Bidirectional Estrogen-Like Effects of Genistein on Murine Experimental Autoimmune Ovarian Disease
Abstract
:1. Introduction
2. Results
2.1. Regularization of the Estrous Cycle by Oral Administration of Genistein
2.2. Estradiol Increased While Those that Are Follicle-Stimulating Hormone and Luteinizing Hormone Decreased after Oral Administration of Genistein
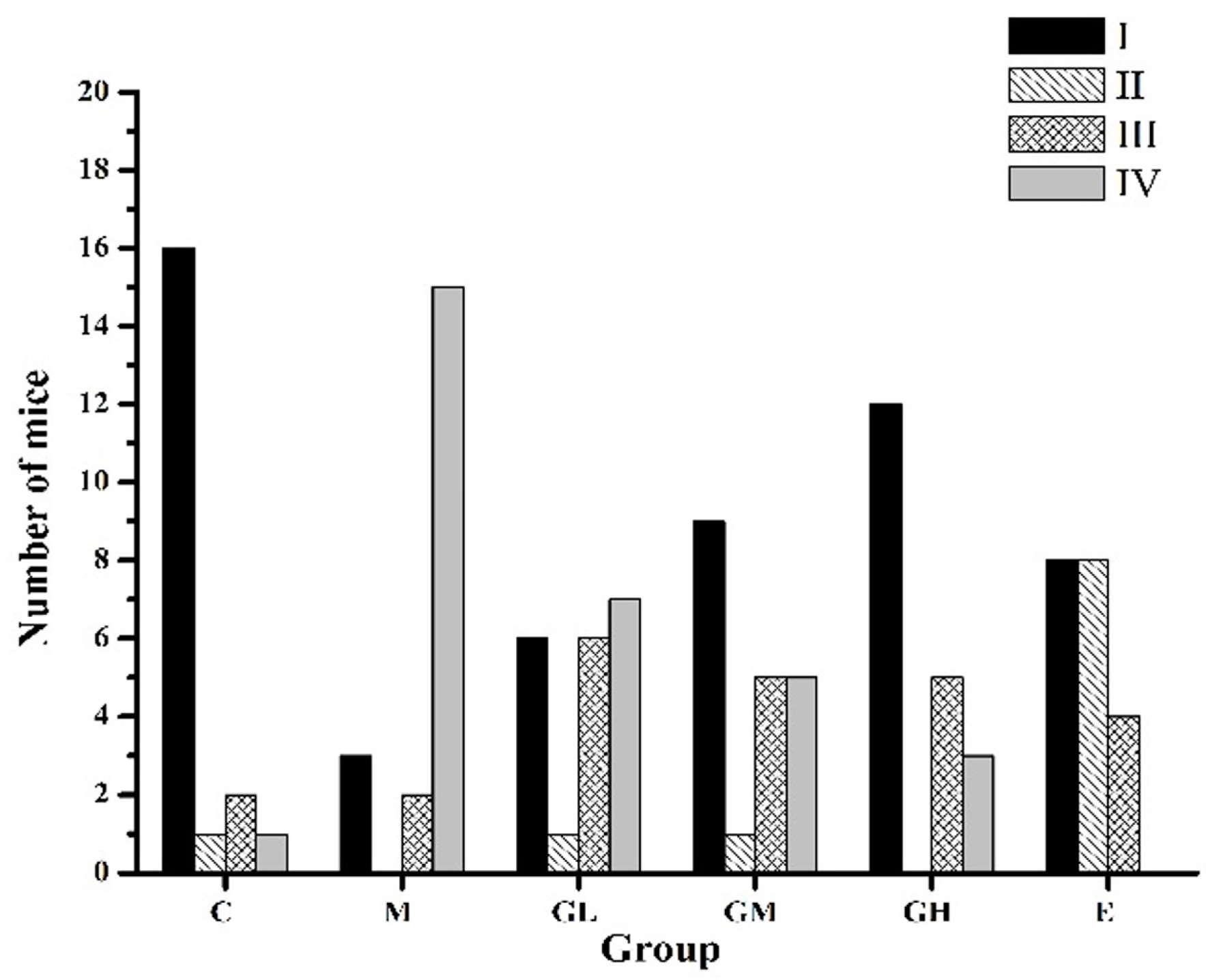
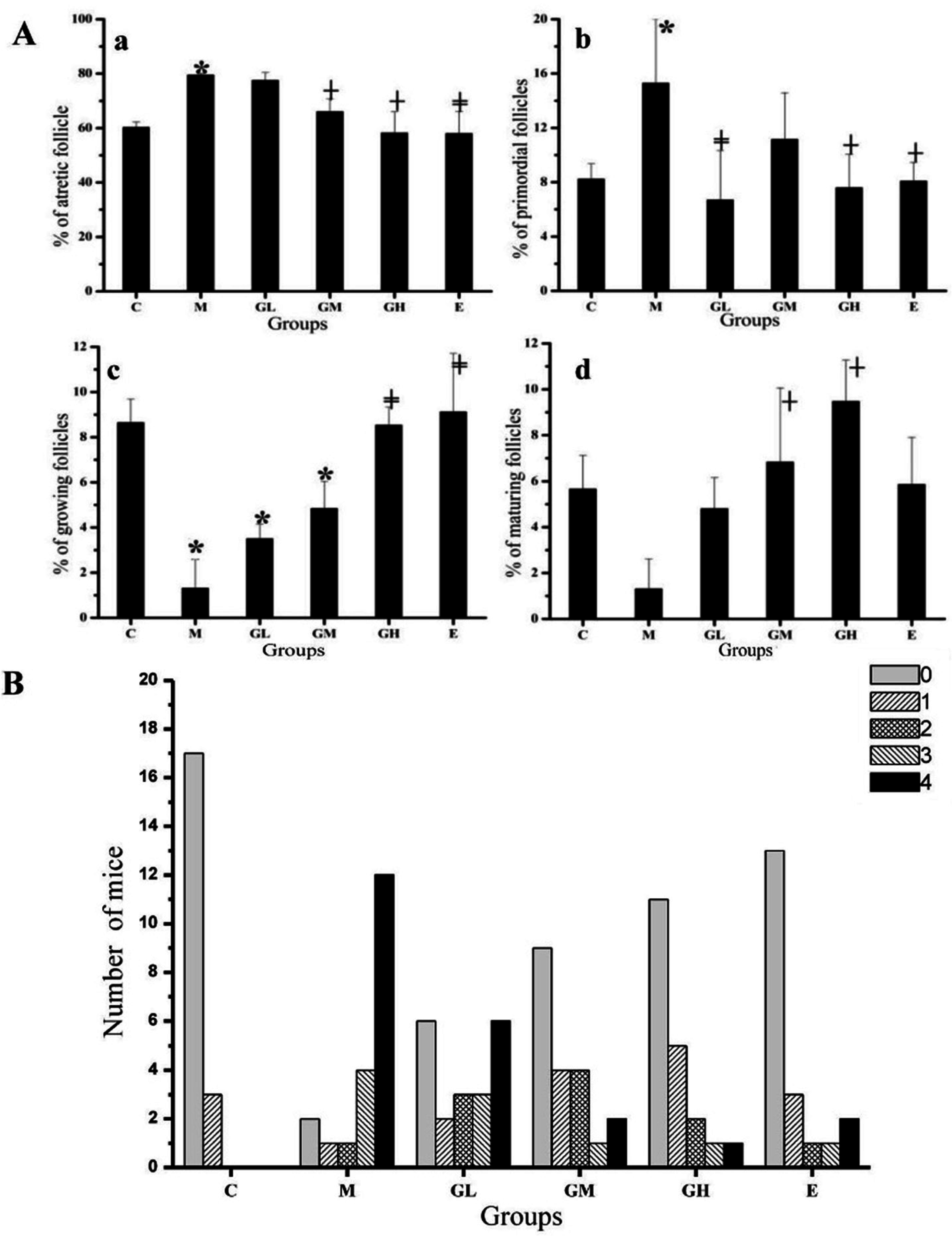
2.3. Decreased Morbidity of Oophoritis after Oral Administration of Genistein
2.4. Proliferative Responses of the Ovary
3. Discussion
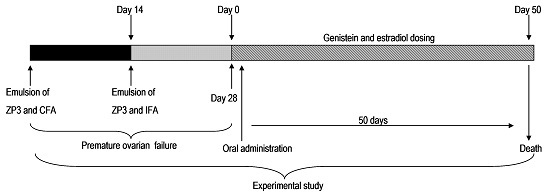
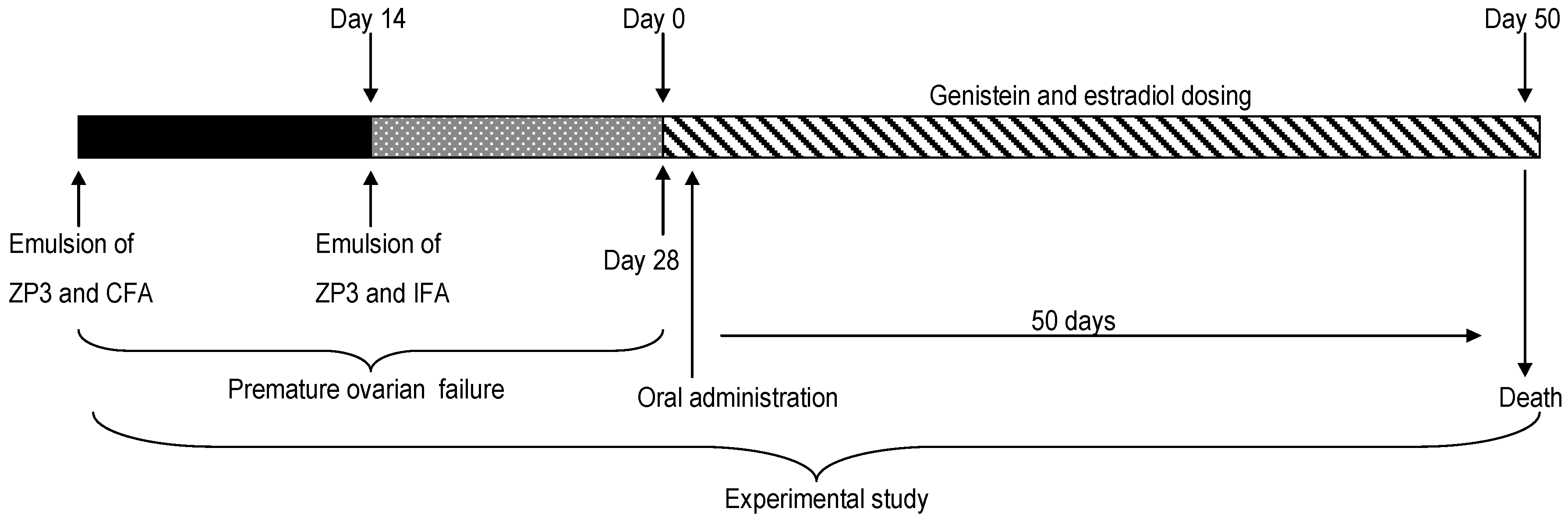
4. Materials and Methods
4.1. Animals and Chemicals
4.2. Estrous Cycle Staging
4.3. Immunization and Induction of Genistein
4.4. Detection of Serum Sex Hormone by Radio Immunity
4.5. Ovarian Histomorphology
4.6. Immunohistochemistry Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AOD | autoimmune ovarian disease |
| POF | premature ovarian failure |
| ZP | zona pellucid |
| C group | control group |
| M group | model group |
| GL group | low-dose of genistein (5 mg/kg body weight) therapeutic group |
| GM group | moderate-dose of genistein (25 mg/kg body weight) therapeutic group |
| GH group | high-dose of genistein (45 mg/kg body weight) therapeutic group |
| E group | estradiol group |
| E2 | estradiol |
| FSH | follicle-stimulating hormone |
| LH | luteinizing hormone |
| T | testosterone |
| PRL | prolactin |
| PCNA | proliferating cell nuclear antigen |
References
- Cordts, E.B.; Santos, M.C.; Peluso, C.; Kayaki, E.A.; Bianco, B.; Barbosa, C.P.; Christofolini, D.M. COMT polymorphism influences decrease of ovarian follicles and emerges as a predictive factor for premature ovarian insufficiency. J. Ovarian Res. 2014, 7, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Meskhi, A.; Seif, M.W. Premature ovarian failure. Curr. Opin. Obstet. Gyn. 2006, 18, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tan, R.; Cui, Y.; Liu, J.; Wu, J. Estrogen receptor α gene (ESR1) polymorphisms associated with idiopathic premature ovarian failure in chinese women. Gynecol. Endocrinol. 