Pharmacologic Treatment Assigned for Niemann Pick Type C1 Disease Partly Changes Behavioral Traits in Wild-Type Mice
Abstract
:1. Introduction
2. Results
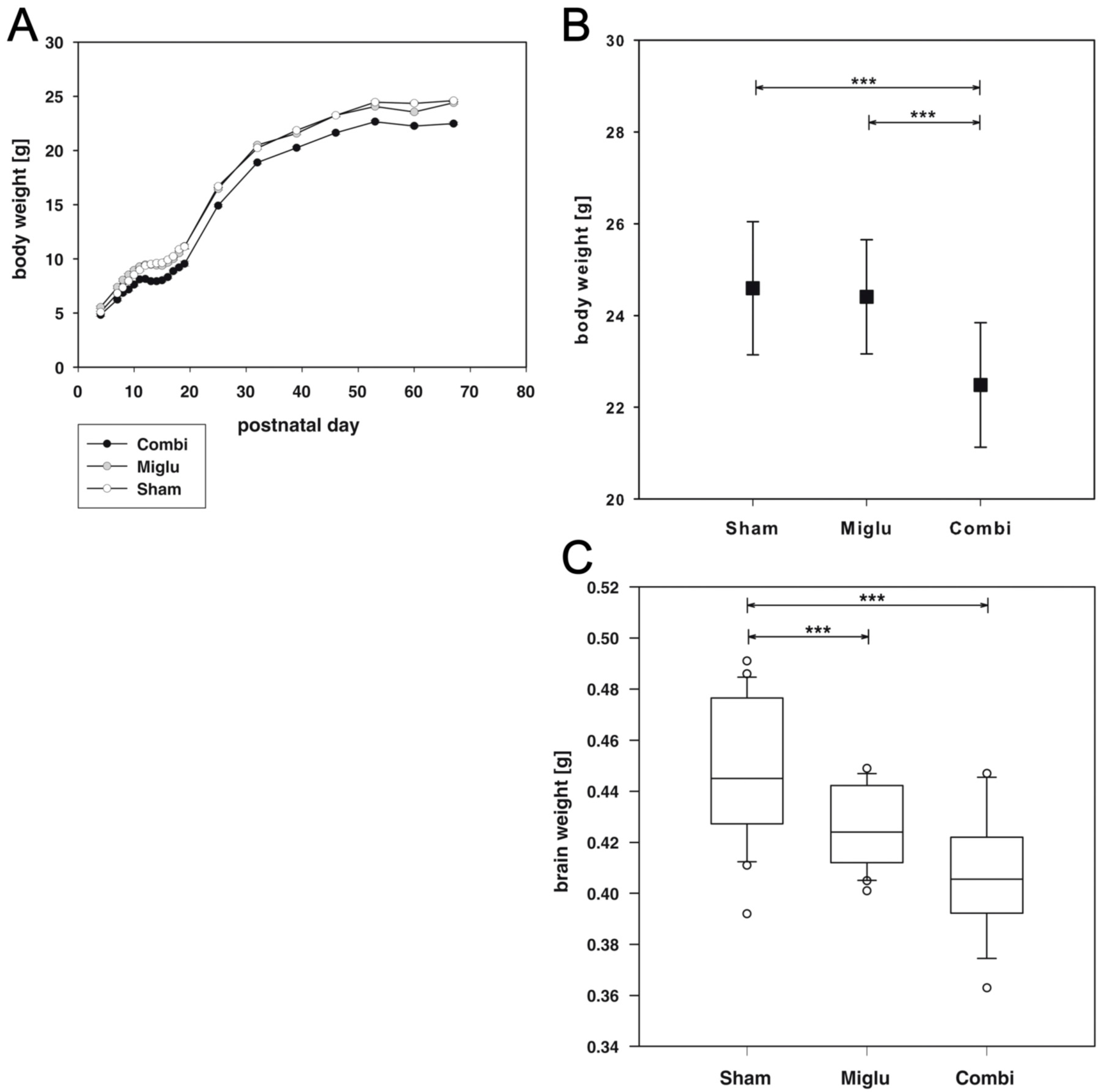
2.1. Reduced Body Weights after Combination Treatment
2.2. Reduced Brain Weights after Miglustat- and Combination Treatment
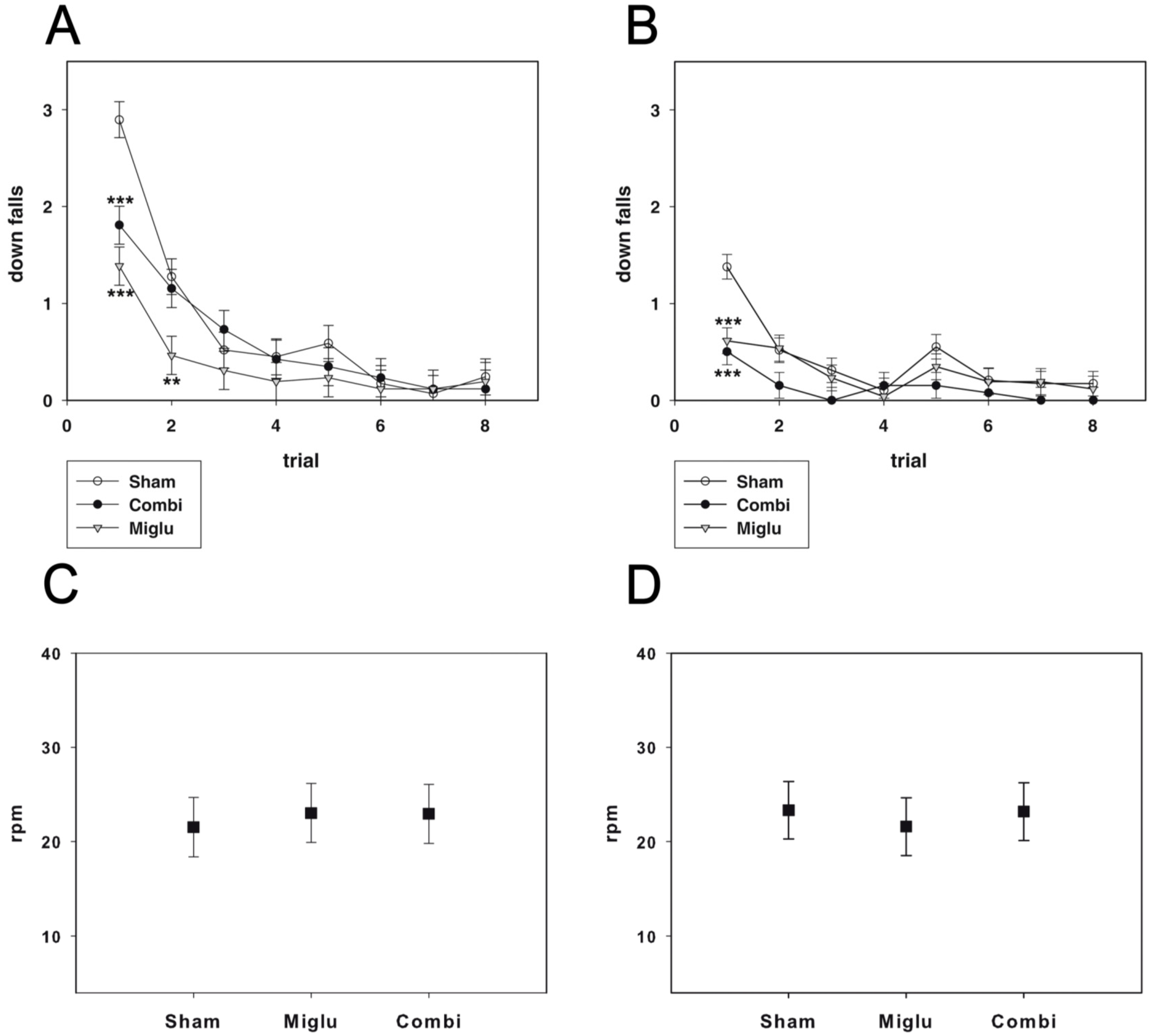
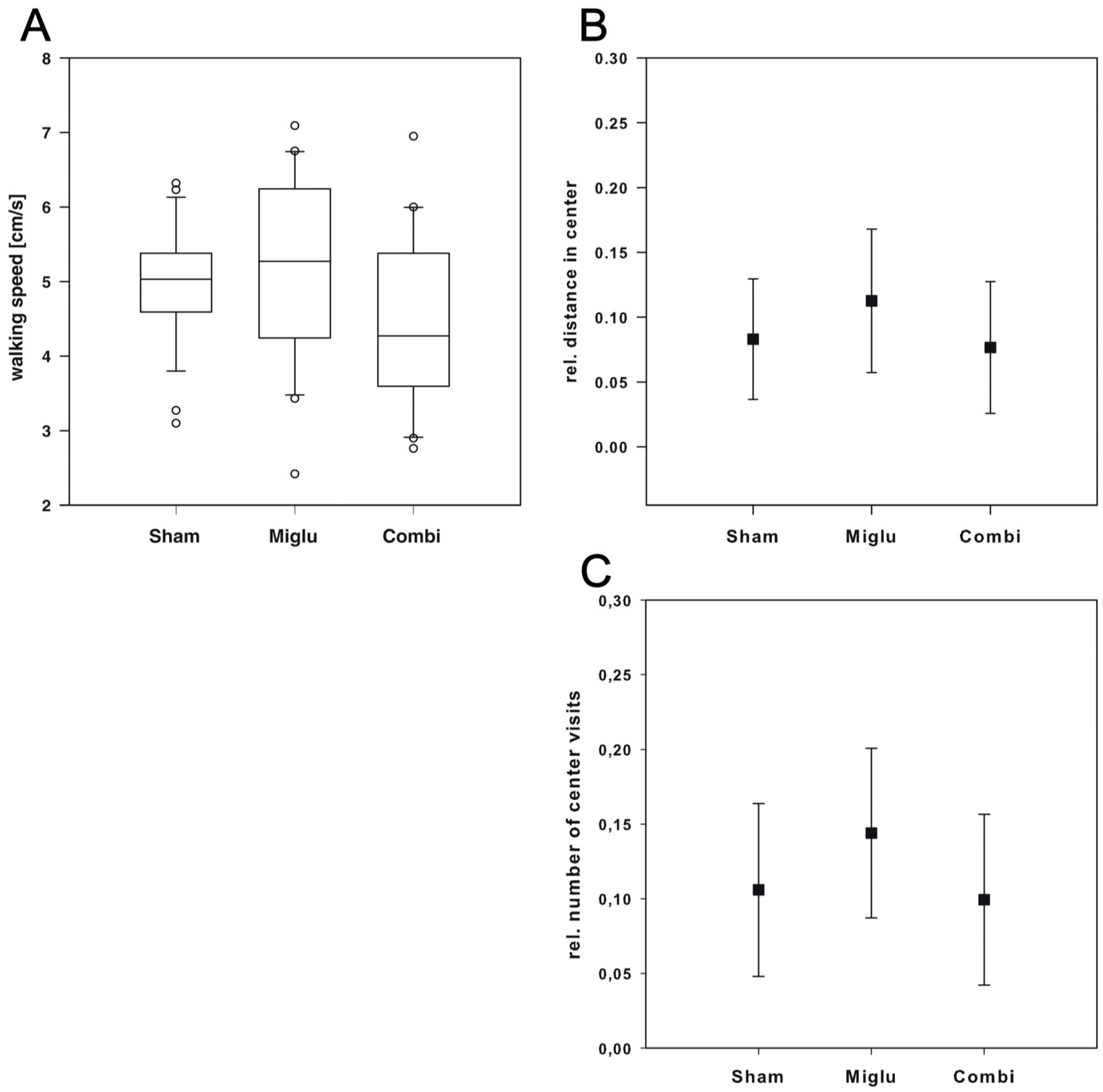
2.3. Miglustat and Combination Treatment Had No Effects on Motor Capabilities
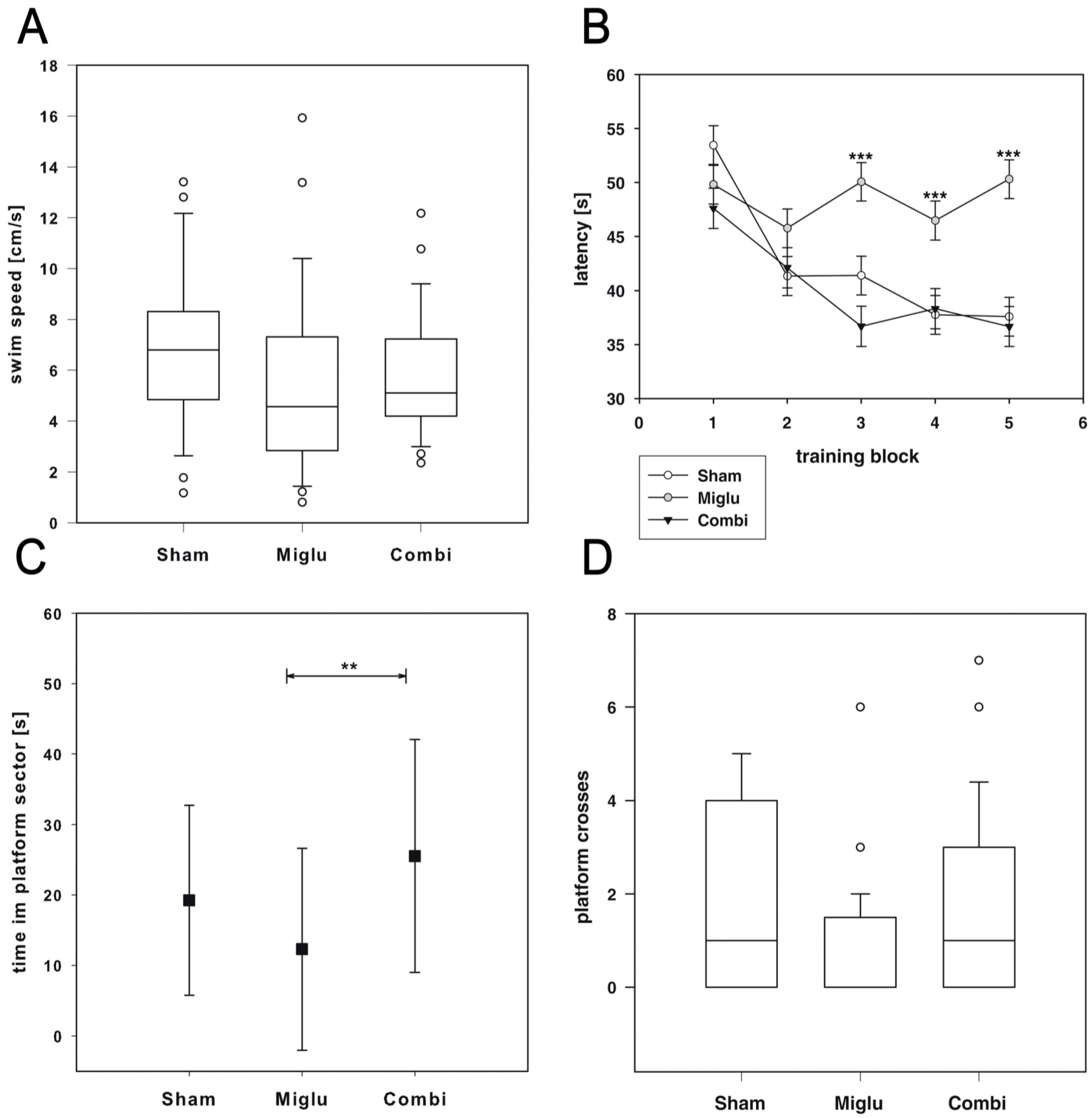
2.4. Impaired Spatial Learning by Miglustat Treatment but Not by Combination Treatment
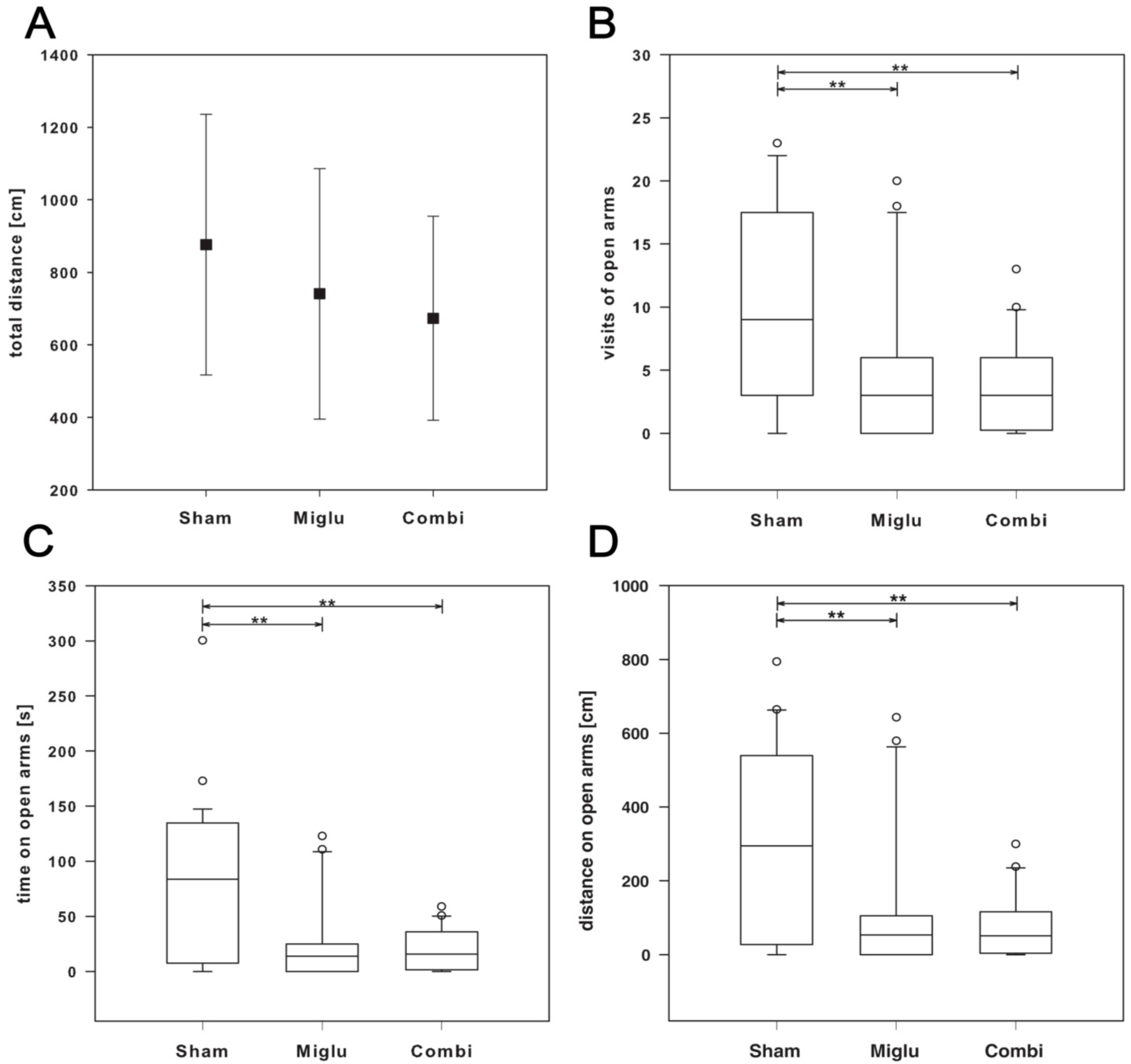
2.5. Enhanced Anxiety by Miglustat and Combination Treatment
2.6. Neither Treatment Influenced Explorative Behavior in Open Field Test
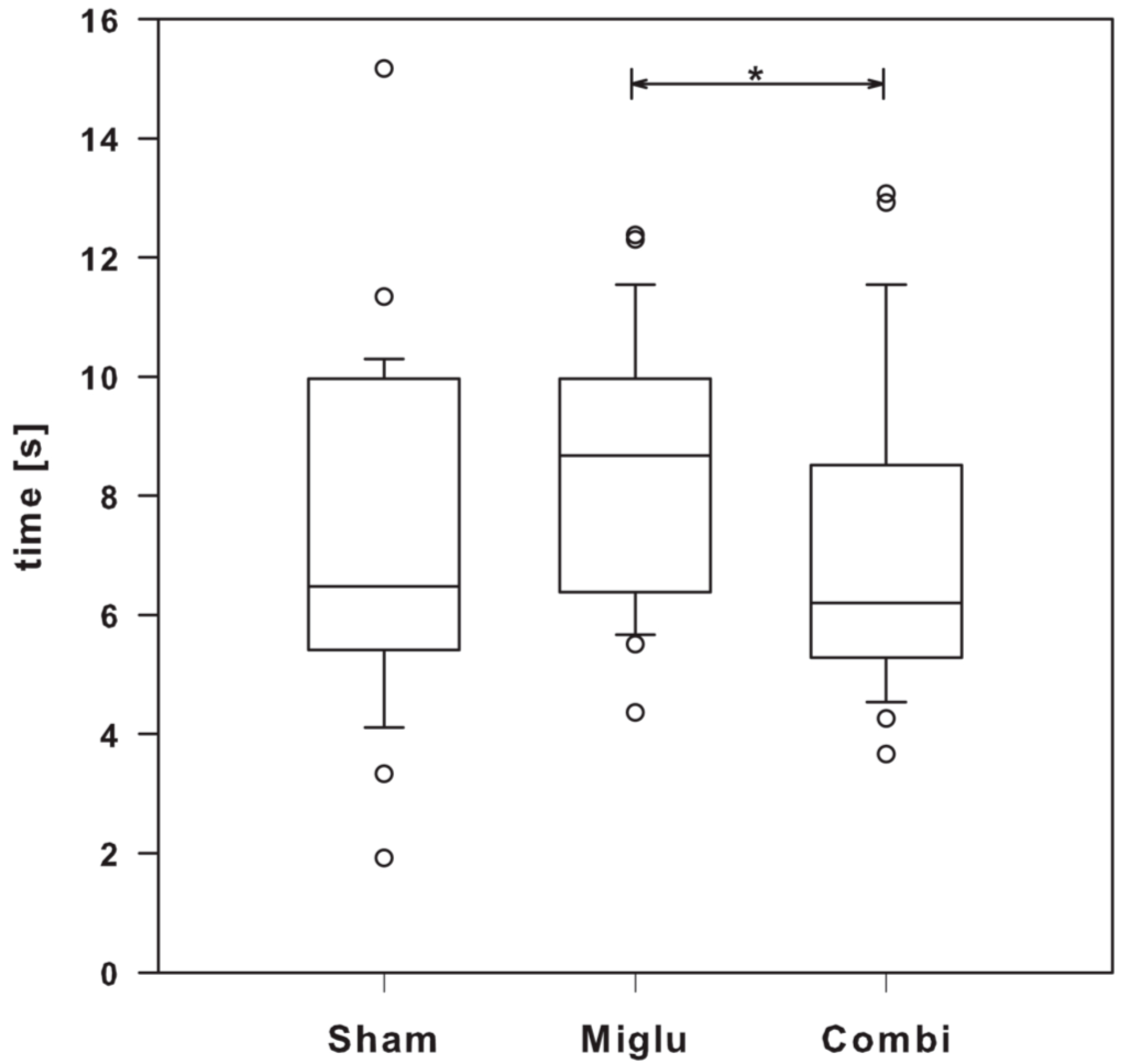
2.7. Miglustat Treatment and Combination Treatment Differed in Pain Sensitivity
3. Discussion
4. Methods
4.1. Animals
4.2. Pharmacologic Treatment
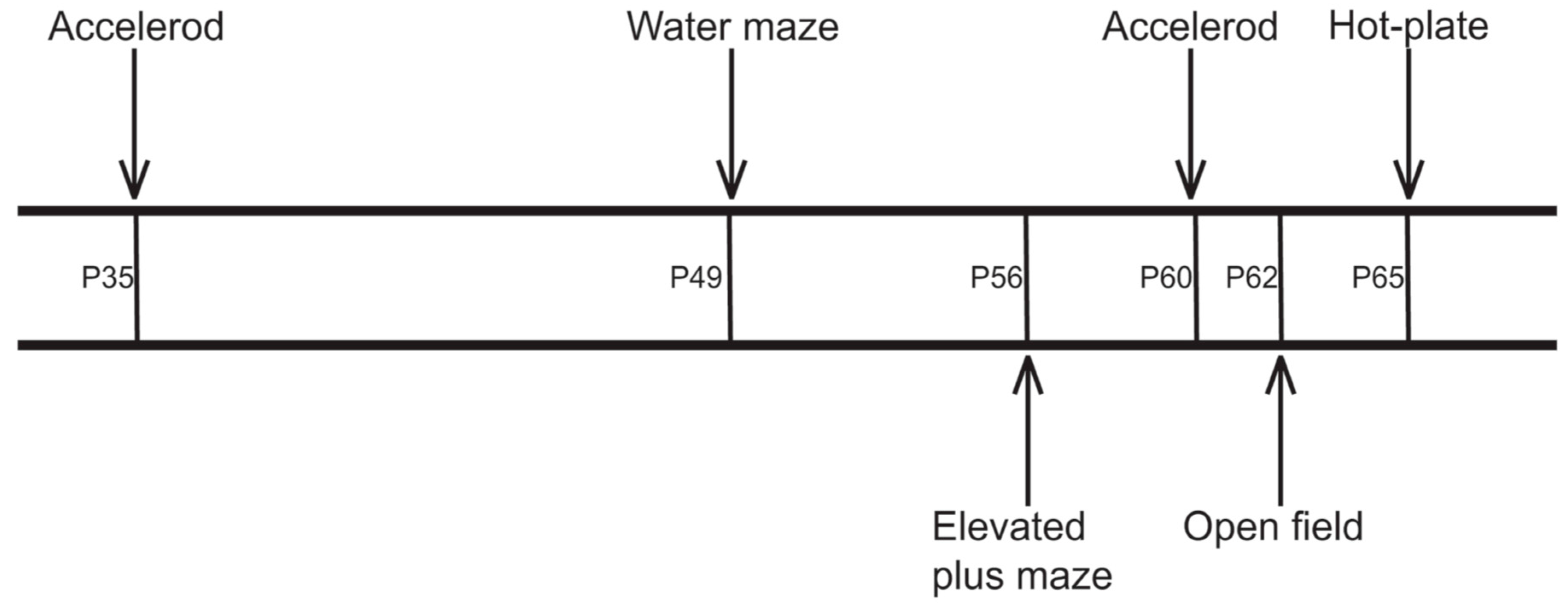
4.3. Behavioral Testing
4.3.1. Accelerod Test
4.3.2. Watermaze Test
4.3.3. Elevated Plus Maze Test
4.3.4. Open Field Test
4.3.5. Hot-Plate Test
4.4. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carstea, E.D.; Morris, J.A.; Coleman, K.G.; Loftus, S.K.; Zhang, D.; Cummings, C.; Gu, J.; Rosenfeld, M.A.; Pavan, W.J.; Krizman, D.B.; et al. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 1997, 277, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, E.B.; Wastney, M.; Patel, S.; Suresh, S.; Cooney, A.M.; Dwyer, N.K.; Roff, C.F.; Ohno, K.; Morris, J.A.; Carstea, E.D.; et al. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 1999, 274, 9627–9635. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.D.; Bhuvaneswaran, C.; Shio, H.; Fowler, S. Lysosome lipid storage disorder in NCTR-BALB/c mice. I. Description of the disease and genetics. Am. J. Pathol. 1982, 108, 140–149. [Google Scholar] [PubMed]
