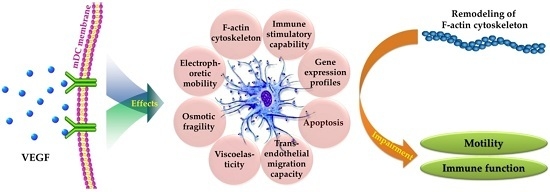
Biophysical Properties and Motility of Human Mature Dendritic Cells Deteriorated by Vascular Endothelial Growth Factor through Cytoskeleton Remodeling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cell Electrophoretic Mobility
2.2. Osmotic Fragility
2.3. Viscoelasticity Analysis
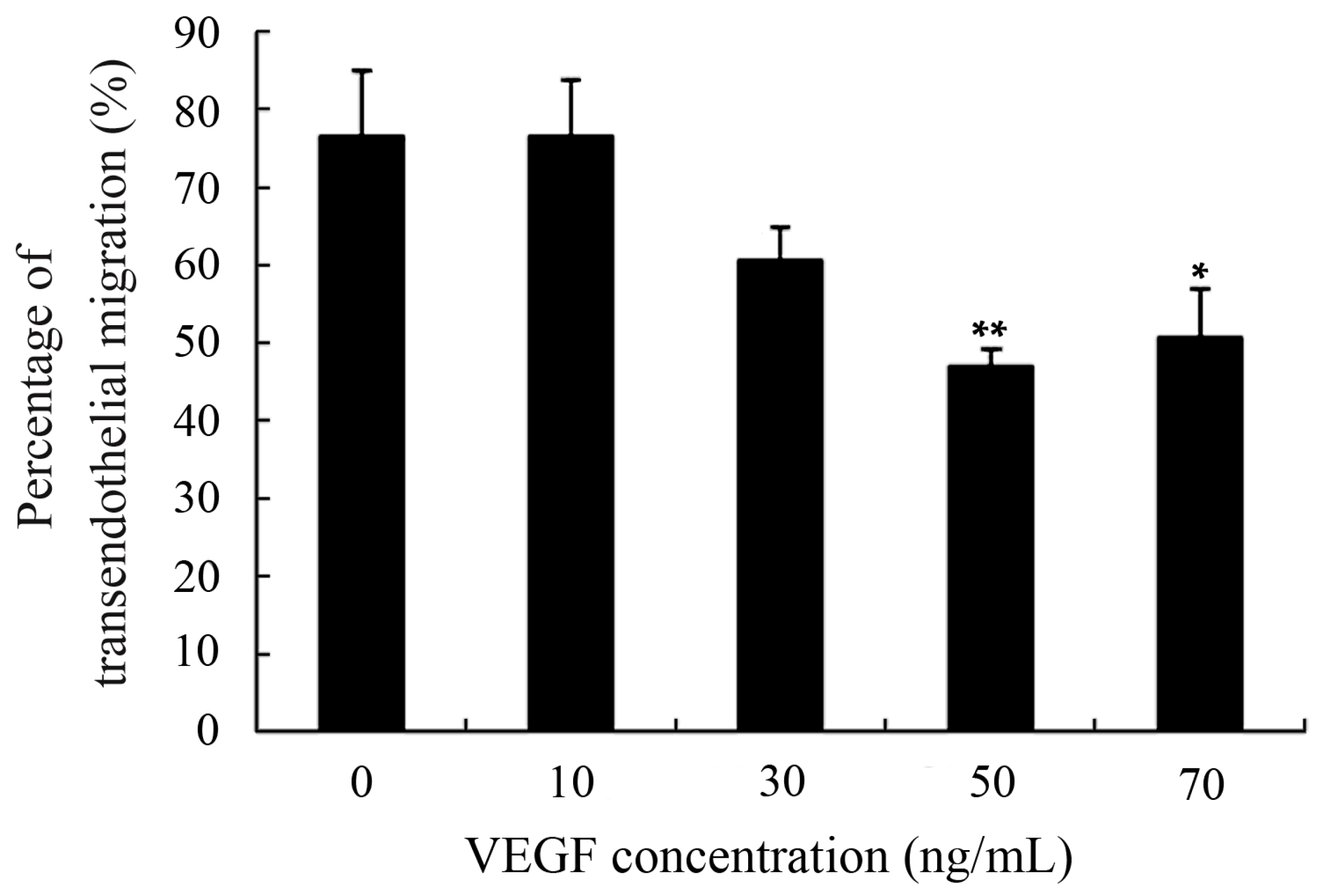
2.4. Capacity of Transendothelial Migration
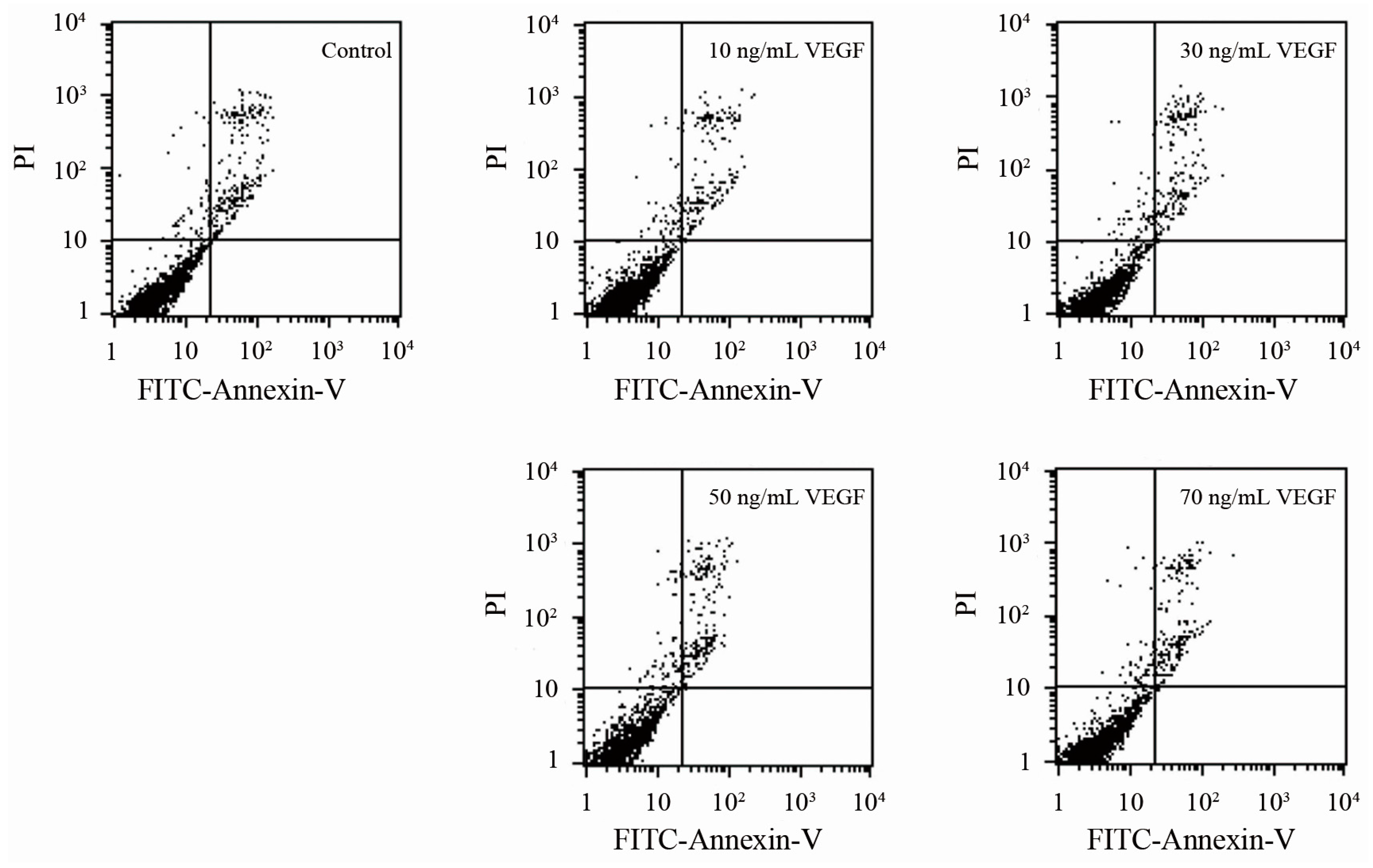
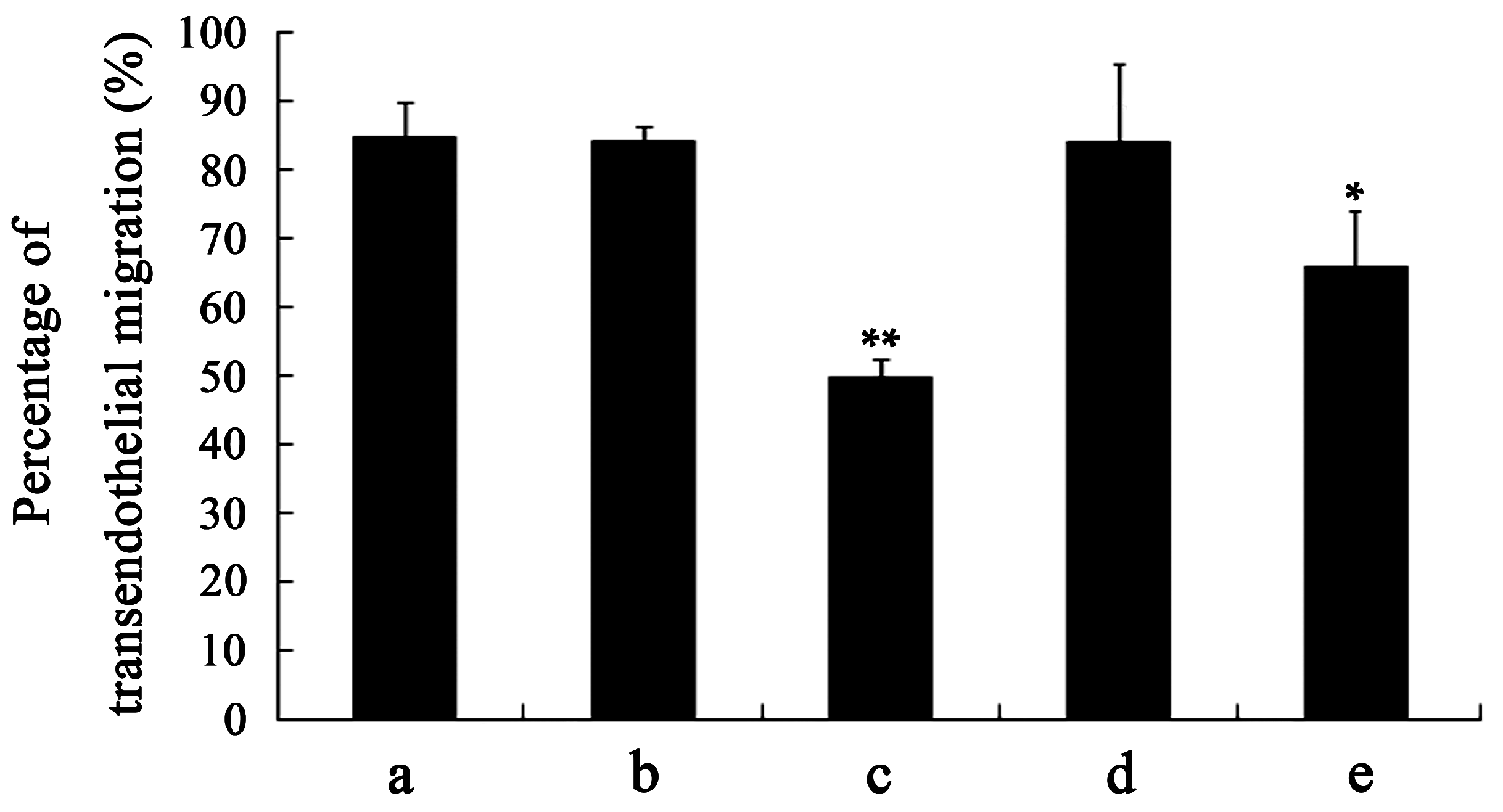
2.5. Analyses of Apoptosis and Specificity of the Effect of VEGF
2.6. Effects of VEGF on F-Actin Cytoskeleton
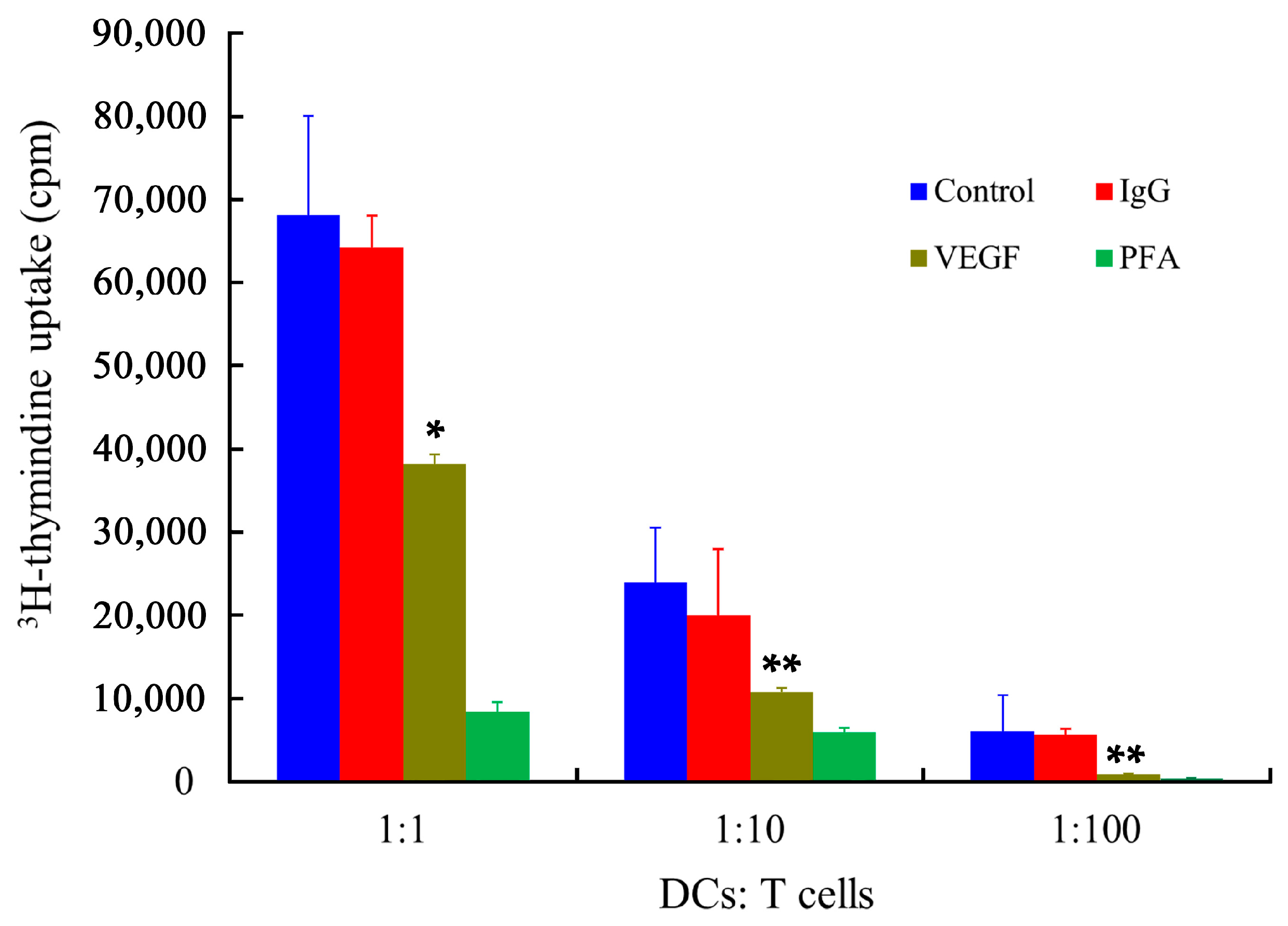
2.7. Stimulatory Capability of mDCs
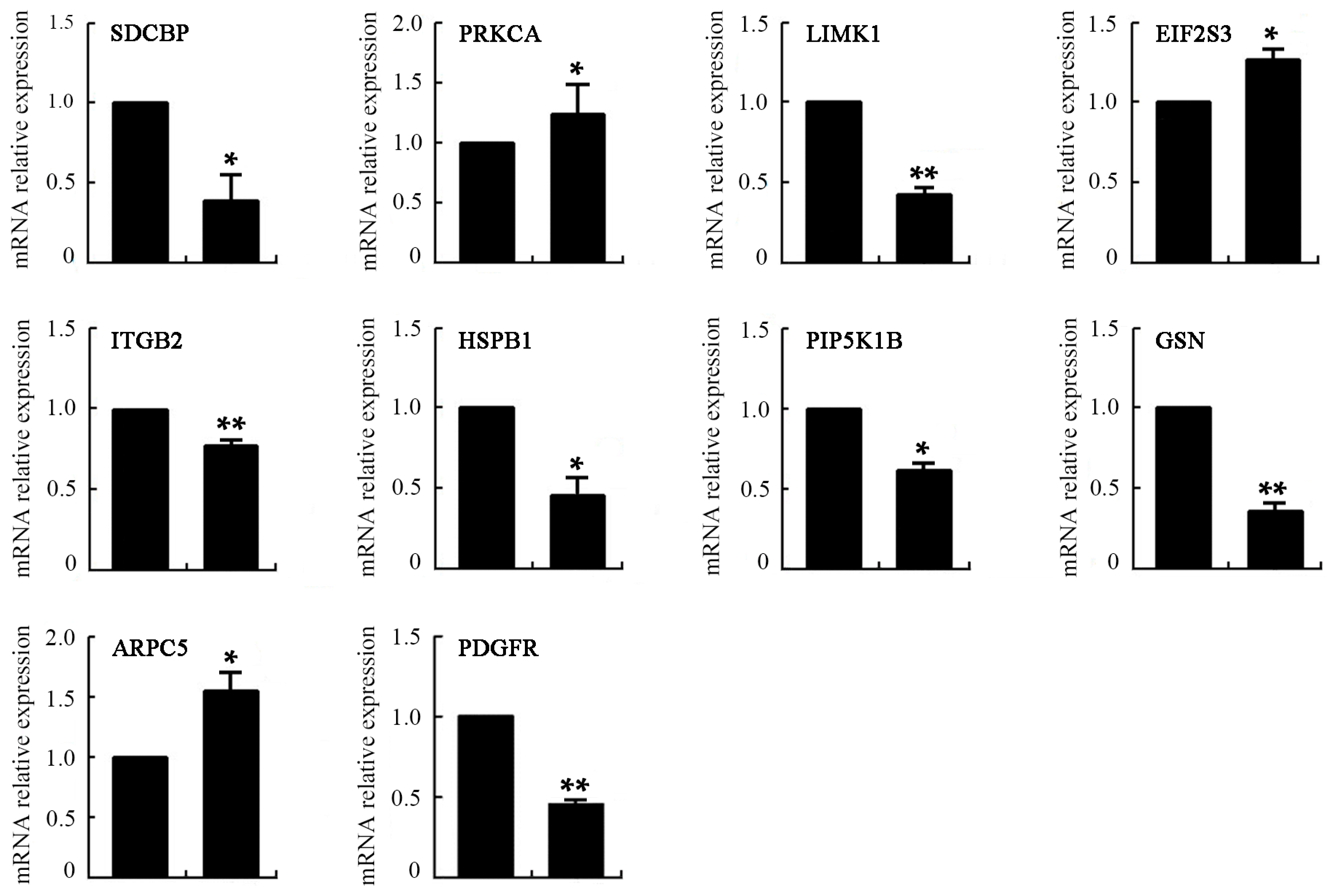
2.8. Differentially Expressed Genes of VEGF-Treated mDCs
3. Materials and Methods
3.1. Isolation of Monocytes and Generation of DCs
3.2. Culture of HY926 Cell Lines
3.3. Treatment of VEGF on mDCs
3.4. Measurement of Cell EPM
3.5. Measurement of Cell Osmotic Fragility
3.6. Micropipette Measurement of Cell Viscoelasticity
3.7. Measurement of mDCs Transmigration in Transwell
3.8. Cell Apoptosis Analysis
3.9. Confocal Laser Scanning Microscopy (CLSM) Analysis
3.10. Mixed Leukocyte Reaction (MLR)
3.11. Microarray and Real-Time PCR Analyses
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steinman, R.M. Decisions about dendritic cells: Past, present, and future. Annu. Rev. Immunol. 2012, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Park, S.H.; Kim, H.Y.; Kwok, S.K. A plasmacytoid dendritic cells-type I interferon axis is critically implicated in the pathogenesis of systemic lupus erythematosus. Int. J. Mol. Sci. 2015, 16, 14158–14170. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.G.; Hassanpour, M.; Tsai, V.W.; Li, H.; Ruitenberg, M.J.; Booth, D.W.; Serrats, J.; Hart, P.H.; Symonds, G.P.; Sawchenko, P.E.; et al. Dendritic cells and multiple sclerosis: Disease, tolerance and therapy. Int. J. Mol. Sci. 2012, 14, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Carranza, P.; Del Río Estrada, P.M.; Díaz Rivera, D.; Ablanedo-Terrazas, Y.; Reyes-Terán, G. Lymph nodes from HIV-infected individuals harbor mature dendritic cells and increased numbers of PD-L1+ conventional dendritic cells. Hum. Immunol. 2016, 77, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Mackern-Oberti, J.P.; Vega, F.; Llanos, C.; Bueno, S.M.; Kalergis, A.M. Targeting dendritic cell function during systemic autoimmunity to restore tolerance. Int. J. Mol. Sci. 2014, 15, 16381–16417. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, Y.; Yang, X.; Rui, K.; Tang, X.; Ma, J.; Chen, J.; Xu, H.; Lu, L.; Wang, S. Ficus carica polysaccharides promote the maturation and function of dendritic cells. Int. J. Mol. Sci. 2014, 15, 12469–12479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrilovich, D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. Immunol. 2004, 4, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Qiao, J.; Fu, Y.X. Immunotherapy and tumor microenvironment. Cancer Lett. 2016, 370, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J.; Angeli, V.; Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005, 5, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Chheda, Z.S.; Sharma, R.K.; Jala, V.R.; Luster, A.D.; Haribabu, B. Chemoattractant receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors. J. Immunol. 2016, 197, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Baranov, M.V.; Ter Beest, M.; Reinieren-Beeren, I.; Cambi, A.; Figdor, C.G.; van den Bogaart, G. Podosomes of dendritic cells facilitate antigen sampling. J. Cell Sci. 2014, 127, 1052–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacken, P.J.; de Vries, I.J.; Torensma, R.; Figdor, C.G. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007, 7, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.; Gomes, C.; Falcão, A.; Cruz, M.T.; Neves, B.M. Antitumor dendritic cell-based vaccines: Lessons from 20 years of clinical trials and future perspectives. Transl. Res. 2016, 168, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Koido, S. Dendritic-tumor fusion cell-based cancer vaccines. Int. J. Mol. Sci. 2016, 17, 828. [Google Scholar] [CrossRef] [PubMed]
- Sabado, R.L.; Bhardwaj, N. Cancer immunotherapy: Dendritic-cell vaccines on the move. Nature 2015, 519, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, J.M.; Yuan, R.; Yi, X.; Li, L.; Gong, W.; Yang, T.; Li, L.; Su, S. Tissue-resident dendritic cells and diseases involving dendritic cell malfunction. Int. Immunopharmacol. 2016, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Legitimo, A.; Failli, A.; Ferrari, P.; Nicolini, A.; Spisni, R.; Miccoli, P.; Consolini, R. Defective generation and maturation of dendritic cells from monocytes in colorectal cancer patients during the course of disease. Int. J. Mol. Sci. 2013, 14, 22022–22041. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zeng, Z.; Yao, W.; Wang, X.; Sun, D.; Ka, W.; Zhang, Y.; Wang, X.; Chen, X.; Zha, Y.; et al. Biomechanical alterations of dendritic cells by co-culturing with K562 CML cells and their potential role in immune escape. J. Biomech. 