Icariin Promotes Tendon-Bone Healing during Repair of Rotator Cuff Tears: A Biomechanical and Histological Study
Abstract
:1. Introduction
2. Results
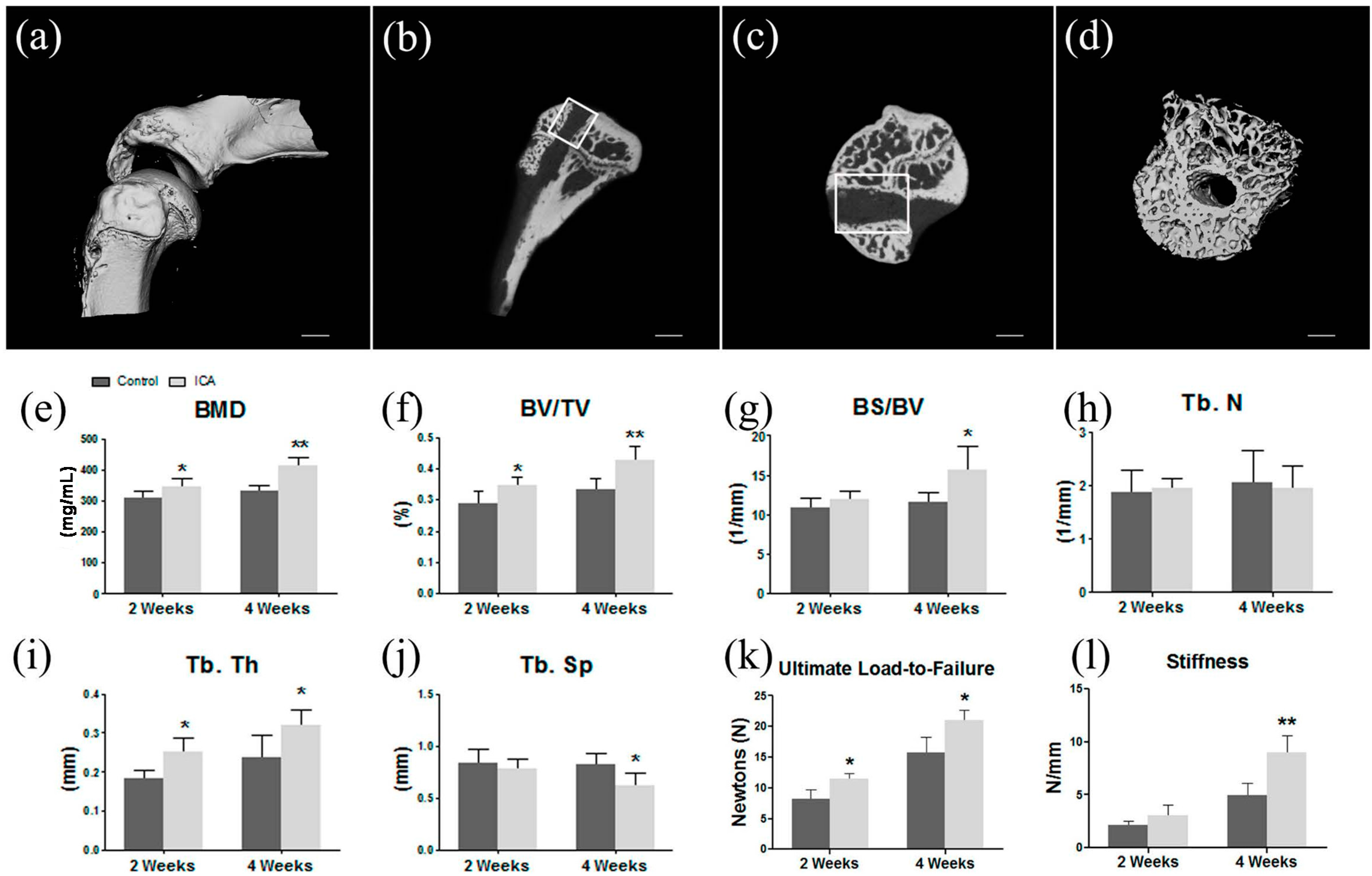
2.1. Micro-CT Analysis Found ICA Enhanced New Bone Formation and Bone Quality
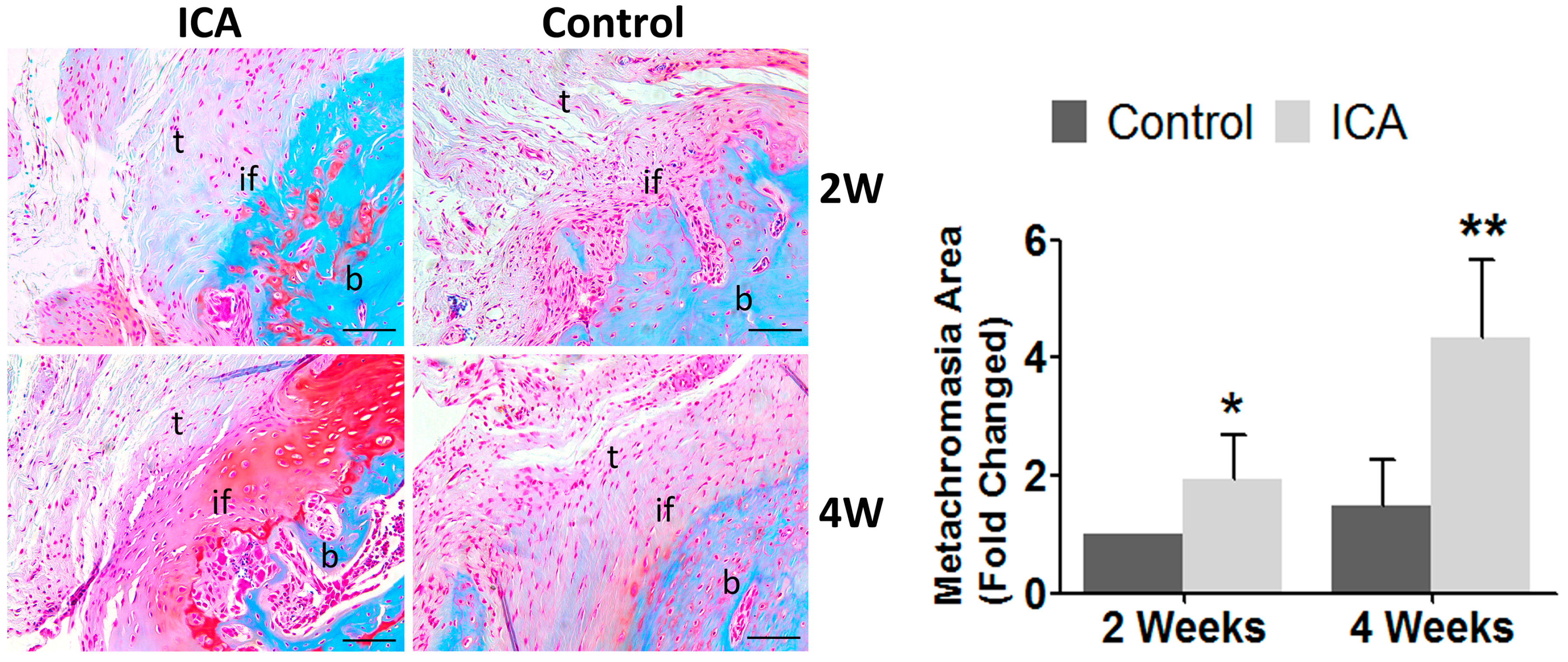
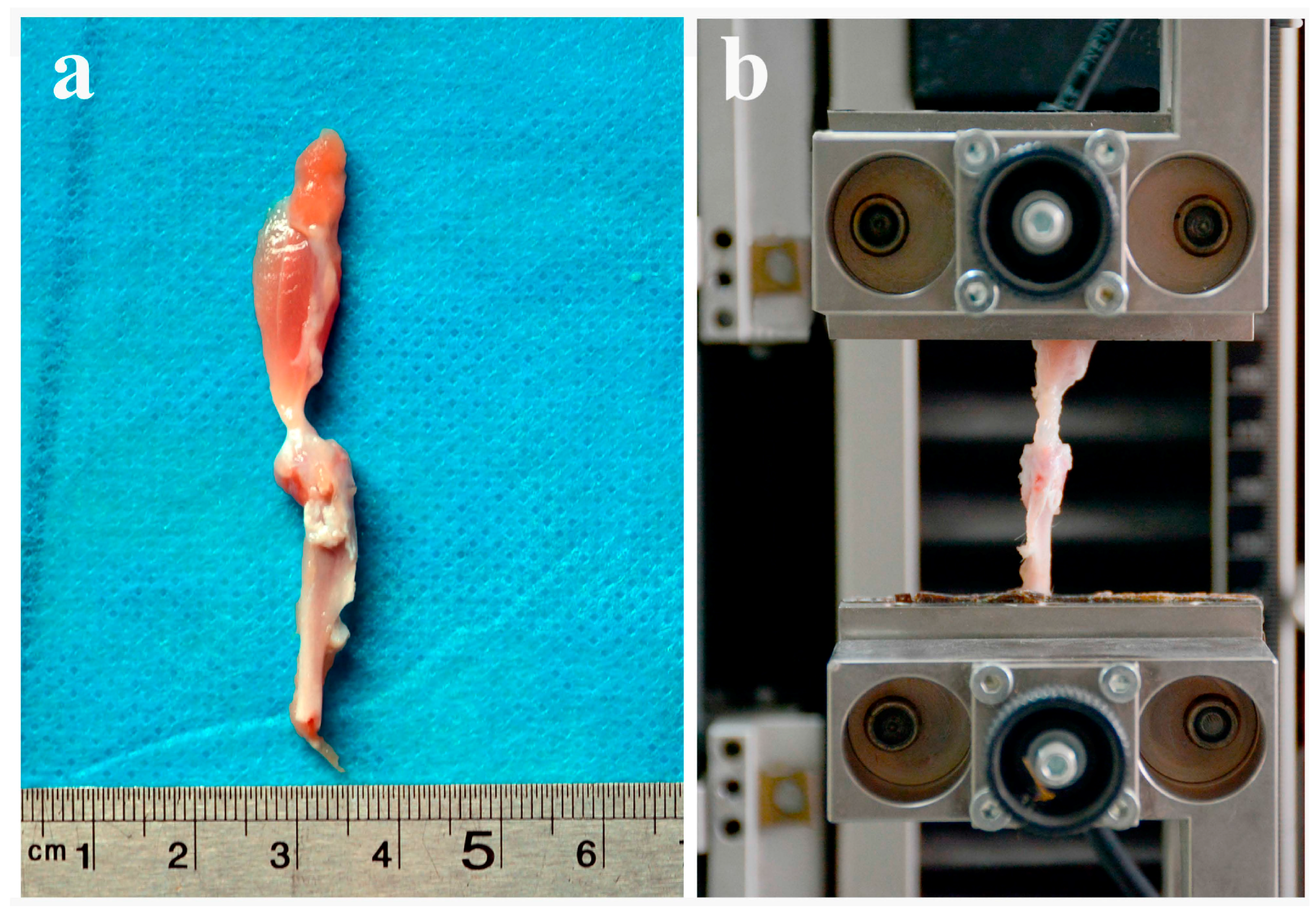
2.2. Biomechanical Testing Showed that ICA Increased the Bonding Strength at the Tendon-Bone Interface
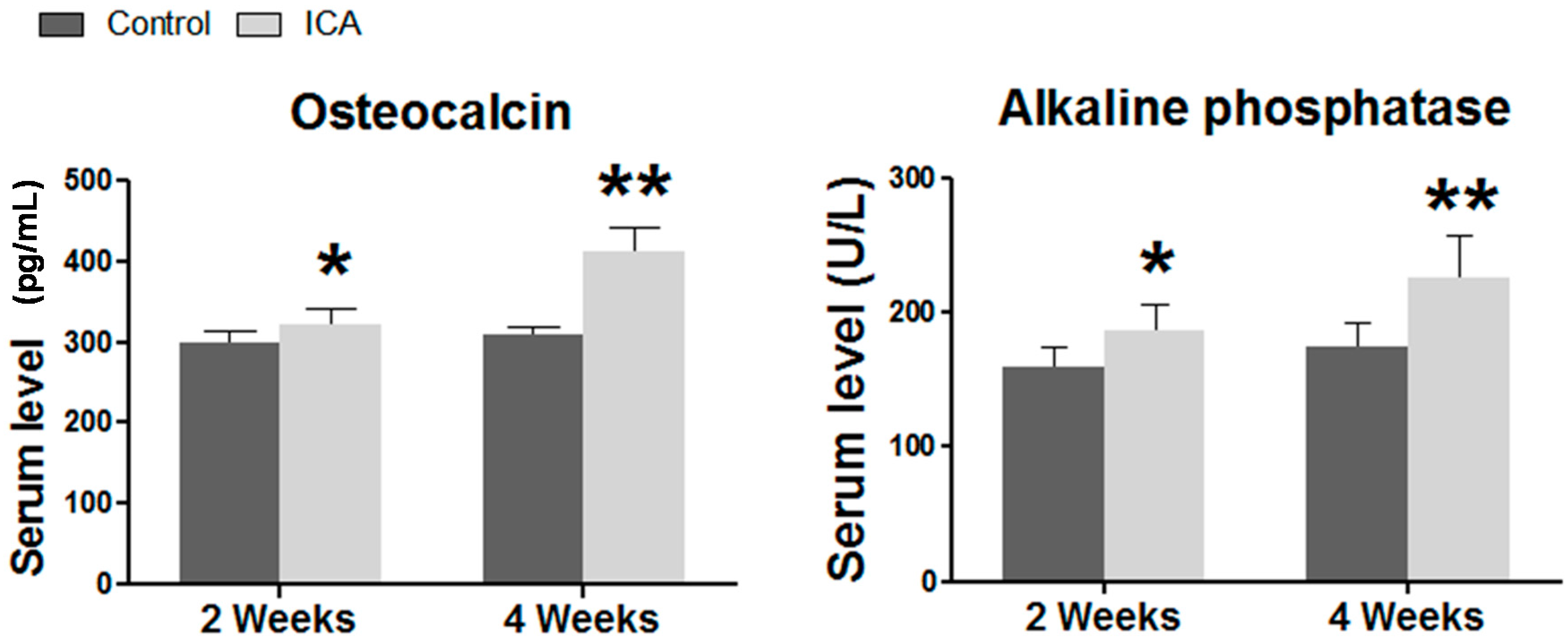
2.3. Serum ELISA Revealed that ICA Increased the Serum Level of Osteogenic Markers, Including Ca, AP, and OCN
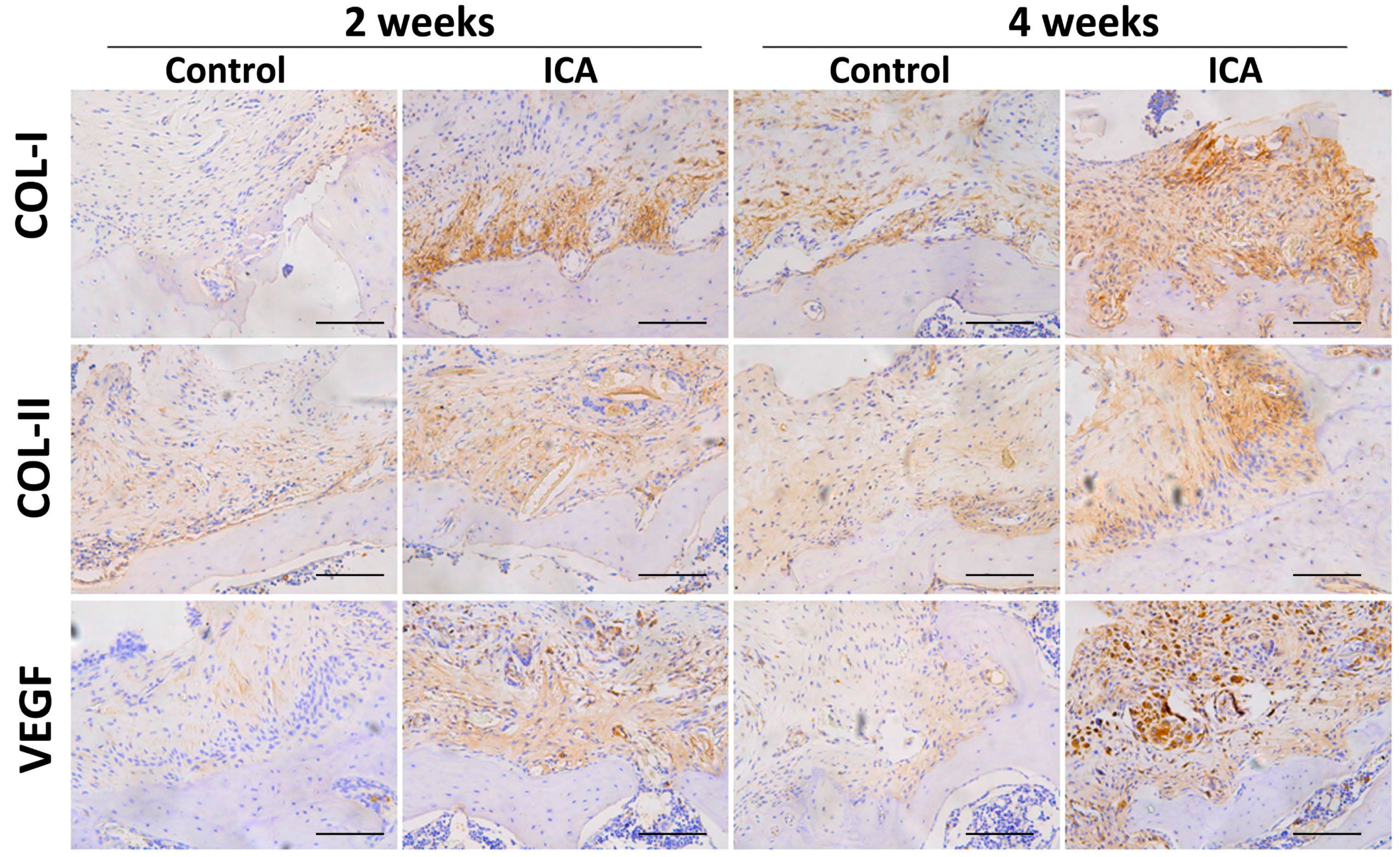
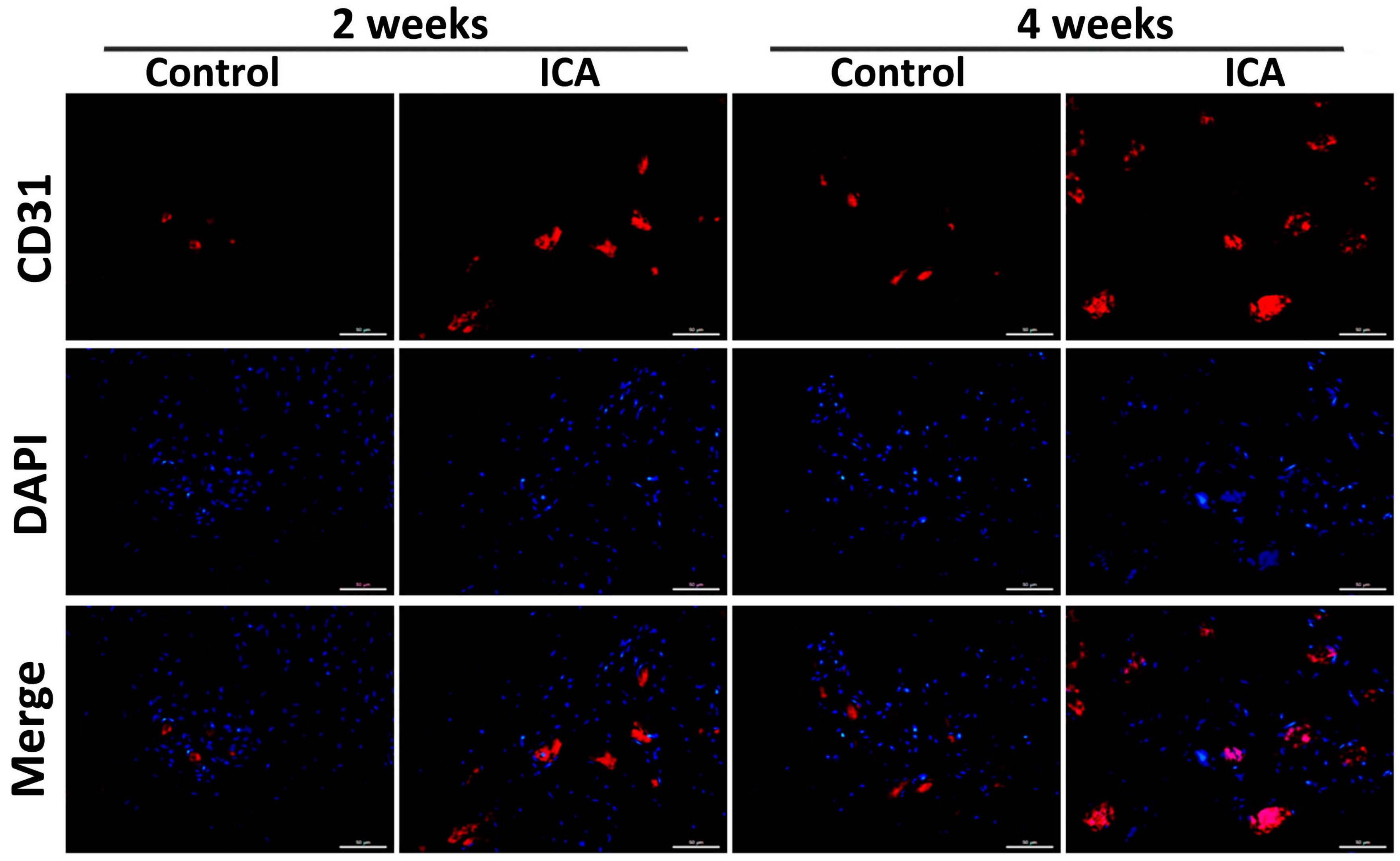
2.4. Immunohistochemical Analysis Showed that ICA Promoted Angiogenesis and Accelerated Tendon-Bone Healing
3. Discussion
4. Materials and Methods
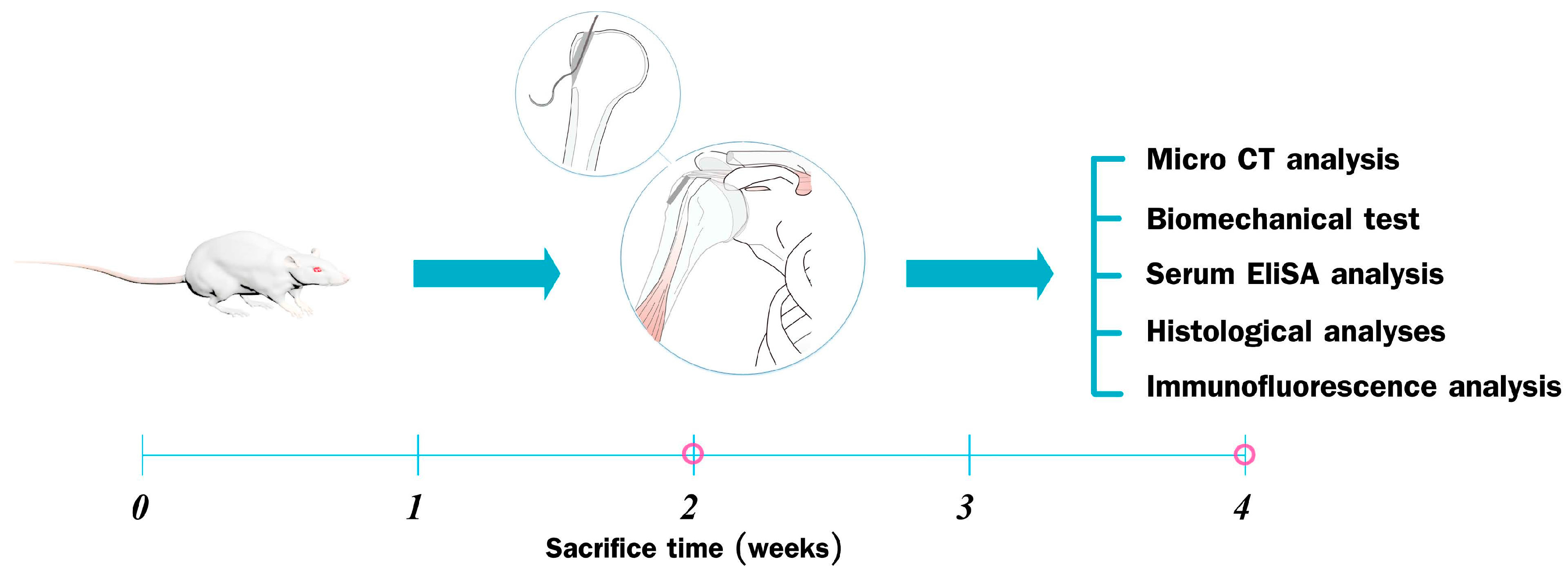
4.1. Study Design
4.2. Surgical Procedure
4.3. Serum Chemistry
4.4. Biomechanical Testing
4.5. Micro-CT Analysis
4.6. Histomorphometry, Immunofluorescence, and Immunocytochemistry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ICA | Icariin |
| SD | Sprague Dawley |
| Ca | Calcium |
| AP | Alkaline Phosphatase |
| OCN | Osteocalcin |
| Col | Collagen |
| VEGF | Vascular Endothelial Growth Factor |
| RCT | Rotator Cuff Tears |
| RC | Rotator Cuff |
| BMD | Bone Mineral Density |
| MSCs | Bone Marrow Stromal Cells |
| ERK | Estrogen Receptor-Mediated Extracellular Signal-Regulated Kinase |
| JNK | c-Jun N-terminal Kinase |
| OPG | Osteoprotegerin |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| ACL | Anterior Cruciate Ligament |
| NS | Normal Saline |
| BS | Bone Surface |
| BV | Bone Volume |
| TV | Total Volume |
| Tb.N | Trabecular Number |
| Tb.Sp | Trabecular Separation |
| Tb.Th | Trabecular Thickness |
References
- Gulotta, L.V.; Nho, S.J.; Dodson, C.C.; Adler, R.S.; Altchek, D.W.; MacGillivray, J.D. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: Part I—Functional outcomes and radiographic healing rates. J. Shoulder Elbow Surg. 2011, 20, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Galatz, L.M.; Ball, C.M.; Teefey, S.A.; Middleton, W.D.; Yamaguchi, K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Jt. Surg. Am. Vol. 2004, 86, 219–224. [Google Scholar]
- Rodeo, S.A.; Potter, H.G.; Kawamura, S.; Turner, A.S.; Kim, H.J.; Atkinson, B.L. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J. Bone Jt. Surg. Am. Vol. 2007, 89, 2485–2497. [Google Scholar] [CrossRef] [PubMed]