2013, 29, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Cordts, E.B.; Christofolini, D.M.; Dos Santos, A.A.; Bianco, B.; Barbosa, C.P. Genetic aspects of premature ovarian failure: A literature review. Arch. Gynecol. Obstet. 2011, 283, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Sassarini, J.; Lumsden, M.A.; Critchley, H.O.D. Sex hormone replacement in ovarian failure—New treatment concepts. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tung, K.S.; Agersborg, S.S.; Alard, P.; Garza, K.M.; Lou, Y.H. Regulatory T-cell, endogenous antigen and neonatal environment in the prevention and induction of autoimmune disease. Immunol. Rev. 2001, 182, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Bagavant, H.; Sharp, C.; Kurth, B.; Tung, K.S. Induction and immunohistology of autoimmune ovarian disease in cynomolgus macaques (macaca fascicularis). Am. J. Pathol. 2002, 160, 141–149. [Google Scholar] [CrossRef]
- Lahoti, A.; Prasannan, L.; Speiser, P.W. Premature ovarian failure. In Abnormal Female Puberty; Springer: New York, NY, USA, 2016; pp. 67–85. [Google Scholar]
- Kelkar, R.L.; Meherji, P.K.; Kadam, S.S.; Gupta, S.K.; Nandedkar, T.D. Circulating auto-antibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J. Reprod. Immunol. 2005, 66, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Hoek, A.; Schoemaker, J.; Drexhage, H. Premature ovarian failure and ovarian autoimmunity 1. Endocr. Rev. 1997, 18, 107–134. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Feng, W.; Li, S.R.; Huang, B.Y. ZP3 peptides administered orally suppress murine experimental autoimmune ovarian disease. J. Reprod. Immunol. 2007, 75, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.X.; Xing, M.M.; Hu, J.L.; Hu, X.J.; Nie, S.P.; Xie, M.Y. Metabolism and health effects of phyto-estrogens. Crit. Rev. Food Sci. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Kostelac, D.; Rechkemmer, G.; Briviba, K. Phytoestrogens modulate binding response of estrogen receptors α and β to the estrogen response element. J. Agric. Food Chem. 2003, 51, 7632–7635. [Google Scholar] [CrossRef] [PubMed]