- Sarna, J.R.; Larouche, M.; Marzban, H.; Sillitoe, R.V.; Rancourt, D.E.; Hawkes, R. Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J. Comp. Neurol. 2003, 456, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Zervas, M.; Somers, K.L.; Thrall, M.A.; Walkley, S.U. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr. Biol. 2001, 11, 1283–1287. [Google Scholar] [CrossRef]
- Patterson, M.C.; Vecchio, D.; Prady, H.; Abel, L.; Wraith, J.E. Miglustat for treatment of Niemann-Pick C disease: A randomised controlled study. Lancet Neurol. 2007, 6, 765–772. [Google Scholar] [CrossRef]
- Pineda, M.; Wraith, J.E.E.; Mengel, E.; Sedel, F.; Hwu, W.-L.L.; Rohrbach, M.; Bembi, B.; Walterfang, M.; Korenke, G.C.C.; Marquardt, T.; et al. Miglustat in patients with Niemann-Pick disease Type C (NP-C): A multicenter observational retrospective cohort study. Mol. Genet. Metab. 2009, 98, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M.; Jeyakumar, M. Substrate reduction therapy. Acta Paediatr. 2008, 97, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Cologna, S.M.; Jiang, X.-S.; Backlund, P.S.; Cluzeau, C.V.M.; Dail, M.K.; Yanjanin, N.M.; Siebel, S.; Toth, C.L.; Jun, H.; Wassif, C.A.; et al. Quantitative proteomic analysis of Niemann-Pick disease, type C1 cerebellum identifies protein biomarkers and provides pathological insight. PLoS ONE 2012, 7, e47845. [Google Scholar] [CrossRef] [PubMed]
- Wraith, J.E.; Vecchio, D.; Jacklin, E.; Abel, L.; Chadha-Boreham, H.; Luzy, C.; Giorgino, R.; Patterson, M.C. Miglustat in adult and juvenile patients with Niemann–Pick disease type C: Long-term data from a clinical trial. Mol. Genet. Metab. 2010, 99, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.D.; Gong, W.; Verot, L.; Mellon, S.H. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 2004, 10, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed]
- Hovakimyan, M.; Maass, F.; Petersen, J.; Holzmann, C.; Witt, M.; Lukas, J.; Frech, M.J.; Hübner, R.; Rolfs, A.; Wree, A. Combined therapy with cyclodextrin/allopregnanolone and miglustat improves motor but not cognitive functions in Niemann-Pick Type C1 mice. Neuroscience 2013, 252, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Maass, F.; Petersen, J.; Hovakimyan, M.; Schmitt, O.; Witt, M.; Hawlitschka, A.; Lukas, J.; Rolfs, A.; Wree, A. Reduced