- Colvin, A.C.; Egorova, N.; Harrison, A.K.; Moskowitz, A.; Flatow, E.L. National trends in rotator cuff repair. J. Bone Jt. Surg. Am. Vol. 2012, 94, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, J.J.; Samagh, S.P.; Schairer, W.; Singh, G.; Valone, F.H., III; Feeley, B.T. Current trends in rotator cuff repair: Surgical technique, setting, and cost. Arthrosc.: J. Arthrosc. Relat. Surg. 2014, 30, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.A.; Vitale, M.G.; Zivin, J.G.; Braman, J.P.; Bigliani, L.U.; Flatow, E.L. Rotator cuff repair: An analysis of utility scores and cost-effectiveness. J. Bone Jt. Surg. Am. Vol. 2007, 16, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Harryman, D.T., II; Mack, L.A.; Wang, K.Y.; Jackins, S.E.; Richardson, M.L.; Matsen, F.A., III. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J. Bone Jt. Surg. Am. Vol. 1991, 73, 982–989. [Google Scholar]
- Lafosse, L.; Brozska, R.; Toussaint, B.; Gobezie, R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J. Bone Jt. Surg. Am. Vol. 2007, 89, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Domb, B.G.; Glousman, R.E.; Brooks, A.; Hansen, M.; Lee, T.Q.; ElAttrache, N.S. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J. Bone Jt. Surg. Am. Vol. 2008, 90, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.O.; Sileo, M.J.; Grossman, M.G.; Serra-Hsu, F. Single-row modified mason-allen vs. double-row arthroscopic rotator cuff repair: A biomechanical and surface area comparison. Arthrosc.: J. Arthrosc. Relat. Surg. 2008, 24, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, M.F.; Froehlich, V.; Ficklscherer, A.; Wegener, B.; Jansson, V.; Muller, P.E. Biomechanical testing of a new knotless suture anchor compared with established anchors for rotator cuff repair. J. Shoulder Elbow Surg. 2008, 17, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Tocci, S.L.; Tashjian, R.Z.; Leventhal, E.; Spenciner, D.B.; Green, A.; Fleming, B.C. Biomechanical comparison of single-row arthroscopic rotator cuff repair technique vs. transosseous repair technique. J. Shoulder Elbow Surg. 2008, 17, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Rodeo, S.A. Growth factors for rotator cuff repair. Clin. Sports Med. 2009, 28, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Gerber, C.; Fuchs, B.; Hodler, J. The results of repair of massive tears of the rotator cuff. J. Bone Jt. Surg. Am. Vol. 2000, 82, 505–515. [Google Scholar]
