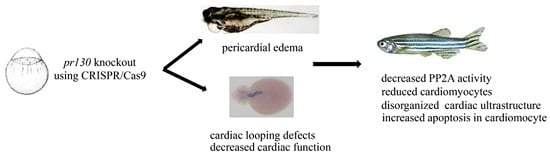
Deletion of Pr130 Interrupts Cardiac Development in Zebrafish
Abstract
:1. Introduction
2. Results
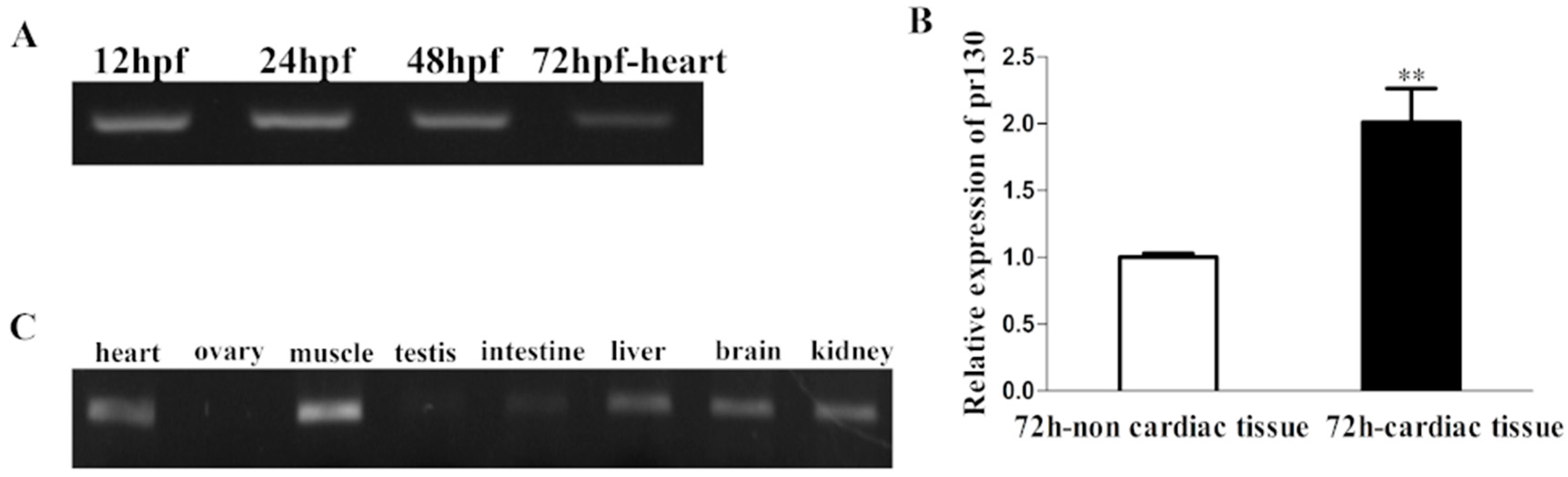
2.1. Expression Patterns of Pr130 in Zebrafish
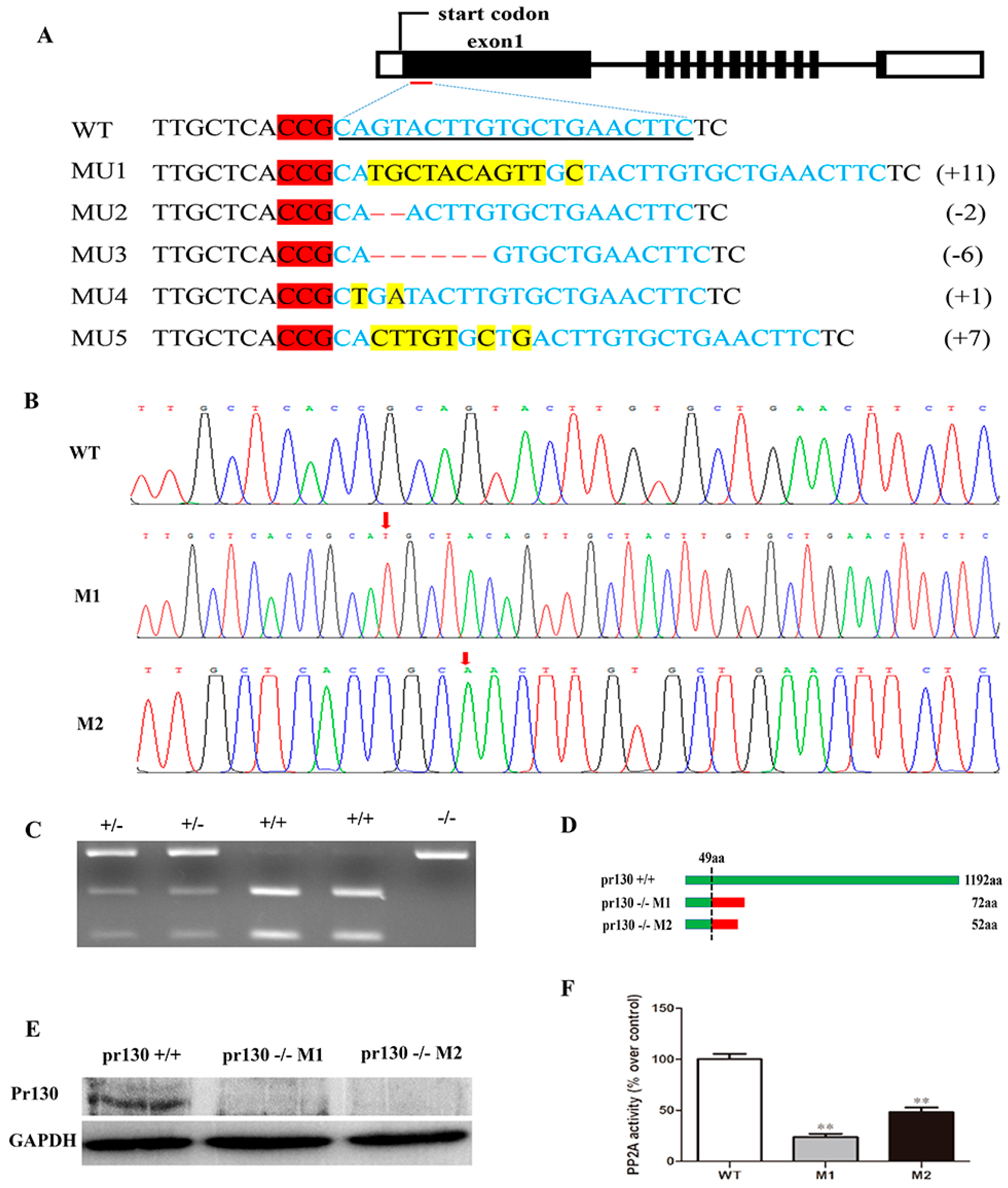
2.2. Deletion of Pr130 Results in Lower PP2A Activity
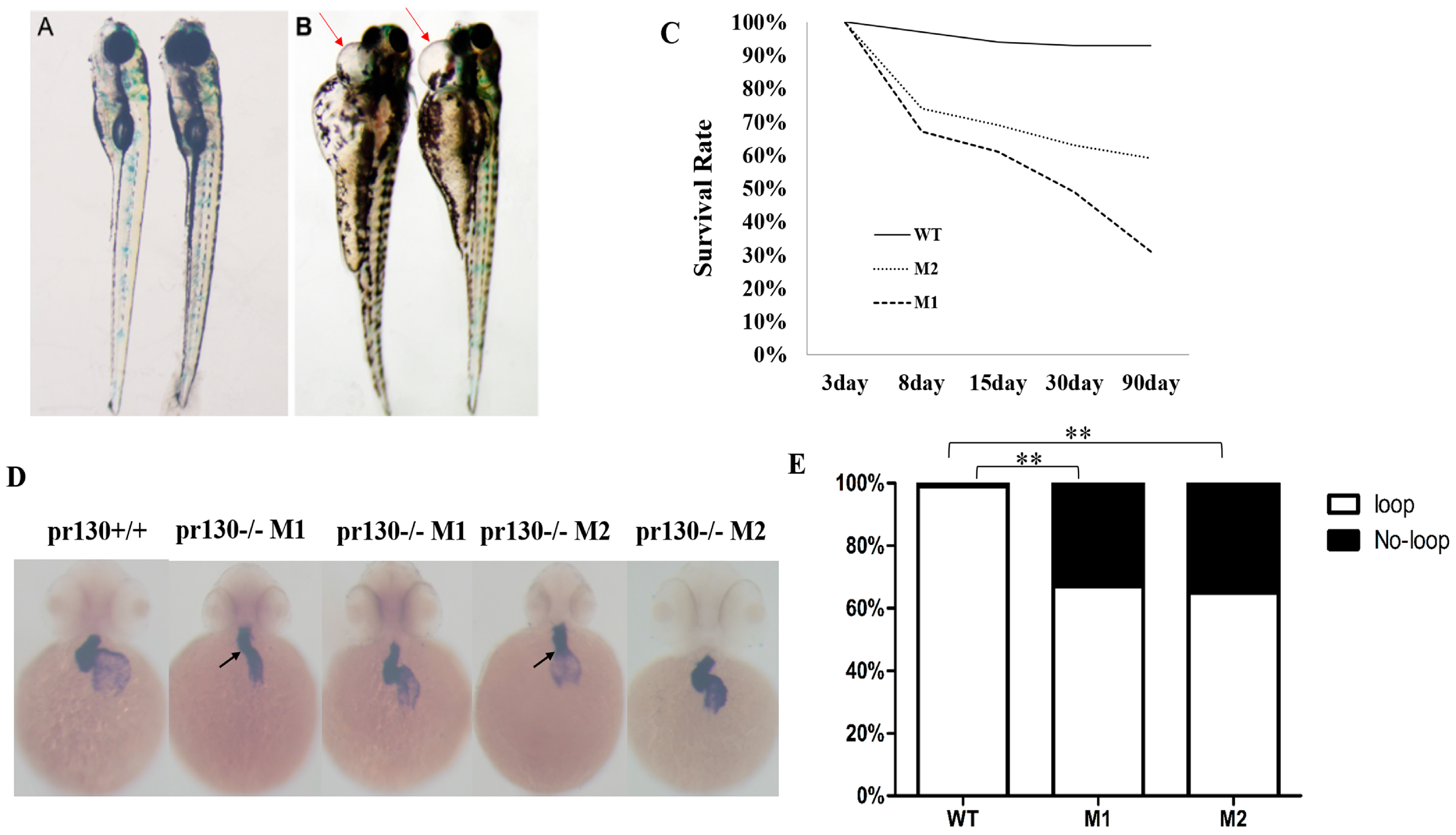
2.3. Increased Mortality and Defective Cardiac Development in Pr130-/- Zebrafish
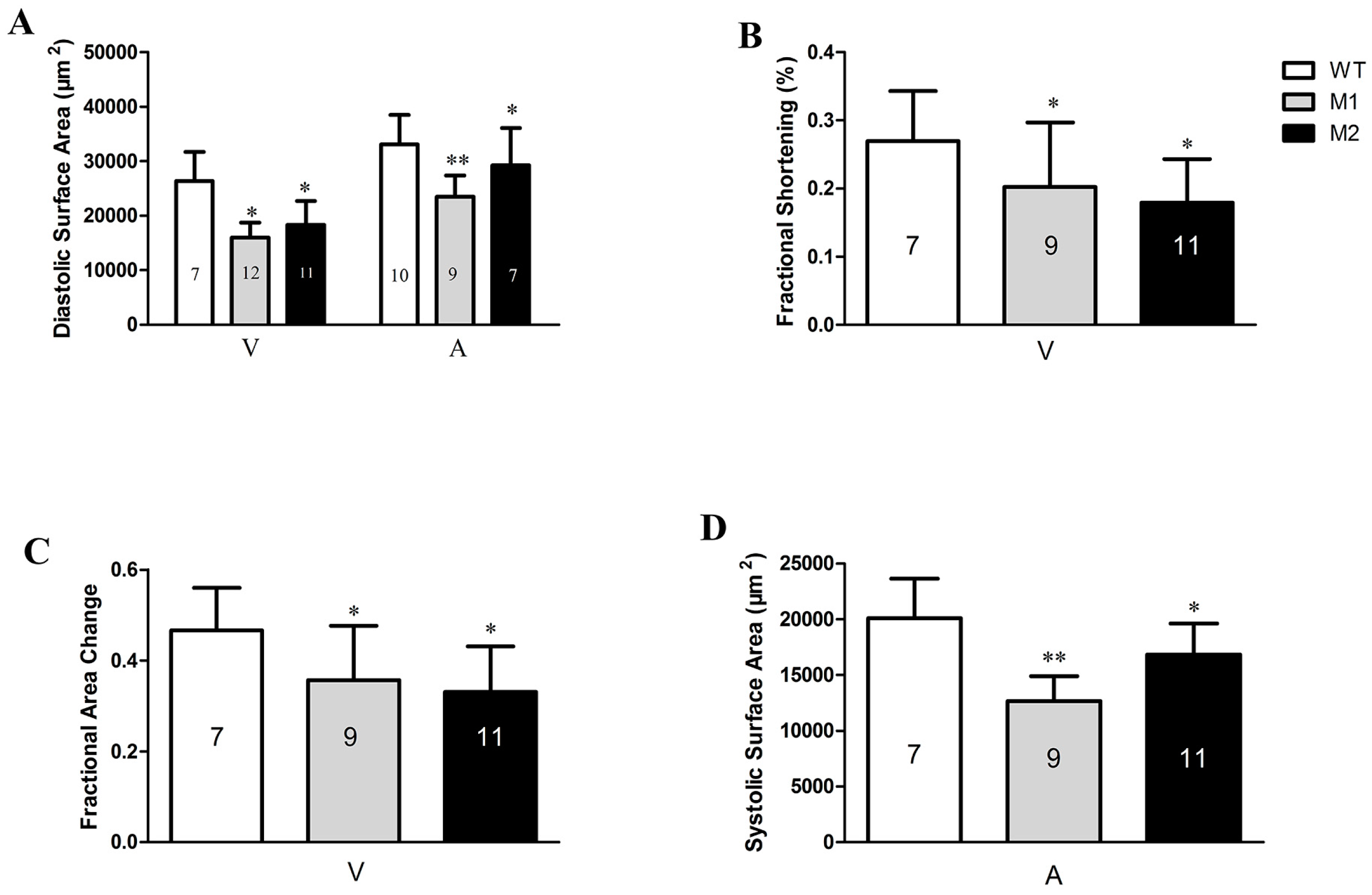
2.4. Altered Cardiac Structure and the Decrease of Cardiomyocytes in Pr130-/- Zebrafish
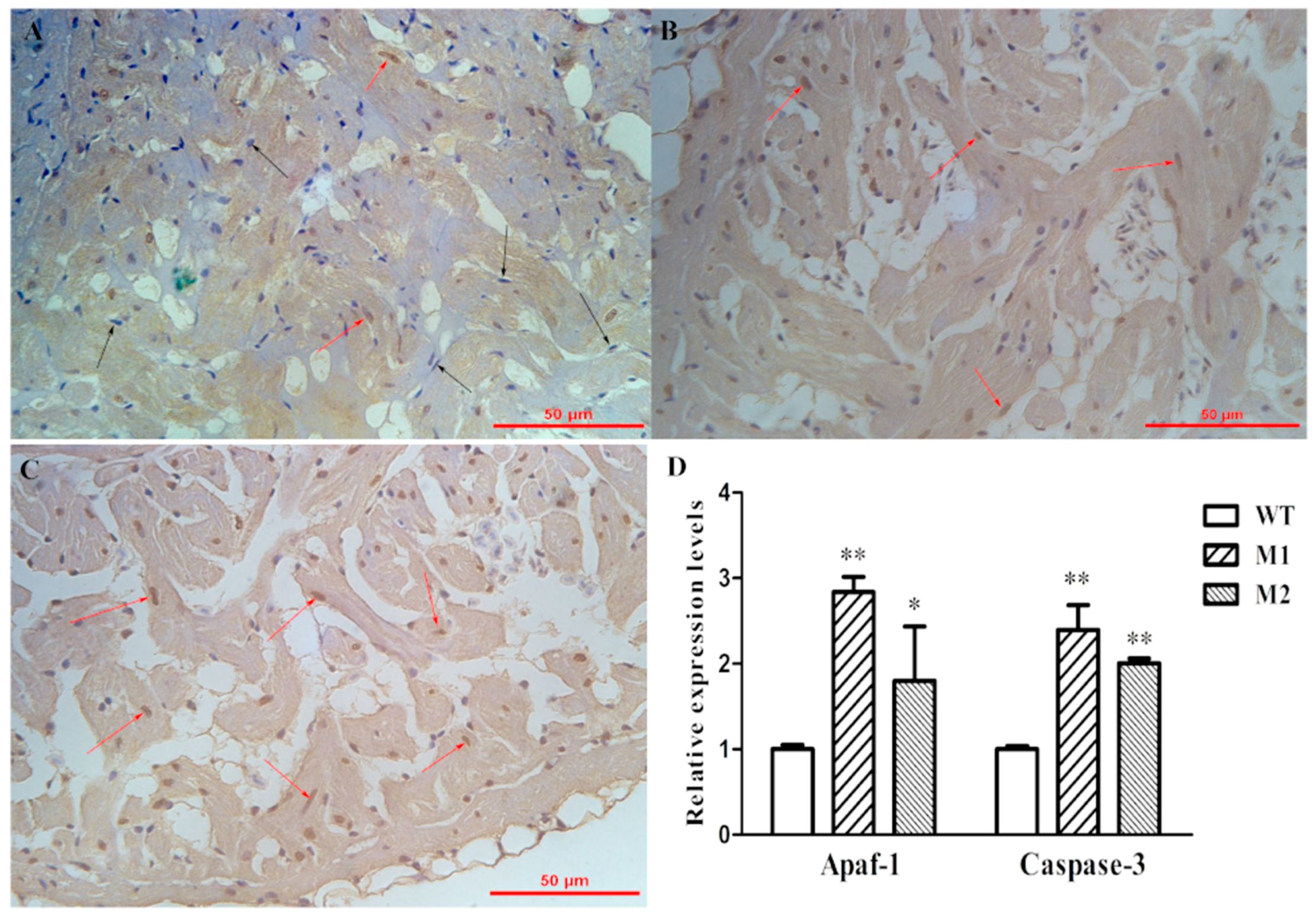
2.5. Increased Apoptosis Caused the Reduction of Cardiomyocytes
3. Discussion
4. Experimental Section
4.1. Zebrafish Maintenance
4.2. Pr130-Knockout by CRISPR/Cas9 System
4.3. RT-PCR and qPCR
4.4. PP2A Phosphatase Activity
4.5. Western Blot Analysis
4.6. WISH
4.7. Histological Studies
4.8. Quantification of the Embryonic Heart Function
4.9. Transmission Electron Microscopy
4.10. Tunel Staining
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lei, M.; Wang, X.; Ke, Y.; Solaro, R.J. Regulation of Ca(2+) transient by PP2A in normal and failing heart. Front Physiol. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.; Schroder, F.; Vogler, D.; Schaefer, A.; Haverich, A.; Schieffer, B.; Korte, T.; Drexler, H. Increased open probability of single cardiac L-type calcium channels in patients with chronic atrial fibrillation. Role of phosphatase 2A. Cardiovasc. Res. 2003, 59, 37–45. [Google Scholar] [CrossRef]
- Zwaenepoel, K.; Louis, J.V.; Goris, J.; Janssens, V. Diversity in genomic organisation, developmental regulation and distribution of the murine pr72/b subunits of protein phosphatase 2A. BMC Genom. 2008, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Serine/threonine phosphatases: Mechanism through structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Lv, B.; Wang, Y. Protein phosphatase 2A mediates oxidative stress induced apoptosis in osteoblasts. Mediat. Inflamm. 2015. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Dewenter, M.; El-Armouche, A.; Dobrev, D. Function and regulation of serine/threonine phosphatases in the healthy and diseased heart. J. Mol. Cell. Cardiol. 2013, 64, 90–98. [Google Scholar] [CrossRef] [PubMed]
- DeGrande, S.T.; Little, S.C.; Nixon, D.J.; Wright, P.; Snyder, J.; Dun, W.; Murphy, N.; Kilic, A.; Higgins, R.; Binkley, P.F.; et al. Molecular mechanisms underlying cardiac protein phosphatase 2A regulation in heart. J. Biol. Chem. 2013, 288, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Creyghton, M.P.; Roel, G.; Eichhorn, P.J.; Vredeveld, L.C.; Destree, O.; Bernards, R. Pr130 