cerebellar neurodegeneration after combined therapy with cyclodextrin/allopregnanolone and miglustat in NPC1: A mouse model of Niemann-Pick type C1 disease. J. Neurosci. Res. 2015, 93, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Palladino, G.; Loizzo, S.; Fortuna, A.; Canterini, S.; Palombi, F.; Erickson, R.P.; Mangia, F.; Fiorenza, M.T. Visual evoked potentials of Niemann-Pick type C1 mice reveal an impairment of the visual pathway that is rescued by 2-hydroxypropyl-β-cyclodextrin. Orphanet J. Rare Dis. 2015, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; Fishman, Y.I.; Puskás, I.; Szemán, J.; Sohajda, T.; McCauliff, L.A.; Sikora, J.; Storch, J.; Vanier, M.T.; Szente, L.; et al. Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann. Clin. Transl. Neurol. 2016, 3, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.; O’Donnell, P.; Fernandez, S.; Vite, C.H. 2-hydroxypropyl-β-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr. Res. 2010, 68, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Crumling, M.A.; Liu, L.; Thomas, P.V.; Benson, J.; Kanicki, A.; Kabara, L.; Hälsey, K.; Dolan, D.; Duncan, R.K. Hearing loss and hair cell death in mice given the cholesterol-chelating agent hydroxypropyl-β-cyclodextrin. PLoS ONE 2012, 7, e53280. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, R.H.; te Vruchte, D.; Lloyd-Evans, E.; Reinkensmeier, G.; Sillence, D.J.; Fernandez-Guillen, L.; Dwek, R.A.; Butters, T.D.; Cox, T.M.; Platt, F.M. Treatment with miglustat reverses the lipid-trafficking defect in Niemann–Pick disease type C. Neurobiol. Dis. 2004, 16, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Muller, A.; Alsop, J.; van Schaik, I.N.; Bembi, B.; Hughes, D. Results From a 9-year Intensive SaFety Surveillance Scheme (IS(3) ) in miglustat (Zavesca(®) )-treated patients. Pharmacoepidemiol. Drug Saf. 2015, 24, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Santos-Lozano, A.; Villamandos García, D.; Sanchis-Gomar, F.; Fiuza-Luces, C.; Pareja-Galeano, H.; Garatachea, N.; Nogales Gadea, G.; Lucia, A. Niemann-Pick disease treatment: A systematic review of clinical trials. Ann. Transl. Med. 2015, 3, 360. [Google Scholar] [CrossRef] [PubMed]
