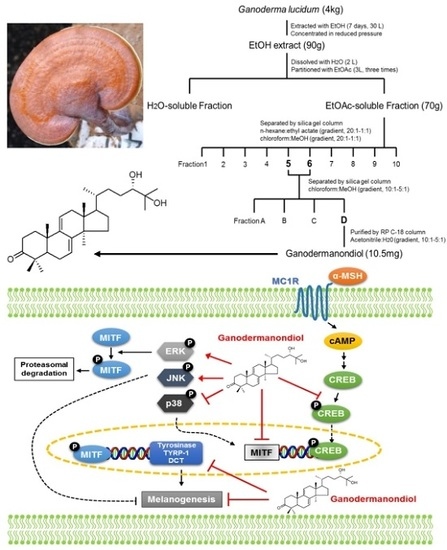
Effects of Ganodermanondiol, a New Melanogenesis Inhibitor from the Medicinal Mushroom Ganoderma lucidum
Abstract
:1. Introduction
2. Results and Discussion
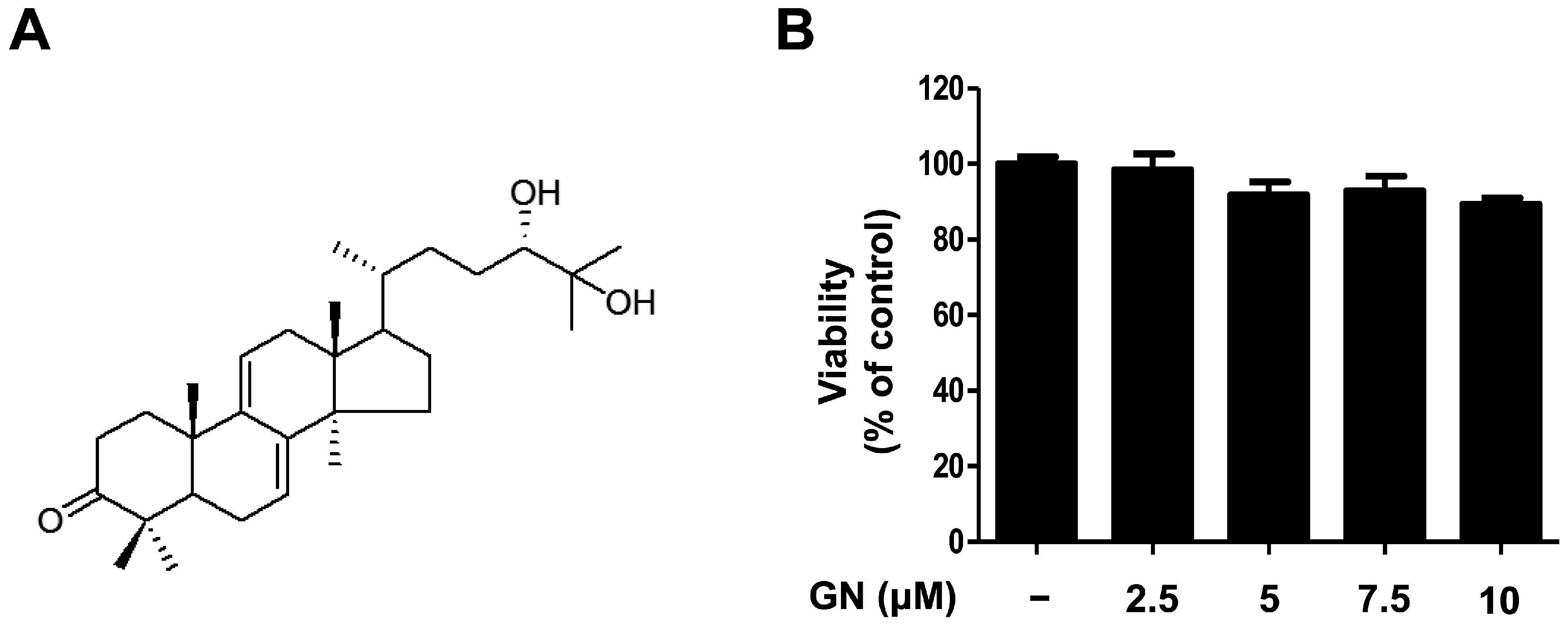
2.1. Chemical Structure and Cytotoxicity of Ganodermanondiol Isolated from Ganoderma lucidum on B16F10 Melanoma Cells
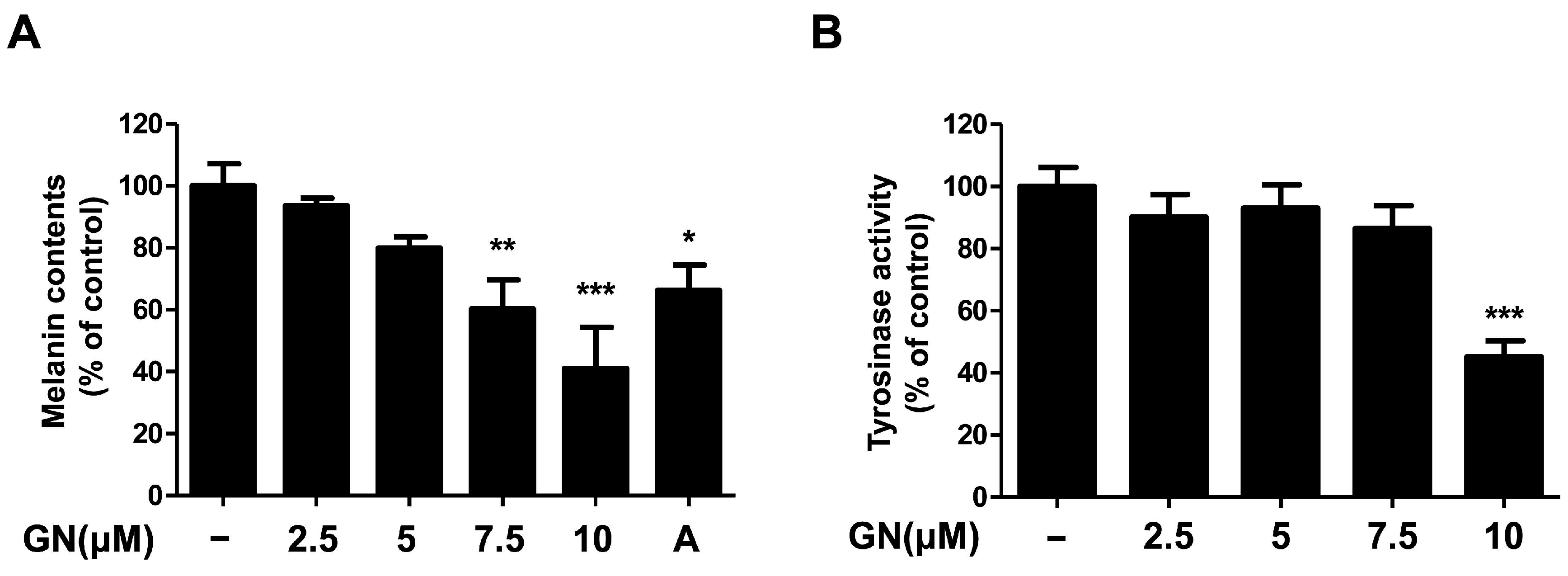
2.2. Effects of Ganodermanondiol on Melanin Contents and Tyrosinase Activity of B16F10 Cells
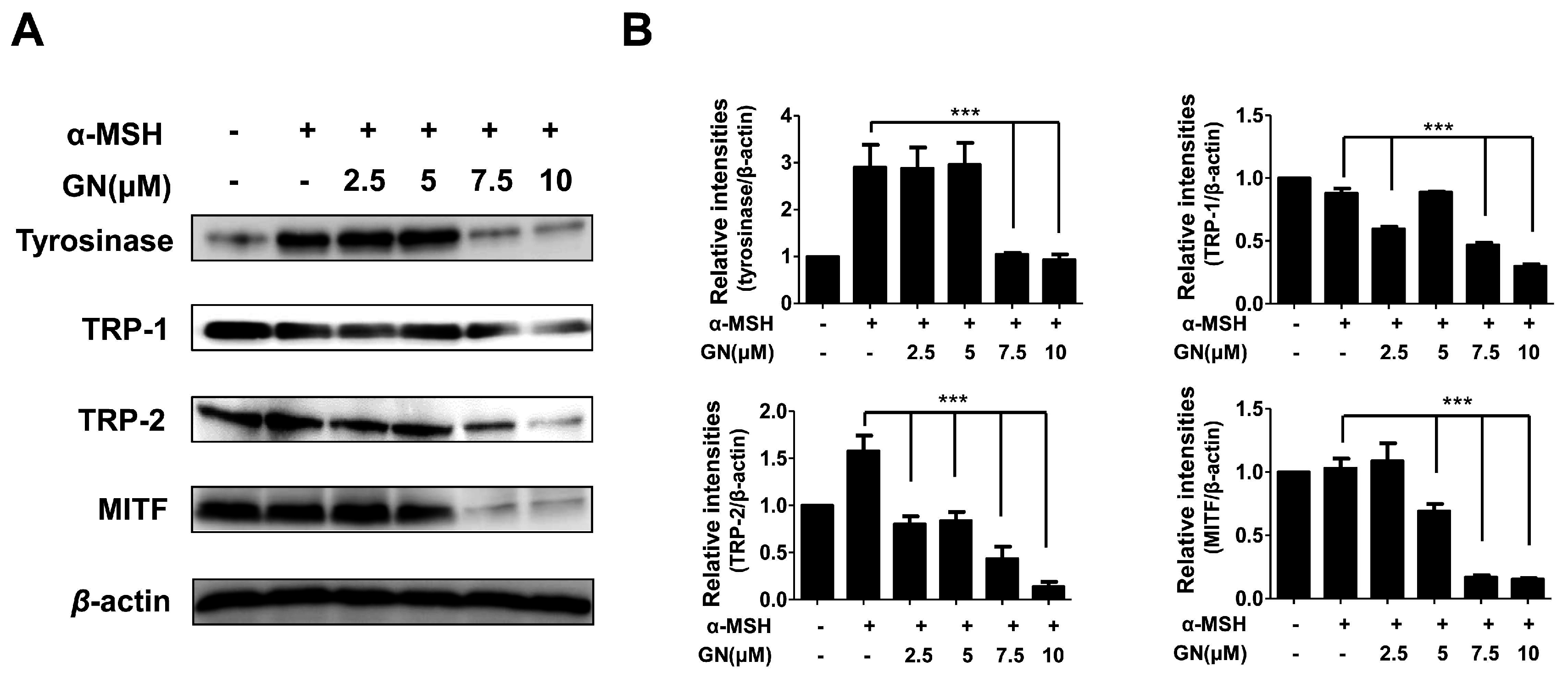
2.3. Effects of Ganodermanondiol on Cellular Melanogenesis-Related Proteins and MITF Protein Expression in B16F10 Cells
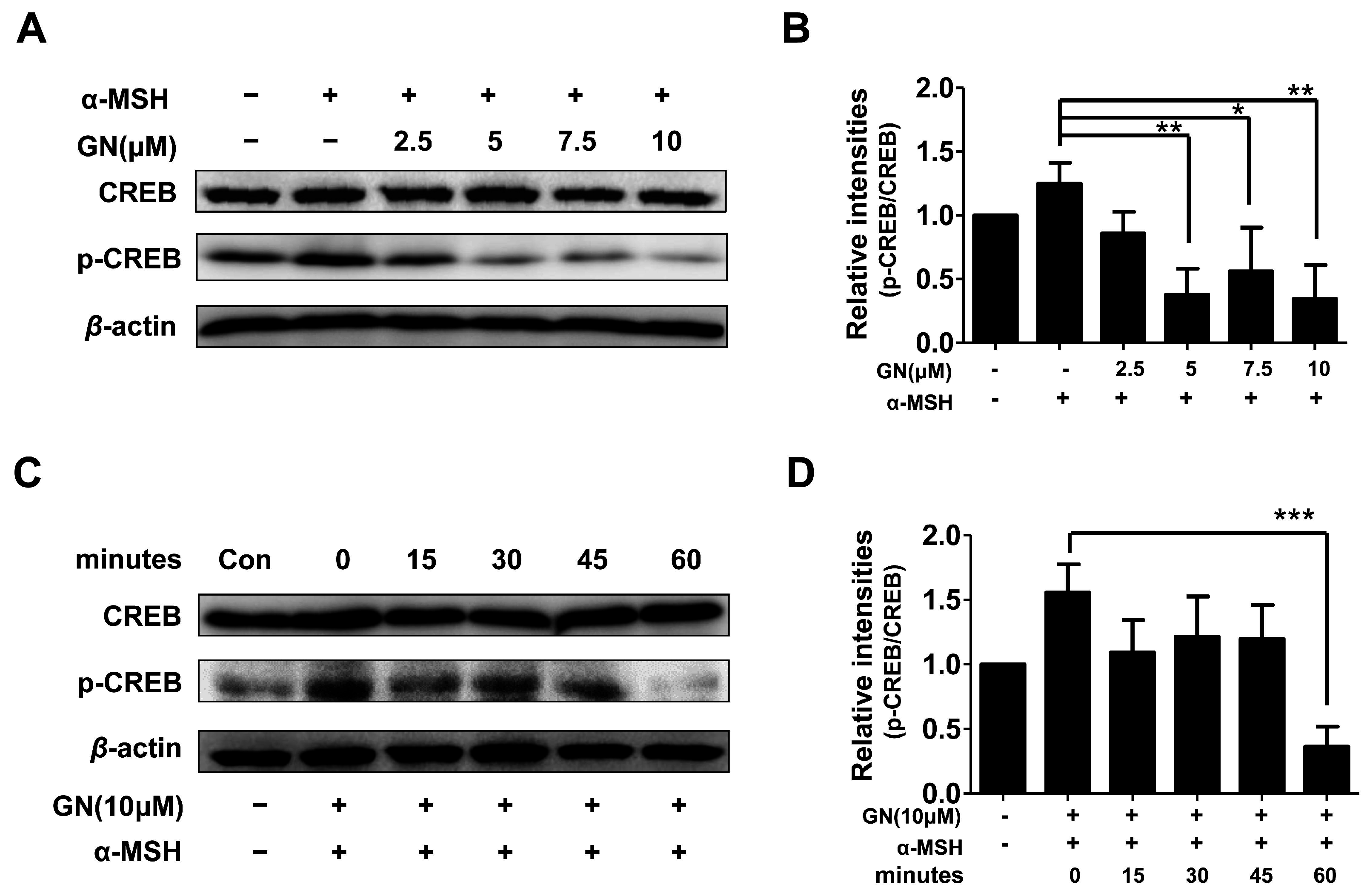
2.4. Effects of Ganodermanondiol on cAMP Response Element Binding Protein (CREB) Phosphorylation in B16F10 Cells
2.5. Effects of Ganodermanondiol on Phosphorylated (p)-p38, p-c-Jun N-Terminal Kinase (JNK) and p-Extracellular Signal-Regulated Kinase (ERK) Protein Levels in B16F10 Cells
3. Materials and Methods
3.1. Reagents
3.2. G. lucidum
Isolation of Ganodermanondiol
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. Tyrosinase Activity in B16F10 Cells
3.6. Melanin Contents of B16F10 Cells
3.7. Western Blot Analysis
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| α-MSH | alpha-melanocyte-stimulating hormone |
| B16F10 | Mus musculus skin melanoma cell |
| HQ | hydroquinone |
| l-DOPA | l-3,4-dihydroxyphenylalanine |
| MITF | microphthalmia-associated transcription factor |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| TRP | tyrosinase-related protein |
| ERK | extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| CREB | cyclic adenosine monophosphate (cAMP) response element binding protein |
References