2010, 43, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Long, J.; Jia, B.; Xu, X.; Zhang, C.; Li, L.; Wen, Z.; Jin, F.; Yao, W.; Zeng, Z. Transforming growth factor-β1 deteriorates microrheological characteristics and motility of mature dendritic cells in concentration-dependent fashion. Clin. Hemorheol. Microcirc. 2014, 56, 25–40. [Google Scholar] [PubMed]
- Escors, D. Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy. New J. Sci. 2014, 2014, 734515. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, X. Dendritic cell vaccines in cancer immunotherapy: From biology to translational medicine. Front. Med. 2011, 5, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Nunes, C.; Amenitsch, H.; Reis, S. Effects of resveratrol on the structure and fluidity of lipid bilayers: A membrane biophysical study. Soft Matter 2016, 12, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Kinnunen, P.K. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006, 16, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Wyss, H.M.; Henderson, J.M.; Byfield, F.J.; Bruggeman, L.A.; Ding, Y.; Huang, C.; Suh, J.H.; Franke, T.; Mele, E.; Pollak, M.R.; et al. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 2011, 300, C397–C405. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Gu, L.; Sun, D.; Ka, W.; Wen, Z.; Chien, S. Wild type p53 gene causes reorganization of cytoskeleton and, therefore, the impaired deformability and difficult migration of murine erythroleukemia cells. Cell Motil. Cytoskeleton 2003, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, X.; Jiang, Y.; Wang, G.; Zhan, J.; Guo, J.; Yao, W.; Sun, D.; Ka, W.; Tang, Y.; et al. Biophysical studies on the differentiation of human CD14+ monocytes into dendritic cells. Cell Biochem. Biophys. 2006, 45, 19–30. [Google Scholar] [CrossRef]
- Zeng, Z.; Yao, W.; Xu, X.; Xu, G.; Long, J.; Wang, X.; Wen, Z.; Chien, S. Hepatocellular carcinoma cells deteriorate the biophysical properties of dendritic cells. Cell Biochem. Biophys. 2009, 55, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Wolgemuth, C.W. Physical mechanisms of cancer in the transition to metastasis. Biophys. J. 2016, 111, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Zhang, S.; Li, Y. Inhibition of vascular endothelial growth factor by small interfering RNA upregulates differentiation, maturation and function of dendritic cells. Exp. Ther. Med. 2015, 9, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Lee, C.H.; Chen, G.S.; Chang, K.L.; Yu, H.S. STAT3-dependent VEGF production from keratinocytes abrogates dendritic cell activation and migration by arsenic: A plausible regional mechanism of immunosuppression in arsenical cancers. Chem. Biol. Interact. 2015, 227, 96–103. [Google Scholar]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [PubMed]