- Aqul, A.; Liu, B.; Ramirez, C.M.; Pieper, A.A.; Estill, S.J.; Burns, D.K.; Liu, B.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 2011, 31, 9404–9413. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Turley, S.D.; Burns, D.K.; Miller, A.M.; Repa, J.J.; Dietschy, J.M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the Npc1−/− mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.M.; Terpack, S.J.; Posey, K.S.; Liu, B.; Ramirez, C.M.; Turley, S.D. Systemic administration of 2-hydroxypropyl-β-cyclodextrin to symptomatic Npc1-deficient mice slows cholesterol sequestration in the major organs and improves liver function. Clin. Exp. Pharmacol. Physiol. 2014, 41, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Ishitsuka, Y.; Yamada, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; Higashi, T.; Motoyama, K.; Arima, H.; Matsuo, M.; et al. Influence of Npc1 genotype on the toxicity of hydroxypropyl-β-cyclodextrin, a potentially therapeutic agent, in Niemann–Pick Type C disease models. Mol. Genet. Metab. Rep. 2014, 1, 19–30. [Google Scholar] [CrossRef]
- Kondo, Y.; Tokumaru, H.; Ishitsuka, Y.; Matsumoto, T.; Taguchi, M.; Motoyama, K.; Higashi, T.; Arima, H.; Matsuo, M.; Higaki, K.; et al. In vitro evaluation of 2-hydroxyalkylated β-cyclodextrins as potential therapeutic agents for Niemann-Pick Type C disease. Mol. Genet. Metab. 2016, 118, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Rufini, S.; Tancredi, V.; Forcina, R.; Grossi, D.; D’Arcangelo, G. Cholesterol depletion inhibits synaptic transmission and synaptic plasticity in rat hippocampus. Exp. Neurol. 2008, 212, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormerod, K.G.; Rogasevskaia, T.P.; Coorssen, J.R.; Mercier, A.J. Cholesterol-independent effects of methyl-β-cyclodextrin on chemical synapses. PLoS ONE 2012, 7, e36395. [Google Scholar] [CrossRef] [PubMed]