- Suetsugi, M.; Su, L.; Karlsberg, K.; Yuan, Y.C.; Chen, S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors 1 national institutes of health grants ES08258 and CA44735. Mol. Cancer Res. 2003, 1, 981–991. [Google Scholar] [PubMed]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor α (ERα) and ERβ in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- McKee, J.; Warber, S.L. Integrative therapies for menopause. South. Med. J. 2005, 98, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Shifren, J.L.; Schiff, I. Role of hormone therapy in the management of menopause. Obstet. Gynecol. 2010, 115, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.K. Functional foods, herbs and nutraceuticals: Towards biochemical mechanisms of healthy aging. Biogerontology 2004, 5, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Brown, J.; Marjoribanks, J.; Kronenberg, F.; Roberts, H.; Eden, J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst. Rev. 2007, 4. [Google Scholar] [CrossRef]
- Xiao, C.W. Health effects of soy protein and isoflavones in humans. J. Nutr. 2008, 138, 1244–1249. [Google Scholar]
- Prasain, J.; Carlson, S.; Wyss, J. Flavonoids and age-related disease: Risk, benefits and critical windows. Maturitas 2010, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Prietsch, R.; Monte, L.D.; Da Silva, F.; Beira, F.; del Pino, F.; Campos, V.; Collares, T.; Pinto, L.; Spanevello, R.; Gamaro, G. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; DeNardo, D.G.; Jacquot, Y.; Laïos, I.; Vidal, D.S.; Zambrana, C.R.; Leclercq, G.; Brown, P.H. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ERα. Breast Cancer Res. Treat. 2006, 99, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Zhou, C.Q.; Rattan, S.; Flaws, J.A. Effects of endocrine-disrupting chemicals on the ovary. Biol. Reprod. 2015, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bagavant, H.; Adams, S.; Terranova, P.; Chang, A.; Kraemer, F.W.; Lou, Y.; Kasai, K.; Luo, A.M.; Tung, K.S. Autoimmune ovarian inflammation triggered by proinflammatory (Th1) T cells is compatible with normal ovarian function in mice. Biol. Reprod. 