- Mimura, K.; Kono, K.; Takahashi, A.; Kawaguchi, Y.; Fujii, H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol. Immunother. 2007, 56, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Laxmanan, S.; Robertson, S.W.; Wang, E.; Lau, J.S.; Briscoe, D.M.; Mukhopadhyay, D. Vascular endothelial growth factor impairs the functional ability of dendritic cells through Id pathways. Biochem. Biophys. Res. Commun. 2005, 334, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Ran, S.; Ishida, T.; Nadaf, S.; Kerr, L.; Carbone, D.P.; Gabrilovich, D.I. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J. Immunol. 1998, 160, 1224–1232. [Google Scholar] [PubMed]
- Slivinsky, G.G.; Hymer, W.C.; Bauer, J.; Morrison, D.R. Cellular electrophoretic mobility data: A first approach to a database. Electrophoresis 1997, 18, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Mempel, T.R.; Henrickson, S.E.; Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004, 427, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xu, X.; Chen, D. Dendritic Cells: Biophysics, Tumor Microenvironment and Chinese Traditional Medicine, SpringerBriefs in Biochemistry and Molecular Biology; Springer: Berlin, Germany, 2015; pp. 11–63. [Google Scholar]
- Benvenuti, F.; Hugues, S.; Walmsley, M.; Ruf, S.; Fetler, L.; Popoff, M.; Tybulewicz, V.L.; Amigorena, S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science 2004, 305, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Delon, J.; Brotz, T.M.; Germain, R.N. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 2002, 296, 1873–1876. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fernández, J.L.; Riol-Blanco, L.; Delgado-Martín, C. What is the function of the dendritic cell side of the immunological synapse? Sci. Signal. 2010, 3, re2. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, P.M.; Trusolino, L. Cancer: The matrix is now in control. Nat. Med. 2005, 11, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.D.; Li, C.; Song, Y.; Lei, Y.M.; Yang, B.X. In situ analysis of FOXP3+ regulatory T cells and myeloid dendritic cells in human colorectal cancer tissue and tumor-draining lymph node. Biomed. Rep. 2013, 1, 207–212. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, N.M.; Mehanna, R.A. Assessment of maturation status of tumor-infiltrating dendritic cells in invasive ductal carcinoma of the breast: Relation with vascular endothelial growth factor expression. Türk Patoloji Derg. 2013, 29, 193–200. [Google Scholar] [PubMed]