- Zervas, M.; Dobrenis, K.; Walkley, S.U. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J. Neuropathol. Exp. Neurol. 2001, 60, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Perez-Poyato, M.S.; O’Callaghan, M.; Vilaseca, M.A.; Pocovi, M.; Domingo, R.; Ruiz Portal, L.; Verdú Pérez, A.; Temudo, T.; Gaspar, A.; et al. Clinical experience with miglustat therapy in pediatric patients with Niemann–Pick disease type C: A case series. Mol. Genet. Metab. 2010, 99, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Héron, B.; Valayannopoulos, V.; Baruteau, J.; Chabrol, B.; Ogier, H.; Latour, P.; Dobbelaere, D.; Eyer, D.; Labarthe, F.; Maurey, H.; et al. Miglustat therapy in the French cohort of paediatric patients with Niemann-Pick disease type C. Orphanet J. Rare Dis. 2012, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmatoug, N.; Burlina, A.; Giraldo, P.; Hendriksz, C.J.; Kuter, D.J.; Mengel, E.; Pastores, G.M. Gastrointestinal disturbances and their management in miglustat-treated patients. J. Inherit. Metab. Dis. 2011, 34, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.C.; Vecchio, D.; Jacklin, E.; Abel, L.; Chadha-Boreham, H.; Luzy, C.; Giorgino, R.; Wraith, J.E. Long-term miglustat therapy in children with Niemann-Pick disease type C. J. Child Neurol. 2010, 25, 300–305. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, G.; Grossi, D.; Racaniello, M.; Cardinale, A.; Zaratti, A.; Rufini, S.; Cutarelli, A.; Tancredi, V.; Merlo, D.; Frank, C. Miglustat Reverts the Impairment of Synaptic Plasticity in a Mouse Model of NPC Disease. Neural Plast. 2016, 2016, 3830424. [Google Scholar] [CrossRef] [PubMed]
- Becher, A.; White, J.H.; McIlhinney, R.A.J. The γ-aminobutyric acid receptor B, but not the metabotropic glutamate receptor type-1, associates with lipid rafts in the rat cerebellum. J. Neurochem. 2008, 79, 787–795. [Google Scholar] [CrossRef]
- Brachet, A.; Norwood, S.; Brouwers, J.F.; Palomer, E.; Helms, J.B.; Dotti, C.G.; Esteban, J.A. LTP-triggered cholesterol redistribution activates Cdc42 and drives AMPA receptor synaptic delivery. J. Cell Biol. 2015, 208, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Comerford, K.B.; Artiss, J.D.; Jen, K.