is a modulator of the wnt-signaling cascade that counters repression of the antagonist naked cuticle. Proc. Natl. Acad. Sci. USA 2006, 103, 5397–5402. [Google Scholar] [CrossRef] [PubMed]
- Novikov, N.; Evans, T. Tmem88a mediates gata-dependent specification of cardiomyocyte progenitors by restricting WNT signaling. Development (Cambridge, England) 2013, 140, 3787–3798. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J. Vitamin D receptor signaling is required for heart development in zebrafish embryo. Biochem. Biophys. Res. Commun. 2016, 470, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Jao, L.E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a crispr nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Sablina, A.A.; Hector, M.; Colpaert, N.; Hahn, W.C. Identification of PP2A complexes and pathways involved in cell transformation. Cancer Res. 2010, 70, 10474–10484. [Google Scholar] [CrossRef] [PubMed]
- Little, S.C.; Curran, J.; Makara, M.A.; Kline, C.F.; Ho, H.T.; Xu, Z.; Wu, X.; Polina, I.; Musa, H.; Meadows, A.M.; et al. Protein phosphatase 2A regulatory subunit B56α limits phosphatase activity in the heart. Sci. Signal. 2015, 8, ra72. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.; Fouquet, B.; Chen, J.N.; Warren, K.S.; Weinstein, B.M.; Meiler, S.E.; Mohideen, M.A.; Neuhauss, S.C.; Solnica-Krezel, L.; Schier, A.F.; et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 1996, 123, 285–292. [Google Scholar] [PubMed]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yu, L.; Liu, C.; Yu, K.; Shi, X.; Yeung, L.W.; Lam, P.K.; Wu, R.S.; Zhou, B. Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat. Toxicol. 2009, 93, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kuster, D.W.; Bawazeer, A.C.; Zaremba, R.; Goebel, M.; Boontje, N.M.; van der Velden, J. Cardiac myosin binding protein c phosphorylation in cardiac disease. J. Muscle Res. Cell Motil. 2012, 33, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Brewis, N.; Ohst, K.; Fields, K.; Rapacciuolo, A.; Chou, D.; Bloor, C.; Dillmann, W.; Rockman, H.; Walter, G. Dilated cardiomyopathy in transgenic mice expressing a mutant a subunit of protein phosphatase 2A. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1307–H1318. [Google Scholar] [PubMed]
- Varadkar, P.; Despres, D.; Kraman, M.; Lozier, J.; Phadke, A.; Nagaraju, K.; McCright, B. The protein phosphatase 2A B56γ regulatory subunit is required for heart development. Dev. Dyn. 2014, 243, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Kirchhefer, U.; Brekle, C.; Eskandar, J.; Isensee, G.; Kucerova, D.; Muller, F.U.; Pinet, F.; Schulte, J.S.; Seidl, M.D.; Boknik, P. Cardiac