- Bouma, G.; Mendoza-Naranjo, A.; Blundell, M.P.; de Falco, E.; Parsley, K.L.; Burns, S.O.; Thrasher, A.J. Cytoskeletal remodeling mediated by WASp in dendritic cells is necessary for normal immune synapse formation and T-cell priming. Blood 2011, 118, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Omotade, O.F.; Myers, K.R.; Zheng, J.Q. Actin cytoskeleton in dendritic spine development and plasticity. Curr. Opin. Neurobiol. 2016, 39, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.T.; Angus, K.L.; Griffiths, G.M. The role of the cytoskeleton at the immunological synapse. Immunol. Rev. 2013, 256, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Chazeau, A.; Giannone, G. Organization and dynamics of the actin cytoskeleton during dendritic spine morphological remodeling. Cell. Mol. Life Sci. 2016, 73, 3053–3073. [Google Scholar] [CrossRef] [PubMed]
- Tourkova, I.L.; Yurkovetsky, Z.R.; Shurin, M.R.; Shurin, G.V. Mechanisms of dendritic cell-induced T cell proliferation in the primary MLR assay. Immunol. Lett. 2001, 78, 75–82. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhang, Y.; Xing, J.; Long, J.; Gu, L.; Wang, X.; Sun, D.; Ka, W.; Yao, W.; et al. Tumor-derived factors impaired motility and immune functions of dendritic cells through derangement of biophysical characteristics and reorganization of cytoskeleton. Cell Motil. Cytoskeleton 2007, 64, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Polchow, B.; Kebbel, K.; Schmiedeknecht, G.; Reichardt, A.; Henrich, W.; Hetzer, R.; Lueders, C. Cryopreservation of human vascular umbilical cord cells under good manufacturing practice conditions for future cell banks. J. Transl. Med. 2012, 10, 98. [Google Scholar] [CrossRef] [PubMed]


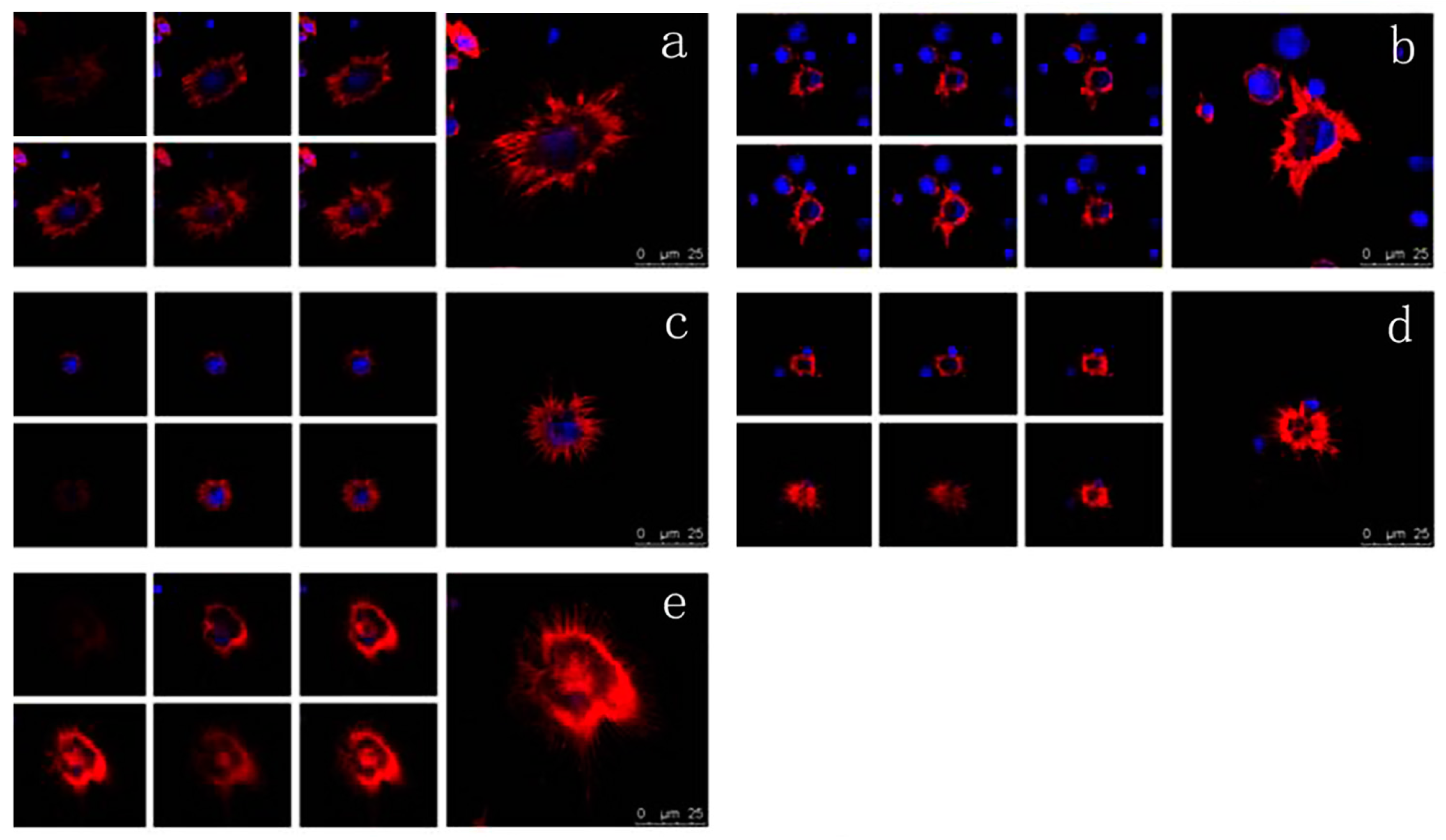
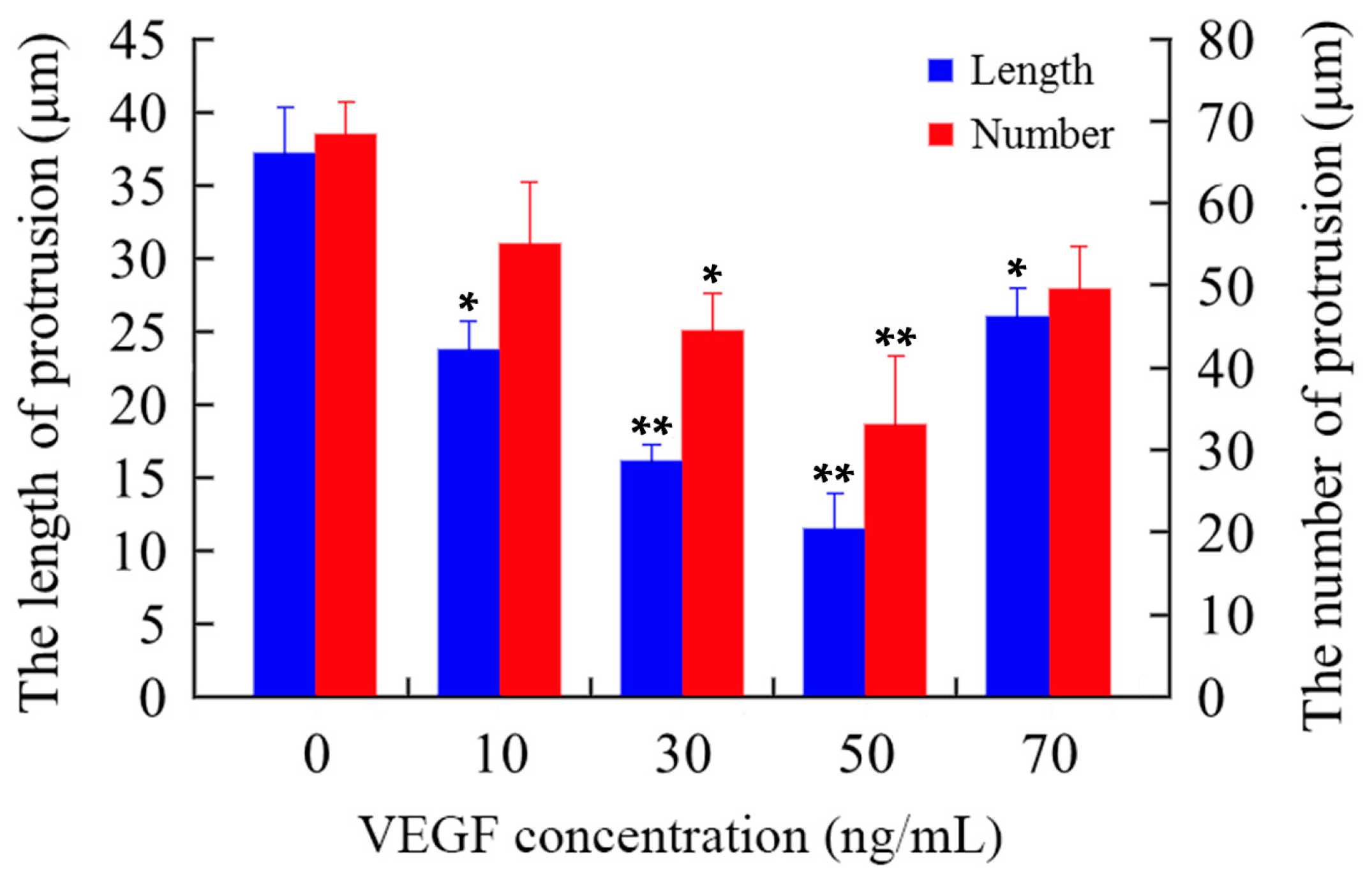
| Concentration of VEGF (ng/mL) | 0 | 10 | 30 | 50 | 70 |
|---|---|---|---|---|---|
| EPM | 3.063 ± 0.292 | 2.775 ± 0.265 | 0.801 ± 0.200 * | 0.729 ± 0.108 ** | 1.801 ± 0.185 |
| GO Term | Count | p-Value | q-Value |
|---|---|---|---|
| GO:0003777 microtubule motor activity | 16 | 9.23 × 10−23 | 1.59 × 10−21 |
| GO:0003779 actin binding | 16 | 7.38 × 10−13 | 7.46 × 10−12 |
| GO:0051015 actin filament binding | 6 | 2.15 × 10−8 | 1.32 × 10−7 |
