubMed]
- Nicchia, G.P.; Nico, B.; Camassa, L.M.; Mola, M.G.; Loh, N.; Dermietzel, R.; Spray, D.C.; Svelto, M.; Frigeri, A. The role of aquaporin-4 in the blood-brain barrier development and integrity: Studies in animal and cell culture models. Neuroscience 2004, 129, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Borgnia, M.; Nielsen, S.; Engel, A.; Agre, P. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 1999, 68, 425–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zador, Z.; Verkman, A.S. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J. Biol. Chem. 2008, 283, 15280–15286. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Manias, J.L.; Stewart, D.J. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009, 118, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.G.; Woodman, S.E.; Park, D.S.; Lisanti, M.P. Caveolin, caveolae, and endothelial cell function. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Lisanti, M.P. The Caveolin genes: From cell biology to medicine. Ann. Med. 2004, 36, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Luoma, J.I.; Boulware, M.I.; Mermelstein, P.G. Caveolin proteins and estrogen signaling in the brain. Mol. Cell. Endocrinol. 2008, 290, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.M.; Mermelstein, P.G. Caveolin regulation of neuronal intracellular signaling. Cell. Mol. Life Sci. 2010, 67, 3785–3795. [Google Scholar] [CrossRef] [PubMed]
- Ikezu, T.; Ueda, H.; Trapp, B.D.; Nishiyama, K.; Sha, J.F.; Volonte, D.; Galbiati, F.; Byrd, A.L.; Bassell, G.; Serizawa, H.; et al. Affinity-purification and characterization of caveolins from the brain: Differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 1998, 804, 177–192. [Google Scholar] [CrossRef]
- Nag, S.; Manias, J.L.; Stewart, D.J. Expression of endothelial phosphorylated caveolin-1 is increased in brain injury. Neuropathol. Appl. Neurobiol. 2009, 35, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Kapadia, A.; Stewart, D.J. Molecular pathogenesis of blood–brain barrier breakdown in acute brain injury. Neuropathol. Appl. Neurobiol. 2011, 37, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Ribatti, D.; Frigeri, A.; Nicchia, G.P.; Corsi, P.; Svelto, M.; Roncali, L. Aquaporin-4 expression during development of the cerebellum. Cerebellum 2002, 1, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Binder, D.K.; Bloch, O.; Auguste, K.; Papadopoulos, M.C. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim. Biophys. Acta 2006, 1758, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.M.; Badaut, J. Aquaporin4: A player in central edema and neuroinflammation. J. Neuroinflamm. 2012, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Nagelhus, E.A.; Ottersen, O.P. Aquaporin-4 and epilepsy. Glia 2012, 60, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Rapôso, C.; Odorissi, P.A.; Oliveira, A.L.; Aoyama, H.; Ferreira, C.V.; Verinaud, L.; Fontana, K.; Ruela-de-Sousa, R.R.; da Cruz-Höfling, M.A. Effect of Phoneutria nigriventer venom on the expression of junctional protein and P-gp efflux pump function in the Blood-Brain Barrier. Neurochem. Res. 2012, 37, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Höfling, M.A.; Zago, G.M.; Melo, L.L.; Rapôso, C. c-FOS and n-NOS reactive neurons in response to circulating Phoneutria nigriventer spider venom. Brain Res. Bull. 2007, 73, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Höfling, M.A.; Rapôso, C.; Verinaud, L.; Zago, G.M. Neuroinflammation and astrocytic reaction in the course of Phoneutria nigriventer (armed-spider) blood-brain barrier (BBB) opening. Neurotoxicology 2009, 30, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.S.; Mendonça, M.C.; da Cruz-Höfling, M.A. eNOS uncoupling in the cerebellum after BBB disruption by exposure to Phoneutria nigriventer spider venom. Toxicon 2015, 104, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Mathiisen, T.M.; Ottersen, O.P. Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with Kir4.1. Neuroscience 2004, 129, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Predescu, D.; Palade, G.E. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am. J. Physiol. 1993, 265, H725–H733. [Google Scholar] [PubMed]
- Rothberg, K.G.; Heuser, J.E.; Donzell, W.C.; Ying, Y.S.; Glenney, J.R.; Anderson, R.G. Caveolin, a protein component of caveolae membrane coats. Cell 1992, 68, 673–682. [Google Scholar] [CrossRef]