function is regulated by B56α-mediated targeting of protein phosphatase 2A (PP2A) to contractile relevant substrates. J. Biol. Chem. 2014, 289, 33862–33873. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Kim, Y.S.; Kim, S.Y.; Huh, Y.; Park, C.; Jeong, J.W. Okadaic acid protects human neuroblastoma SH-SY5Y cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis. Neurosci. Lett. 2009, 449, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Haneji, T.; Hirashima, K.; Teramachi, J.; Morimoto, H. Okadaic acid activates the PKR pathway and induces apoptosis through PKR stimulation in mg63 osteoblast-like cells. Int. J. Oncol. 2013, 42, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lou, Q.; He, J.; Yin, Z. Role of zebrafish lbx2 in embryonic lateral line development. PLoS ONE 2011, 6, e29515. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.D.; Maeder, M.L.; Reyon, D.; Voytas, D.F.; Joung, J.K.; Dobbs, D. ZiFiT (Zinc Finger Targeter): An updated zinc finger engineering tool. Nucleic Acids Res. 2010, 38, W462–W468. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Sun, C.; Gao, L.; Zhu, D.; Xu, X.; Zhu, X.; Xiong, J.W.; Xi, J.J. Genome editing with RNA-guided cas9 nuclease in zebrafish embryos. Cell Res. 2013, 23, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.G.; MacRae, C.A. Purification of hearts from zebrafish embryos. Biotechniques 2006, 40, 274, 276, 278. [Google Scholar] [PubMed]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Burrows, J.T.; Pearson, B.J.; Scott, I.C. An in vivo requirement for the mediator subunit MED14 in the maintenance of stem cell populations. Stem Cell Rep. 2015, 4, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Berdougo, E.; Coleman, H.; Lee, D.H.; Stainier, D.Y.; Yelon, D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development 2003, 130, 6121–6129. [Google Scholar] [CrossRef] [PubMed]
- Ocorr, K.; Fink, M.; Cammarato, A.; Bernstein, S.; Bodmer, R. Semi-automated optical heartbeat analysis of small hearts. J. Vis. Exp. 2009. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Callol-Massot, C.; Chu, A.; Ruiz-Lozano, P.; Izpisua-Belmonte, J.C.; Giles, W.; Bodmer, R.; Ocorr, K. A new method for detection and quantification of heartbeat parameters in drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 2009, 46, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Ocorr, K.; Perrin, L.; Lim, H.Y.; Qian, L.; Wu, X.; Bodmer, R. Genetic control of heart function and aging in drosophila. Trends Cardiovasc. Med. 2007, 17, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lafontant, P.J.; Behzad, A.R.; Brown, E.; Landry, P.; Hu, N.; Burns, A.R. Cardiac myocyte diversity and a fibroblast network in the junctional region of the zebrafish heart revealed by transmission and serial block-face scanning electron microscopy. PLoS ONE 2013, 8, e72388. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Sun, H.; Xie, P.; Wang, J.; Zhang, G.; Chen, N.; Yan, W.; Li, G. The role of apoptosis in MCLR-induced developmental toxicity in zebrafish embryos. Aquat. Toxicol. 2014, 149, 25–32. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, Z.; Gan, X.; Zhai, G.; Gao, J.; Xiong, C.; Qiu, X.; Wang, X.; Yin, Z.; Zheng, F. Deletion of Pr130 Interrupts Cardiac Development in Zebrafish. Int. J. Mol. Sci. 2016, 17, 1746. https://doi.org/10.3390/ijms17111746
Yang J, Li Z, Gan X, Zhai G, Gao J, Xiong C, Qiu X, Wang X, Yin Z, Zheng F. Deletion of Pr130 Interrupts Cardiac Development in Zebrafish. International Journal of Molecular Sciences. 2016; 17(11):1746. https://doi.org/10.3390/ijms17111746
Chicago/Turabian StyleYang, Jie, Zuhua Li, Xuedong Gan, Gang Zhai, Jiajia Gao, Chenling Xiong, Xueping Qiu, Xuebin Wang, Zhan Yin, and Fang Zheng. 2016. "Deletion of Pr130 Interrupts Cardiac Development in Zebrafish" International Journal of Molecular Sciences 17, no. 11: 1746. https://doi.org/10.3390/ijms17111746