| GO:0005200 structural constituent of cytoskeleton | 6 | 7.26 × 10−7 | 3.56 × 10−6 |
| GO:0008017 microtubule binding | 5 | 2.59 × 10−6 | 1.12 × 10−5 |
| GO:0051059 NF-kappaB binding | 4 | 3.08 × 10−6 | 1.32 × 10−5 |
| GO:0005089 Rho guanyl-nucleotide exchange factor activity | 5 | 1.44 × 10−5 | 5.31 × 10−5 |
| GO:0015631 tubulin binding | 4 | 2.28 × 10−4 | 5.67 × 10−4 |
| GO:0050431 transforming growth factor beta binding | 2 | 7.75 × 10−4 | 0.00159 |
| GO:0003774 motor activity | 4 | 0.003258 | 0.005246 |
| GO:0030616 transforming growth factor beta receptor, common-partner cytoplasmic mediator activity | 1 | 0.004199 | 0.005246 |
| GO:0070123 transforming growth factor beta receptor activity, type III | 1 | 0.004199 | 0.005246 |
| GO:0050839 cell adhesion molecule binding | 2 | 0.004958 | 0.006071 |
| GO:0005100 Rho GTPase activator activity | 2 | 0.005356 | 0.006509 |
| GO:0046332 SMAD binding | 2 | 0.010646 | 0.010604 |
| GO:0005522 profilin binding | 1 | 0.02082 | 0.016575 |
| GO:0030898 actin-dependent ATPase activity | 1 | 0.024932 | 0.01898 |
| GO:0030675 Rac GTPase activator activity | 1 | 0.033104 | 0.023774 |
| GO:0005072 transforming growth factor beta receptor, cytoplasmic mediator activity | 1 | 0.045234 | 0.030536 |
| GO:0042288 MHC class I protein binding | 1 | 0.053235 | 0.034859 |
| GO:0008093 cytoskeletal adaptor activity | 1 | 0.053235 | 0.034859 |
| GO:0000146 microfilament motor activity | 1 | 0.06117 | 0.039009 |
| GO:0042287 MHC protein binding | 1 | 0.096063 | 0.056973 |
| GO:0017048 Rho GTPase binding | 1 | 0.107403 | 0.062869 |
| GO:0008092 cytoskeletal protein binding | 3 | 0.299076 | 0.160163 |
| GO Term | Count | p-Value | q-Value |
|---|---|---|---|
| GO:0003779 actin binding | 39 | 1.09 × 10−44 | 4.76 × 10−43 |
| GO:0046332 SMAD binding | 9 | 3.39 × 10−14 | 4.02 × 10−13 |
| GO:0032395 MHC class II receptor activity | 6 | 1.32 × 10−11 | 1.24 × 10−10 |
| GO:0034713 type I transforming growth factor beta receptor binding | 4 | 9.52 × 10−9 | 6.15 × 10−8 |
| GO:0051015 actin filament binding | 6 | 1.80 × 10−8 | 1.12 × 10−7 |
| GO:0005200 structural constituent of cytoskeleton | 6 | 6.11 × 10−7 | 3.03 × 10−6 |
| GO:0003774 motor activity | 7 | 2.37 × 10−6 | 9.99 × 10−6 |
| GO:0005021 vascular endothelial growth factor receptor activity | 3 | 3.72 × 10−6 | 1.53 × 10−5 |
| GO:0050431 transforming growth factor beta binding | 3 | 7.93 × 10−6 | 3.04 × 10−5 |
| GO:0048365 Rac GTPase binding | 3 | 7.93 × 10−6 | 3.04 × 10−5 |
| GO:0030617 transforming growth factor beta receptor, inhibitory cytoplasmic mediator activity | 2 | 1.66 × 10−5 | 5.64 × 10−5 |