1999, 61, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rebar, R.W. Premature ovarian failure. Obstet. Gynecol. 2009, 113, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, W.W.; Macmillan, K.L.; Hansen, P.J.; Drost, M. Concepts for regulation of corpus luteum function by the conceptus and ovarian follicles to improve fertility. Theriogenology 1989, 31, 149–164. [Google Scholar] [CrossRef]
- Thomas, H.; Nasim, M.; Sarraf, C.; Alison, M.; Love, S.; Lambert, H.; Price, P. Proliferating cell nuclear antigen (PNCA) immunostaining—A prognostic factor in ovarian cancer? Br. J. Cancer 1995, 71, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Rhim, S.H.; Millar, S.; Robey, F.; Luo, A.; Lou, Y.; Yule, T.; Allen, P.; Dean, J.; Tung, K. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J. Clin. Investig. 1992, 89, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Tung, K.; Lou, Y.; Garza, K.; Teuscher, C. Autoimmune ovarian disease: Mechanism of disease induction and prevention. Curr. Opin. Immunol. 1997, 9, 839–845. [Google Scholar] [CrossRef]
- Zhuang, X.L.; Fu, Y.C.; Xu, J.J.; Kong, X.X.; Chen, Z.G.; Luo, L.L. Effects of genistein on ovarian follicular development and ovarian life span in rats. Fitoterapia 2010, 81, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Takatsuka, N.; Inaba, S.; Kawakami, N.; Shimizu, H. Effect of soymilk consumption on serum estrogen concentrations in premenopausal japanese women. J. Natl. Cancer Inst. 1998, 90, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.M.; Merz, B.E.; Xu, X.; Nagel, T.C.; Phipps, W.R.; Kurzer, M.S. Soy isoflavones exert modest hormonal effects in premenopausal women 1. J. Clin. Endocr. Metab. 1999, 84, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Stanczyk, F.; Hendrich, S.; Murphy, P.; Zhang, C.; Wan, P.; Pike, M. Effects of soy foods on ovarian function in premenopausal women. Br. J. Cancer. 2000, 82, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Zin, S.R.M.; Omar, S.Z.; Khan, N.L.A.; Musameh, N.I.; Das, S.; Kassim, N.M. Effects of the phytoestrogen genistein on the development of the reproductive system of Sprague Dawley rats. Clinics 2013, 68, 253–262. [Google Scholar] [CrossRef]