-L.C.; Karakas, S.E. The Beneficial Effects α-Cyclodextrin on Blood Lipids and Weight Loss in Healthy Humans. Obesity 2011, 19, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, P.A.; Fletcher, E.; Elserafy, E.; Artiss, J.D.; Jen, K.-L.C. The Effect of α-Cyclodextrin on postprandial lipid and glycemic responses to a fat-containing meal. Metabolism 2013, 62, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Rodal, S.K.; Skretting, G.; Garred, O.; Vilhardt, F.; van Deurs, B.; Sandvig, K. Extraction of Cholesterol with Methyl-β-Cyclodextrin Perturbs formation of Clathrin-coated Endocytic Vesicles. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int. J. Pharm. 2007, 338, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hatzi, P.; Mourtas, S.; Klepetsanis, P.G.; Antimisiaris, S.G. Integrity of liposomes in presence of cyclodextrins: Effect of liposome type and lipid composition. Int. J. Pharm. 2007, 333, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Treiber, A.; Morand, O.; Clozel, M. The pharmacokinetics and tissue distribution of the glucosylceramide synthase inhibitor miglustat in the rat. Xenobiotica 2007, 37, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.H.; Almeida-Coburn, K.L.; Louza, M.P.; Cerqueira, D.M.; Aguiar, D.P.; Silva-Cardoso, L.; Mendes, F.A.; Andrade, L.R.; Einicker-Lamas, M.; Atella, G.C.; et al. Plasma membrane cholesterol depletion disrupts prechordal plate and affects early forebrain patterning. Dev. Biol. 2012, 365, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S. Sequence of myelinization in the brain of the albino rat. A. Cerebral cortex, thalamus and related structures. J. Comp. Neurol. 1963, 121, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, U. Observations on the postnatal development of neuronal structures in the lateral geniculate nucleus of the rat by electron microscopy. J. Ultrastruct. Res. 1967, 17, 158–175. [Google Scholar] [CrossRef]
- D’Hooge, R.; de Deyn, P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef]
- Royle, S.J.; Collins, F.C.; Rupniak, H.T.; Barnes, J.C.; Anderson, R. Behavioural analysis and susceptibility to CNS injury of four inbred strains of mice. Brain Res. 1999, 816, 337–349. [Google Scholar] [CrossRef]