| GO:0042289 MHC class II protein binding | 2 | 4.96 × 10−5 | 1.51 × 10−4 |
| GO:0003823 antigen binding | 6 | 8.13 × 10−5 | 2.34 × 10−4 |
| GO:0050839 cell adhesion molecule binding | 3 | 1.45 × 10−4 | 3.87 × 10−4 |
| GO:0017048 Rho GTPase binding | 3 | 1.84 × 10−4 | 4.57 × 10−4 |
| GO:0030274 LIM domain binding | 2 | 2.46 × 10−4 | 5.91 × 10−4 |
| GO:0008017 microtubule binding | 3 | 0.001215 | 0.002335 |
| GO:0045296 cadherin binding | 2 | 0.001256 | 0.002393 |
| GO:0008093 cytoskeletal adaptor activity | 2 | 0.001256 | 0.002393 |
| GO:0000146 microfilament motor activity | 2 | 0.001682 | 0.003063 |
| GO:0043183 vascular endothelial growth factor receptor 1 binding | 1 | 0.004075 | 0.005707 |
| GO:0043184 vascular endothelial growth factor receptor 2 binding | 1 | 0.004075 | 0.005707 |
| GO:0051059 NF-kappaB binding | 2 | 0.004315 | 0.005707 |
| GO:0005100 Rho GTPase activator activity | 2 | 0.005054 | 0.006579 |
| GO:0004920 interleukin-10 receptor activity | 1 | 0.008134 | 0.00876 |
| GO:0042805 actinin binding | 1 | 0.016201 | 0.014943 |
| GO:0005522 profilin binding | 1 | 0.020211 | 0.017634 |
| GO:0030618 transforming growth factor beta receptor, pathway-specific cytoplasmic mediator activity | 1 | 0.020211 | 0.017634 |
| GO:0005025 transforming growth factor beta receptor activity, type I | 1 | 0.024204 | 0.019893 |
| GO:0030675 Rac GTPase activator activity | 1 | 0.032141 | 0.025015 |
| GO:0003777 microtubule motor activity | 2 | 0.039709 | 0.029659 |
| GO:0005072 transforming growth factor beta receptor, cytoplasmic mediator activity | 1 | 0.043925 | 0.032045 |
| GO:0005024 transforming growth factor beta receptor activity | 1 | 0.063249 | 0.043469 |
| GO:0042287 MHC protein binding | 1 | 0.093358 | 0.06095 |
| GO:0008092 cytoskeletal protein binding | 4 | 0.117053 | 0.074554 |
| Pathway | Up-Regulated Genes | Down-Regulated Genes | ||||
|---|---|---|---|---|---|---|
| Count | p-Value | q-Value | Count | p-Value | q-Value | |
| T cell receptor signaling pathway | 19 | 1.07 × 10−12 | 5.63 × 10−12 | 5 | 0.096195 | 0.026281 |
| Leukocyte transendothelial migration | 13 | 1.39 × 10−6 | 2.56 × 10−6 | 17 | 1.75 × 10−9 | 2.65 × 10−9 |
| Regulation of actin cytoskeleton | 17 | 3.30 × 10−6 | 5.31 × 10−6 | 27 | 6.92 × 10−13 | 1.86 × 10−12 |
| Cell adhesion molecules (CAMs) | 13 | 4.40 × 10−6 | 6.97 × 10−6 | 34 | 3.15 × 10−26 | 4.09 × 10−25 |
| Focal adhesion | 15 | 2.60 × 10−5 | 3.36 × 10−5 | 32 | 5.62 × 10−18 | 2.83 × 10−17 |