- Bishop, J.; Klepps, S.; Lo, I.K.; Bird, J.; Gladstone, J.N.; Flatow, E.L. Cuff integrity after arthroscopic vs. open rotator cuff repair: A prospective study. J. Shoulder Elbow Surg. 2006, 15, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Venkateswaran, B.; Even, T.; Ravenscroft, M.; Copeland, S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J. Bone Jt. Surg. Br. Vol. 2008, 90, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Nho, S.J.; Delos, D.; Yadav, H.; Pensak, M.; Romeo, A.A.; Warren, R.F.; MacGillivray, J.D. Biomechanical and biologic augmentation for the treatment of massive rotator cuff tears. Am. J. Sports Med. 2010, 38, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Seeherman, H.J.; Archambault, J.M.; Rodeo, S.A.; Turner, A.S.; Zekas, L.; D'Augusta, D.; Li, X.J.; Smith, E.; Wozney, J.M. RHBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J. Bone Jt. Surg. Am. Vol. 2008, 90, 2206–2219. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.P.; Chiou, Y.L.; Lin, C.Y. Hyperbaric oxygen-stimulated proliferation and growth of osteoblasts may be mediated through the FGF-2/MEK/ERK 1/2/NF-κAB and PKC/JNK pathways. Connect. Tissue Res. 2010, 51, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Nian, H.; Ma, M.H.; Nian, S.S.; Xu, L.L. Antiosteoporotic activity of icariin in ovariectomized rats. Phytomed.: Int. J. Phytother. Phytopharmacol. 2009, 16, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.K.; Chen, W.F.; Lai, W.P.; Leung, P.C.; Wang, X.L.; Yao, X.S.; Wong, M.S. Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor-dependent osteoblastic functions in UMR 106 cells. Br. J. Pharmacol. 2010, 159, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Sharan, K.; Siddiqui, J.A.; Swarnkar, G.; Maurya, R.; Chattopadhyay, N. Role of phytochemicals in the prevention of menopausal bone loss: Evidence from in vitro and in vivo, human interventional and pharma-cokinetic studies. Curr. Med. Chem. 2009, 16, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, L.; Shi, Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: A 24-month randomized, double-blind and placebo-controlled trial. J. Bone Miner. Res. 2007, 22, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.K.; Guo, X.Y.; Ge, B.F.; Zhen, P.; Ma, X.N.; Zhou, J.; Ma, H.P.; Xian, C.J.; Chen, K.M. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone 2014, 66, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.G.; Chen, K.M.; Xian, C.J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J. Cell. Physiol. 2013, 228, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhao, J.; Zhang, X.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H.; Liu, M.H. Icariin isolated from epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and CBFA1 expression. Phytomed.: Int. J. Phytother. Phytopharmacol. 2010, 17, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H. Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF-κAB regulated HIF-1α and PGE(2) synthesis. Phytomed.: Int. J. Phytother. Phytopharmacol. 2011, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Xu, H.; Zhao, Y.J.; Tang, D.Z.; Xu, G.H.; Holz, J.; Wang, J.; Cheng, S.D.; Shi, Q.; Wang, Y.J. Icariin augments bone formation and reverses the phenotypes of osteoprotegerin-deficient mice through the activation of Wnt/β-catenin-BMP signaling. Evid.-Based Complement. Altern. Med. 2013, 2013, 652317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ohba, S.; Komiyama, Y.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin: A potential osteoinductive compound for bone tissue engineering. Tissue Eng. Part A 2010, 16, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.B.; Kawamura, S.; Deng, X.H.; Ying, L.; Schneidkraut, J.; Hays, P.; Rodeo, S.A. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: A study of RHBMP-2 and noggin. Am. J. Sports Med. 2007, 35, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.N.; Kim, H.M.; Sakiyama-Elbert, S.; Galatz, L.M.; Havlioglu, N.; Thomopoulos, S. Sustained delivery of transforming growth factor β three enhances tendon-to-bone healing in a rat model. J. Orthop. Res. 2011, 29, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kuroda, R.; Ishida, K.; Kubo, S.; Matsumoto, T.; Mifune, Y.; Kinoshita, K.; Tei, K.; Akisue, T.; Tabata, Y.; et al. Enhancement of tendon-bone osteointegration of anterior cruciate ligament graft using granulocyte colony-stimulating factor. Am. J. Sports Med. 2008, 36, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Mifune, Y.; Inui, A.; Sakata, R.; Muto, T.; Takase, F.; Ueda, Y.; Kataoka, T.; Kokubu, T.; Kuroda, R.; et al. Rotator cuff repair using cell sheets derived from human rotator cuff in a rat model. J. Orthop. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.J.; Wicker, J.F.; Dines, J.S.; Bonasser, L.; Razzano, P.; Dines, D.M.; Grande, D.A. Histologic stages of healing correlate with restoration of tensile strength in a model of experimental tendon repair. HSS J. 2010, 6, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Serrat, M.A.; Efaw, M.L.; Williams, R.M. Hindlimb heating increases vascular access of large molecules to murine tibial growth plates measured by in vivo multiphoton imaging. J. Appl. Physiol. 2014, 116, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Odgren, P.R.; Witwicka, H.; Reyes-Gutierrez, P. The cast of clasts: Catabolism and vascular invasion during bone growth, repair, and disease by osteoclasts, chondroclasts, and septoclasts. Connect. Tissue Res. 2016, 57, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Mifune, Y.; Matsumoto, T.; Ota, S.; Nishimori, M.; Usas, A.; Kopf, S.