- Xiao, G.Y.; Liu, I.H.; Cheng, C.C.; Chang, C.C.; Lee, Y.H.; Cheng, W.T.K.; Wu, S.C. Amniotic fluid stem cells prevent follicle atresia and rescue fertility of mice with premature ovarian failure induced by chemotherapy. PLoS ONE 2014, 9, e106538. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Cline, J.M.; Wood, C.E. The mammary glands of macaques. Toxicol. Pathol. 2008, 36, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Chen, L.Y.; Wang, F.; Wu, Y.Q.; Su, J.Q.; Huang, X.H.; Cheng, Y.; Wang, Z.C. Expression of hypoxia-inducible factor-1α during ovarian follicular growth and development in Sprague-Dawley rats. Genet. Mol. Res. 2015, 14, 5896–5909. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.M.; Agersborg, S.S.; Baker, E.; Tung, K.S. Persistence of physiological self antigen is required for the regulation of self tolerance. J. Immunol. 2000, 164, 3982–3989. [Google Scholar] [CrossRef] [PubMed]

| Time/Day | Group | E2/pg·mL−1 | LH/mIU·mL−1 | FSH/mIU·mL−1 | PRL/ng·mL−1 | T/ng·mL−1 |
|---|---|---|---|---|---|---|
| 1st | C | 9.41 ± 0.45 | 30.15 ± 2.34 | 9.33 ± 1.01 | 9.28 ± 0.65 | 0.531 ± 0.062 |
| M | 5.24 ± 0.90 a | 36.51 ± 0.51 aa | 9.90 ± 0.54 | 7.09 ± 0.65 | 0.544 ± 0.072 | |
| GL | 7.66 ± 0.36 | 31.41 ± 2.43 b | 8.68 ± 0.71 | 7.07 ± 1.02 | 0.448 ± 0.045 | |
| GM | 8.39 ± 0.22 | 31.65 ± 2.07 b | 7.15 ± 0.23 | 10.77 ± 0.20 b | 0.514 ± 0.055 | |
| GH | 9.17 ± 3.15 b | 34.23 ± 0.87 | 5.69 ± 1.71 aa,bb | 8.48 ± 0.36 | 0.492 ± 0.021 | |
| E | 6.45 ± 0.79 | 32.02 ± 1.03 b | 8.24 ± 0.52 | 8.74 ± 0.42 | 0.421 ± 0.018 | |
| 20th | C | 13.10 ± 8.57 | 31.50 ± 2.08 b | 7.60 ± 1.86 b | 9.01 ± 1.36 | 0.468 ± 0.006 |
| M | 4.66 ± 0.56 aa | 36.33 ± 1.41 | 10.05 ± 1.11 | 7.45 ± 0.97 | 0.484 ± 0.020 | |
| GL | 9.09 ± 3.27 | 29.80 ± 1.94 bb,c | 7.57 ± 0.92 | 9.36 ± 1.39 | 0.463 ± 0.046 | |
| GM | 5.21 ± 0.85 aa | 36.39 ± 1.83 a | 8.96 ± 0.66 | 8.43 ± 1.08 | 0.432 ± 0.042 | |