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-Tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferrer, Á.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochem. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Jimbow, K.; Obata, H.; Pathak, M.A.; Fitzpatrick, T.B. Mechanism of depigmentation by hydroquinone. J. Investig. Dermatol. 1974, 62, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.A.; Fitzpatrick, T.B.; Kraus, E.W. Usefulness of retinoic acid in the treatment of melasma. J. Am. Acad. Dermatol. 1986, 15, 894–899. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wood, J.W. A possible mechanism of action for azelaic acid in the human epidermis. Arch. Dermatol. Res. 1990, 282, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Chazarra, S.; García-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar] [PubMed]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim. Biophys. Acta 1991, 1073, 85–90. [Google Scholar] [CrossRef]
- Decaprio, A.P. The toxicology of hydroquinone—Relevance to occupational and environmental exposure. Crit. Rev. Toxicol. 1999, 29, 283–330. [Google Scholar] [CrossRef] [PubMed]
- McGregor, D. Hydroquinone: An evaluation of the human risks from its carcinogenic and mutagenic properties. Crit. Rev. Toxicol. 2007, 37, 887–914. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H.S. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Aburjai, T.; Natsheh, F.M. Palnts used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; Lin, R.D.; Hsu, F.L.; Huang, Y.H.; Chang, H.C.; Huang, C.Y.; Lee, M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.S.; Pawar, H.A. Herbal cosmetics and cosmeceuticals: An overview. Nat. Prod. Chem. Res. 2015, 3, 170. [Google Scholar] [CrossRef]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2003; pp. 11–12. [Google Scholar]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma lucidum (Lingzhi or Reishi). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Watchel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 1–31. [Google Scholar]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [PubMed]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.; Bisen, P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.C.; Tsai, M.L.; Chen, C.C.; Chang, S.J.; Tseng, C.H. Effects on tyrosinase activity by the extracts of Ganoderma lucidum and related mushrooms. Mycopathologia 2008, 166, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Nakamura, N.; Miyashiro, H.; Bae, K.W.; Hattori, M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998, 46, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Gao, J.J.; Hattori, M.; Lee, H.K.; Kim, Y.H. Anticomplement activity of terpenoids from the spores of Ganoderma lucidum. Planta Med. 2001, 67, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lee, D.S.; Kang, Y.; Yao, N.Q.; An, R.B.; Kim, Y.C. Protective effect of ganodermanondiol isolated from the Lingzhi mushroom against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 2013, 53, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yokokawa, Y.; Hatao, M.; Naganuma, M.; Tomita, Y. Comparison of the melanogenesis in human black and light brown melanocytes. J. Dermatol. Sci. 1997, 14, 199–206. [Google Scholar] [CrossRef]
- Fang, D.; Kute, T.; Setaluri, V. Regulation of tyrosinase-related protein-2 (TYRP2) in human melanocytes: Relationship to growth and morphology. Pigment Cell Melanoma Res. 2001, 14, 132–139. [Google Scholar] [CrossRef]
- Shibahara, S.; Yasumoto, K.I.; Amae, S.; Udono, T.; Watanabe, K.I.; Saito, H.; Takeda, K. Regulation of pigment cell-specific gene expression by MITF. Pigment Cell Melanoma Res. 2000, 13, 98–102. [Google Scholar] [CrossRef]