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Therapeutic potential of anterior cruciate ligament-derived stem cells for anterior cruciate ligament reconstruction. Cell Transplant. 2012, 21, 1651–1665. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Matsumoto, T.; Kubo, S.; Matsushita, T.; Sasaki, H.; Nishizawa, Y.; Matsuzaki, T.; Saito, T.; Nishida, K.; Tabata, Y.; et al. Local administration of low-dose simvastatin-conjugated gelatin hydrogel for tendon-bone healing in anterior cruciate ligament reconstruction. Tissue Eng. Part A 2013, 19, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kubo, S.; Sasaki, K.; Kawakami, Y.; Oka, S.; Sasaki, H.; Takayama, K.; Tei, K.; Matsushita, T.; Mifune, Y.; et al. Acceleration of tendon-bone healing of anterior cruciate ligament graft using autologous ruptured tissue. Am. J. Sports Med. 2012, 40, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Soslowsky, L.J.; Carpenter, J.E.; DeBano, C.M.; Banerji, I.; Moalli, M.R. Development and use of an animal model for investigations on rotator cuff disease. J. Shoulder Elbow Surg. 1996, 5, 383–392. [Google Scholar] [CrossRef]
- Bhattacharya, V.; Shi, Q.; Ishida, A.; Sauvage, L.R.; Hammond, W.P.; Wu, M.H. Administration of granulocyte colony-stimulating factor enhances endothelialization and microvessel formation in small-caliber synthetic vascular grafts. J. Vasc. Surg. 2000, 32, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Chu, K.; Lee, S.T.; Kim, S.J.; Sinn, D.I.; Kim, S.U.; Kim, M.; Roh, J.K. Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with stat activation. Brain Res. 2006, 1073–1074, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Atesok, K.; Fu, F.H.; Wolf, M.R.; Ochi, M.; Jazrawi, L.M.; Doral, M.N.; Lubowitz, J.H.; Rodeo, S.A. Augmentation of tendon-to-bone healing. J. Bone Jt. Surg. Am. Vol. 2014, 96, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tao, Y.; Ping, Z.; Zhang, W.; Hu, X.; Wang, Y.; Wang, L.; Shi, J.; Wu, X.; Yang, H.; et al. Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/β-catenin signaling pathway in vivo and in vitro. Sci. Rep. 2016, 6, 23827. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.J.; Tohyama, H.; Kondo, E.; Yoshikawa, T.; Muneta, T.; Shinomiya, K.; Yasuda, K. Effects of local administration of vascular endothelial growth factor on properties of the in situ frozen-thawed anterior cruciate ligament in rabbits. Am. J. Sports Med. 2006, 34, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Tohyama, H.; Katsura, T.; Kondo, E.; Kotani, Y.; Matsumoto, H.; Toyama, Y.; Yasuda, K. Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am. J. Sports Med. 2006, 34, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Wang, Z.; Xin, F.; Xin, H. Wnt5a is associated with the differentiation of bone marrow mesenchymal stem cells in vascular calcification by connecting with different receptors. Mol. Med. Rep. 2014, 10, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Huang, H.Y.; Pai, C.H. Shock wave-enhanced neovascularization at the tendon-bone junction: An experiment in dogs. J. Foot Ankle Surg. 2002, 41, 16–22. [Google Scholar] [CrossRef]
- Chung, B.H.; Kim, J.D.; Kim, C.K.; Kim, J.H.; Won, M.H.; Lee, H.S.; Dong, M.S.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/AKT/eNOS-dependent signal pathways in human endothelial cells. Biochem. Biophys. Res. Commun. 2008, 376, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Le, A.W.; Shan, L.; Wang, Z.H.; Dai, X.Y.; Xiao, T.H.; Zuo, R. Effects of icariin on the expression of ER, VEGF, and KDR in the endometrial cells of thin endometrium. Genet. Mol. Res. 2015, 14, 11250–11258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Chen, K.M.; Ge, B.F.; Zhen, P.; Gao, Y.H.; Ma, H.P. Comparison research with icariin and genistein by anti-inflammatory reaction and angiogenesis pathway to inhibit bone loss on ovariectomized rats. J. Chin. Med. Mater. 2014, 37, 627–631. [Google Scholar]
- Xin, H.; Zhou, F.; Liu, T.; Li, G.Y.; Liu, J.; Gao, Z.Z.; Bai, G.Y.; Lu, H.; Xin, Z.C. Icariin ameliorates streptozotocin-induced diabetic retinopathy in vitro and in vivo. Int. J. Mol. Sci. 2012, 13, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.S.; Callaci, J.J.; Juknelis, D.; Marra, G.; Tonino, P.; Freedman, K.B.; Wezeman, F.H. The effect of corticosteroid on collagen expression in injured rotator cuff tendon. J. Bone Jt. Surg. Am. Vol. 2006, 88, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Corazza, O.; Martinotti, G.; Santacroce, R.; Chillemi, E.; Di Giannantonio, M.; Schifano, F.; Cellek, S. Sexual enhancement products for sale online: Raising awareness of the psychoactive effects of yohimbine, maca, horny goat weed, and Ginkgo biloba. BioMed Res. Int. 2014, 2014, 841798. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhou, W.; Liu, S.; Chen, P.; Wu, H. Icariin stimulates the proliferation of rat bone mesenchymal stem cells via ERK and p38 MAPK signaling. Int. J. Clin. Exp. Med. 2015, 8, 7125–7133. [Google Scholar] [PubMed]