- Shajahan, A.N.; Timblin, B.K.; Sandoval, R.; Tiruppathi, C.; Malik, A.B.; Minshall, R.D. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 2004, 279, 20392–20400. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhang, Z.; Xue, Y.; Wang, P.; Liu, Y. Mechanisms of the increase in the permeability of the blood-tumor barrier obtained by combining low-frequency ultrasound irradiation with small-dose bradykinin. J. Neurooncol. 2009, 94, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.E.; Oh, P.; Pinney, E.; Allard, J. Fillipin-sensitive caveolae-mediated transport in endothelium: Reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 1994, 127, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, S.; Lee, T.; Lee, H.; Chen, S.; Shyue, S. Caveolin-1 deletion reduces early brain injury after experimental intracerebral hemorrhage. Am. J. Pathol. 2011, 178, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Steinhäuser, C. Ion channels in glial cells. Brain Res. Rev. 2000, 32, 380–412. [Google Scholar] [CrossRef]
- Wen, H.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Agre, P.; Ottersen, O.P.; Nielsen, S. Ontogeny of water transport in rat brain: Postnatal expression of the aquaporin-4 water channel. Eur. J. Neurosci. 1999, 11, 934–945. [Google Scholar] [CrossRef]
- Lassiale, S.; Valamanesh, F.; Klein, C.; Hicks, D.; Abitbol, M.; Versaux-Botteri, C. Changes in aquaporin-4 and Kir4.1 expression in rats inherited retinal dystrophy. Exp. Eye Res. 2016, 148, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.S.; Seldin, M.; Lee, D.J.; Seifert, G.; Steinhäuser, C.; Binder, D.K. Laminar-specific and developmental expression of aquaporin-4 in the mouse hippocampus. Neuroscience 2011, 178, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.L.; Park, H.; Yi, H.; Boo, Y.C.; Sorescu, G.P.; Sykes, M.; Jo, H. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1113–H1122. [Google Scholar] [CrossRef] [PubMed]
- Nassoy, P.; Lamaze, C. Stressing caveolae new role in cell mechanics. Trends Cell Biol. 2012, 22, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; del Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Bruckner, S.R.; Sengoku, T.; Geddes, J.W.; Estus, S. Glutamate regulates caveolin expression in rat hippocampal neurons. J. Neursci. 2003, 72, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zanchet, E.M.; Cury, Y. Peripheral tachykinin and excitatory amino acid receptors mediate hyperalgesia induced by Phoneutria nigriventer venom. Eur. J. Pharmacol. 2003, 467, 111–118. [Google Scholar] [CrossRef]
- Silva, F.R.; Batista, E.M.; Gomez, M.V.; Kushmerick, C.; da Silva, J.F.; Cordeiro, M.N.; Vieira, L.B.; Ribeiro, F.M. The Phoneutria nigriventer spider toxin, PnTx-4-5, promotes neuronal survival by blocking NMDA receptors. Toxicon 2016, 112, 16–21. [Google Scholar] [CrossRef] [PubMed]
- García-Cardeñas, G.; Martasek, P.; Masters, B.S.; Skidd, P.M.; Couet, J.; Li, S.; Lisanti, M.P.; Sessa, W.C. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J. Biol. Chem. 1997, 272, 25437–25440. [Google Scholar] [CrossRef]
- Santhanam, A.V.; d’Uscio, L.V.; Smith, L.A.; Katusic, Z.S. Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the central microvessels of HPH-1 mice. J. Neurochem. 2012, 122, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Ferriero, D.M. Neonatal brain injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.W. Free and open source software for manipulation of digital images. AJR Am. J. Roentgenol. 2009, 192, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Moro, O. MIP-1β, a novel biomarker for in vitro sensitization test using human monocytic cell line. Toxicol. In Vitro 2006, 20, 736–742. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, E.S.; Stávale, L.M.; Mendonça, M.C.P.; Coope, A.; Cruz-Höfling, M.A.d. Age-Related Modulations of AQP4 and Caveolin-1 in the Hippocampus Predispose the Toxic Effect of Phoneutria nigriventer Spider Venom. Int. J. Mol. Sci. 2016, 17, 1462. https://doi.org/10.3390/ijms17111462
Soares ES, Stávale LM, Mendonça MCP, Coope A, Cruz-Höfling MAd. Age-Related Modulations of AQP4 and Caveolin-1 in the Hippocampus Predispose the Toxic Effect of Phoneutria nigriventer Spider Venom. International Journal of Molecular Sciences. 2016; 17(11):1462. https://doi.org/10.3390/ijms17111462
Chicago/Turabian StyleSoares, Edilene S., Leila M. Stávale, Monique C. P. Mendonça, Andressa Coope, and Maria Alice da Cruz-Höfling. 2016. "Age-Related Modulations of AQP4 and Caveolin-1 in the Hippocampus Predispose the Toxic Effect of Phoneutria nigriventer Spider Venom" International Journal of Molecular Sciences 17, no. 11: 1462. https://doi.org/10.3390/ijms17111462