- Luger, T.A.; Scholzen, T.; Grabbe, S. The role of alpha-melanocyte-stimulating hormone in cutaneous biology. J. Investig. Dermatol. Symp. Proc. 1997, 2, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Buscà, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Melanoma Res. 2000, 13, 60–69. [Google Scholar] [CrossRef]
- Bentley, N.J.; Eisen, T.; Goding, C.R. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol. Cell. Biol. 1994, 14, 7996–8006. [Google Scholar] [CrossRef] [PubMed]
- Yavuzer, U.; Keenan, E.; Lowings, P.; Vachtenheim, J.; Currie, G.; Goding, C.R. The Microphthalmia gene product interacts with the retinoblastoma protein in vitro and is a target for deregulation of melanocyte-specific transcription. Oncogene 1995, 10, 123–134. [Google Scholar] [PubMed]
- Bertolotto, C.; Bille, K.; Ortonne, J.P.; Ballotti, R. Regulation of tyrosinase gene expression by cAMP in B16 melanoma cells involves two CATGTG motifs surrounding the TATA box: Implication of the microphthalmia gene product. J. Cell Biol. 1996, 134, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Yim, N.H.; Im, M.; Jung, Y.P.; Liang, C.; Cho, W.K.; Ma, J.Y. Ssanghwa-tang, an oriental herbal cocktail, exerts anti-melanogenic activity by suppression of the p38 MAPK and PKA signaling pathways in B16F10 cells. BMC Complement. Altern. Med. 2013, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.F.; Huang, C.C.; Lee, M.Y.; Lin, Y.S. Fermented broth in tyrosinase- and melanogenesis inhibition. Molecules 2014, 19, 13122–13135. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, H.N.; Kim, Y.R.; Choi, W.Y.; Choi, Y.H.; Shin, H.K.; Choi, B.T. Partially purified components of Nardostachys chinensis suppress melanin synthesis through ERK and Akt signaling pathway with cAMP down-regulation in B16F10 cells. J. Ethnopharmacol. 