- Van Dam, D.; Lenders, G.; de Deyn, P.P. Effect of Morris water maze diameter on visual-spatial learning in different mouse strains. Neurobiol. Learn. Mem. 2006, 85, 164–172. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Zavesca. Summary ofF Product Characteristics. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000435/WC500046726.pdf (accessed on 16 June 2016).
- Nonaka, N.; Farr, S.A.; Nakamachi, T.; Morley, J.E.; Nakamura, M.; Shioda, S.; Banks, W.A. Intranasal administration of PACAP: Uptake by brain and regional brain targeting with cyclodextrins. Peptides 2012, 36, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.; Garrud, P.; Rawlins, J.N.; O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 1982, 297, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- File, S.E. The contribution of behavioural studies to the neuropharmacology of anxiety. Neuropharmacology 1987, 26, 877–886. [Google Scholar] [CrossRef]
- Crawley, J.N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 1985, 9, 37–44. [Google Scholar] [CrossRef]
- Defries, J.C.; Hegmann, J.P.; Weir, M.W. Open-field behavior in mice: Evidence for a major gene effect mediated by the visual system. Science 1966, 154, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Eikelis, N.; Van Den Buuse, M. Cardiovascular responses to open-field stress in rats: Sex differences and effects of gonadal hormones. Stress 2000, 3, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-field Test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef] [PubMed]
- Denenberg, V.H. Open-field behavior in the rat: What does it mean? Ann. N. Y. Acad. Sci. 1969, 159, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Sudakov, S.K.; Nazarova, G.A.; Alekseeva, E.V.; Bashkatova, V.G. Estimation of the Level of Anxiety in Rats: Differences in Results of Open-field Test, Elevated Plus-Maze Test, and Vogel’s Conflict Test. Bull. Exp. Biol. Med. 2013, 155, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, R.; Strazielle, C. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J. Neurosci. Methods 2008, 171, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Pick, C.G.; Cheng, J.; Paul, D.; Pasternak, G.W. Genetic influences in opioid analgesic sensitivity in mice. Brain Res. 1991, 566, 295–298. [Google Scholar] [CrossRef]