| Primary immunodeficiency | 6 | 7.30 × 10−5 | 8.11 × 10−5 | 7 | 1.02 × 10−5 | 7.72 × 10−6 |
| Antigen processing and presentation | 8 | 5.09 × 10−4 | 4.63 × 10−4 | 21 | 4.90 × 10−16 | 2.12 × 10−15 |
| B cell receptor signaling pathway | 7 | 9.03 × 10−4 | 7.70 × 10−4 | 14 | 1.05 × 10−9 | 1.65 × 10−9 |
| VEGF signaling pathway | 5 | 0.020369 | 0.011837 | 8 | 2.87 × 10−4 | 1.55 × 10−4 |
| TGF-beta signaling pathway | 5 | 0.033979 | 0.018626 | 11 | 3.72 × 10−6 | 3.10 × 10−6 |
| MAPK signaling pathway | 5 | 0.54 × 10−4 | 0.81 × 10−4 | 33 | 4.95 × 10−15 | 1.98 × 10−14 |
| Gene | Primers | PCR Product (bp) | Tm (°C) |
|---|---|---|---|
| SDCBP | 5′-GGGTTTGCAGAAAAAGCAGT-3′ | 94 | 58 |
| 5′-GAGCGGTTCCTTGTGGG-3′ | |||
| PRKCA | 5′-CAAATTCATGGCACCTCTTG-3′ | 91 | 59 |
| 5′-CGAGGTGAAGGACCACAAAT-3′ | |||
| LIMK1 | 5′-TAGTACTGGTGCGACAGGGA-3′ | 105 | 60 |
| 5′-GGAGAGGAAGGAAGCGAGTT-3′ | |||
| EIF2S3 | 5′-TGGCTTGATTGTCACTCCTC-3′ | 109 | 60 |
| 5′-TTTTGACCAATCCAGTGTGC-3′ | |||
| ITGB2 | 5′-TGCTGACCTTGAACTTCGTG-3′ | 108 | 60 |
| 5′-GGACTCCAGCACACCGAG-3′ | |||
| HSPB1 | 5′-GTGAAGCACCGGGAGATGTA-3′ | 113 | 60 |
| 5′-AGCTGACGGTCAAGACCAAG-3′ | |||
| PIP5K1B | 5′-AGAAAAGGCCCTCAAATGCT-3′ | 100 | 59 |
| 5′-GTGAGATGAGGAAGCCAAGG-3′ | |||
| GSN | 5′-CTTCCCTGCCTTGAGGAACT-3′ | 108 | 60 |
| 5′-CAGCAGCAGGTAGTGCTCAT-3′ | |||
| ARPC5 | 5′-CCACTTCCTGCCAGACTACAC-3′ | 100 | 60 |
| 5′-GCCCGTCTGACAATAGCAGT-3′ | |||
| PDGFR | 5′-CGAATGGTCACCCGAGTTT-3′ | 105 | 61 |
| 5′-CTTTAAGAAGGCCACGGTGA-3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.-Q.; Xue, H.; Long, J.-H.; Wang, Y.; Jia, Y.; Qiu, W.; Zhou, J.; Wen, Z.-Y.; Yao, W.-J.; Zeng, Z. Biophysical Properties and Motility of Human Mature Dendritic Cells Deteriorated by Vascular Endothelial Growth Factor through Cytoskeleton Remodeling. Int. J. Mol. Sci. 2016, 17, 1756. https://doi.org/10.3390/ijms17111756
Hu Z-Q, Xue H, Long J-H, Wang Y, Jia Y, Qiu W, Zhou J, Wen Z-Y, Yao W-J, Zeng Z. Biophysical Properties and Motility of Human Mature Dendritic Cells Deteriorated by Vascular Endothelial Growth Factor through Cytoskeleton Remodeling. International Journal of Molecular Sciences. 2016; 17(11):1756. https://doi.org/10.3390/ijms17111756
Chicago/Turabian StyleHu, Zu-Quan, Hui Xue, Jin-Hua Long, Yun Wang, Yi Jia, Wei Qiu, Jing Zhou, Zong-Yao Wen, Wei-Juan Yao, and Zhu Zeng. 2016. "Biophysical Properties and Motility of Human Mature Dendritic Cells Deteriorated by Vascular Endothelial Growth Factor through Cytoskeleton Remodeling" International Journal of Molecular Sciences 17, no. 11: 1756. https://doi.org/10.3390/ijms17111756