2011, 137, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: Perspective of anti-pigmenting agents. Int. J. Mol. Sci. 2014, 15, 8293–8315. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Andersen, R.; Maibach, H.I. What do we know about depigmenting agents? Cosmet. Toilet. 2015, 130, 26–29. [Google Scholar]
- Valverde, P.; Benedito, E.; Solano, F.; Oaknin, S.; Lozano, J.A.; García-Borrón, J.C. Melatonin antagonizes α-melanocyte-stimulating hormone enhancement of melanogenesis in mouse melanoma cells by blocking the hormone-induced accumulation of the C Locus tyrosinase. Eur. J. Biochem. 1995, 232, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell. Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Jeong, I.Y.; Shin, H.E.; Song, S.G.; Kim, N.D.; Jung, S.H.; Hong, J.T.; Lee, S.H.; Han, S.B.; Kim, Y.S. Downregulation of melanocyte-specific facultative melanogenesis by 4-hydroxy-3-methoxycinnamaldehyde acting as a cAMP antagonist. J. Investig. Dermatol. 2014, 134, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, W.J.; Chang, S.E.; Lee, G.Y. Hesperidin, a popular antioxidant inhibits melanogenesis via Erk1/2 mediated MITF degradation. Int. J. Mol. Sci. 2015, 16, 18384–18395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.Y.; Yin, W.H.; Wang, M.R.; Dang, Y.Y.; Ye, X.Y. Andrographolide suppresses melanin synthesis through Akt/GSK3β/β-catenin signal pathway. J. Dermatol. Sci. 2015, 79, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Choi, W.H.; Baek, S.H.; Woo, W.H. Effect of quercetin on melanogenesis in melan-a melanocyte cells. Korean J. Pharmacogn. 2002, 33, 245–251. [Google Scholar]