| GH | 4.67 ± 0.45 aa | 32.56 ± 2.19 b | 6.84 ± 0.26 bb | 8.25 ± 0.67 | 0.479 ± 0.026 | |
| E | 4.79 ± 0.14 aa | 34.72 ± 3.98 | 8.71 ± 0.30 | 9.39 ± 0.74 | 0.457 ± 0.045 | |
| 30th | C | 4.32 ± 0.37 | 30.61 ± 0.37 | 8.36 ± 0.92 | 8.56 ± 1.23 | 0.397 ± 0.192 |
| M | 3.01 ± 0.45 a | 36.87 ± 0.56 aa | 10.32 ± 0.12 a | 8.44 ± 0.75 | 0.437 ± 0.016 | |
| GL | 4.13 ± 0.22 | 32.35 ± 1.31 b | 8.89 ± 0.23 | 8.56 ± 0.52 | 0.414 ± 0.013 | |
| GM | 4.13 ± 0.81 | 36.73 ± 2.57 aa | 8.65 ± 0.42 | 7.23 ± 0.02 c | 0.449 ± 0.028 | |
| GH | 4.38 ± 0.57 b | 31.93 ± 0.90 b | 7.50 ± 0.19 b | 8.59 ± 0.39 | 0.691 ± 0.234 a,c | |
| E | 4.29 ± 0.16 b | 34.05 ± 0.61 | 8.48 ± 0.91 | 9.87 ± 1.20 | 0.399 ± 0.035 | |
| 50th | C | 4.42 ± 0.32 | 30.23 ± 1.62 | 9.02 ± 0.36 | 10.21 ± 1.31 | 0.470 ± 0.043 |
| M | 3.03 ± 0.75 a | 34.65 ± 0.91 a | 11.35 ± 0.44 a | 7.52 ± 0.34 a,c | 0.486 ± 0.018 | |
| GL | 4.93 ± 0.78 b,c | 31.50 ± 1.96 b | 10.97 ± 0.93 | 8.76 ± 0.38 | 0.471 ± 0.010 | |
| GM | 4.54 ± 0.35 b | 30.15 ± 1.28 b,c | 8.78 ± 0.29 b | 7.78 ± 0.32 a,c | 0.461 ± 0.032 | |
| GH | 3.84 ± 0.24 | 34.68 ± 1.43 a | 8.14 ± 0.77 bb | 8.86 ± 0.56 | 0.469 ± 0.025 | |
| E | 3.51 ± 0.13 | 33.65 ± 1.16 | 9.34 ± 0.41 b | 9.98 ± 0.91 | 0.448 ± 0.019 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Q.; Wang, Y.; Li, N.; Zhu, K.; Hu, J.; Wang, S.; Zhu, F.; Nie, S. Bidirectional Estrogen-Like Effects of Genistein on Murine Experimental Autoimmune Ovarian Disease. Int. J. Mol. Sci. 2016, 17, 1855. https://doi.org/10.3390/ijms17111855
Ding Q, Wang Y, Li N, Zhu K, Hu J, Wang S, Zhu F, Nie S. Bidirectional Estrogen-Like Effects of Genistein on Murine Experimental Autoimmune Ovarian Disease. International Journal of Molecular Sciences. 2016; 17(11):1855. https://doi.org/10.3390/ijms17111855
Chicago/Turabian StyleDing, Qiao, Yuxiao Wang, Na Li, Kexue Zhu, Jielun Hu, Sunan Wang, Fan Zhu, and Shaoping Nie. 2016. "Bidirectional Estrogen-Like Effects of Genistein on Murine Experimental Autoimmune Ovarian Disease" International Journal of Molecular Sciences 17, no. 11: 1855. https://doi.org/10.3390/ijms17111855