- Zeng, L.; Wang, W.; Rong, X.F.; Zhong, Y.; Jia, P.; Zhou, G.Q.; Li, R.H. Chondroprotective effects and multi-target mechanisms of icariin in IL-1 β-induced human sw 1353 chondrosarcoma cells and a rat osteoarthritis model. Int. Immunopharmacol. 2014, 18, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.-X.; Gao, P.; Xu, H.-B. The safety evaluation of herba epimedii water extract. Carcinog. Teratog. Mutagen. 2006, 18, 439–442. [Google Scholar]
- Li, M.; Zhang, N.D.; Wang, Y.; Han, T.; Jiang, Y.P.; Rahman, K.; Qin, L.P.; Xin, H.L.; Zhang, Q.Y. Coordinate regulatory osteogenesis effects of icariin, timosaponin b II and ferulic acid from traditional chinese medicine formulas on UMR-106 osteoblastic cells and osteoblasts in neonatal rat calvaria cultures. J. Ethnopharmacol. 2016, 185, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Shen, J.; Wang, M.; Cui, J.; Wang, Y.; Zhu, S.; Zhang, W.; Yang, H.; Xu, Y.; Geng, D. Icariin protects against titanium particle-induced osteolysis and inflammatory response in a mouse calvarial model. Biomaterials 2015, 60, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wignall, N.; Brown, E.S. An open-label pilot study of icariin for co-morbid bipolar and alcohol use disorder. Am. J. Drug Alcohol Abuse 2016, 42, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Galli, G.V.; Dal Cero, E.; Belluti, F.; Matera, R.; Zironi, E.; Pagliuca, G.; Bosisio, E. Potent inhibition of human phosphodiesterase-5 by icariin derivatives. J. Natl. Prod. 2008, 71, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xin, Z.C.; Liu, W.J.; Yang, Y.M.; Liu, G.; Chen, L.; Fu, J.; Wang, L.L. Effects of icariin on the erectile function and expression of nitrogen oxide synthase isoforms in corpus cavernosum of arterigenic erectile dysfunction rat model. Zhonghua Yi Xue Za Zhi 2004, 84, 954–957. [Google Scholar] [PubMed]
- Zhang, J.; Wang, Y.B.; Ma, C.G.; Liu, T.; Li, W.R.; Gong, Y.Q.; Xin, Z.C. Icarisid ii, a PDE5 inhibitor from epimedium wanshanense, increases cellular cgmp by enhancing NOS in diabetic ed rats corpus cavernosum tissue. Andrologia 2012, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Angeline, M.E.; Ma, R.; Pascual-Garrido, C.; Voigt, C.; Deng, X.H.; Warren, R.F.; Rodeo, S.A. Effect of diet-induced vitamin D deficiency on rotator cuff healing in a rat model. Am. J. Sports Med. 2014, 42, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, S.; Sandmann, G.H.; Walz, L.; Hoppe, H.; Beitzel, K.; Wexel, G.; Tian, W.; Winter, G.; Imhoff, A.B. Refixation of the supraspinatus tendon in a rat model—Influence of continuous growth factor application on tendon structure. J. Orthop. Res. 2013, 31, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, S.; Walz, L.; Sandmann, G.H.; Hoppe, H.; Beitzel, K.; Wexel, G.; Battmann, A.; Vogt, S.; Hinterwimmer, S.; Imhoff, A.B. Rotator cuff changes in a full thickness tear rat model: Verification of the optimal time interval until reconstruction for comparison to the healing process of chronic lesions in humans. Arch. Orthop. Trauma Surg. 2011, 131, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Soslowsky, L.J.; Thomopoulos, S.; Tun, S.; Flanagan, C.L.; Keefer, C.C.; Mastaw, J.; Carpenter, J.E. Neer award 1999: Overuse activity injures the supraspinatus tendon in an animal model: A histologic and biomechanical study. J. Shoulder Elbow Surg. 2000, 9, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Kovacevic, D.; Montgomery, S.; Ehteshami, J.R.; Packer, J.D.; Rodeo, S.A. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am. J. Sports Med. 2010, 38, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Hettrich, C.M.; Rodeo, S.A.; Hannafin, J.A.; Ehteshami, J.; Shubin Stein, B.E. The effect of muscle paralysis using botox on the healing of tendon to bone in a rat model. J. Shoulder Elbow Surg. 2011, 20, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mtor in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Fox, A.J.; Kovacevic, D.; Deng, X.H.; Warren, R.F.; Rodeo, S.A. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am. J. Sports Med. 2010, 38, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, D.; Fox, A.J.; Bedi, A.; Ying, L.; Deng, X.H.; Warren, R.F.; Rodeo, S.A. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am. J. Sports Med. 2011, 39, 811–819. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Zhang, W.; Wang, S.; Jiang, S.; Yu, Y.; Chen, E.; Xue, D.; Chen, J.; He, R. Icariin Promotes Tendon-Bone Healing during Repair of Rotator Cuff Tears: A Biomechanical and Histological Study. Int. J. Mol. Sci. 2016, 17, 1780. https://doi.org/10.3390/ijms17111780
Ye C, Zhang W, Wang S, Jiang S, Yu Y, Chen E, Xue D, Chen J, He R. Icariin Promotes Tendon-Bone Healing during Repair of Rotator Cuff Tears: A Biomechanical and Histological Study. International Journal of Molecular Sciences. 2016; 17(11):1780. https://doi.org/10.3390/ijms17111780
Chicago/Turabian StyleYe, Chenyi, Wei Zhang, Shengdong Wang, Shuai Jiang, Yuanbin Yu, Erman Chen, Deting Xue, Jianzhong Chen, and Rongxin He. 2016. "Icariin Promotes Tendon-Bone Healing during Repair of Rotator Cuff Tears: A Biomechanical and Histological Study" International Journal of Molecular Sciences 17, no. 11: 1780. https://doi.org/10.3390/ijms17111780