- Chun, H.J.; Hwang, S.G.; Kim, C.K.; Jeon, B.H.; Baek, S.H.; Woo, W.H. In vitro modulation of proliferation and melanization of B16/F10 melanoma cells by quercetin. J. Pharm. Soc. Korea 2002, 46, 75–80. [Google Scholar]
- Kim, Y.J. Hyperin and quercetin modulate oxidative stress-induced melanogenesis. Biol. Pharm. Bull. 2012, 35, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, S.; Matsuzaki, K.; Kitatani, K. Quercetin stimulates melanogenesis in hair follicle melanocyte of the mouse. Tokai J. Exp. Clin. Med. 2013, 38, 129–134. [Google Scholar] [PubMed]
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Osamura, R.Y. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and normal human melanocytes. Pigment Cell Melanoma Res. 2004, 17, 66–73. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.K.; Brożyna, A.A.; Janjetovic, Z.; Brooks, D.L.P.; Schwab, L.P.; Skobowiat, C.; Jóźwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jóźwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Kim, H.-I.; Kim, J.-H.; Kwon, O.-C.; Son, E.-S.; Lee, C.-S.; Park, Y.-J. Effects of Ganodermanondiol, a New Melanogenesis Inhibitor from the Medicinal Mushroom Ganoderma lucidum. Int. J. Mol. Sci. 2016, 17, 1798. https://doi.org/10.3390/ijms17111798
Kim J-W, Kim H-I, Kim J-H, Kwon O-C, Son E-S, Lee C-S, Park Y-J. Effects of Ganodermanondiol, a New Melanogenesis Inhibitor from the Medicinal Mushroom Ganoderma lucidum. International Journal of Molecular Sciences. 2016; 17(11):1798. https://doi.org/10.3390/ijms17111798
Chicago/Turabian StyleKim, Ji-Woong, Hong-Il Kim, Jong-Hyeon Kim, O-Chul Kwon, Eun-Suk Son, Chang-Soo Lee, and Young-Jin Park. 2016. "Effects of Ganodermanondiol, a New Melanogenesis Inhibitor from the Medicinal Mushroom Ganoderma lucidum" International Journal of Molecular Sciences 17, no. 11: 1798. https://doi.org/10.3390/ijms17111798