- Hollak, C.E.M.; Hughes, D.; van Schaik, I.N.; Schwierin, B.; Bembi, B. Miglustat (Zavesca) in type 1 Gaucher disease: 5-year results of a post-authorisation safety surveillance programme. Pharmacoepidemiol. Drug Saf. 2009, 18, 770–777. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). European Medicines Agency (EMA). Available online: http://www.ema.europa.eu/ (accessed on 16 June 2016).
- Vance, J.E.; Karten, B. Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J. Lipid Res. 2014, 55, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Urbach, Y.K.; Bode, F.J.; Nguyen, H.P.; Riess, O.; von Hörsten, S. Neurobehavioral Tests in Rat Models of Degenerative Brain Diseases. In Rat Genomics: Methods and Protocols; Anegon, I., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 333–356. [Google Scholar]
- Van Gaalen, M.M.; Steckler, T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav. Brain Res. 2000, 115, 95–106. [Google Scholar] [CrossRef]
- Karl, T.; Pabst, R.; von Hörsten, S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 2003, 55, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.J.; Roberts, D.J. A rotarod suitable for quantitative measurements of motor incoordination in naive mice. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1968, 259, 211. [Google Scholar] [CrossRef] [PubMed]
- Bogo, V.; Hill, T.A.; Young, R.W. Comparison of accelerod and rotarod sensitivity in detecting ethanol- and acrylamide-induced performance decrement in rats: Review of experimental considerations of rotating rod systems. Neurotoxicology 1981, 2, 765–787. [Google Scholar] [PubMed]
- Hall, C. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J. Comp. Psychol. 1934, 18, 385–403. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlegel, V.; Thieme, M.; Holzmann, C.; Witt, M.; Grittner, U.; Rolfs, A.; Wree, A. Pharmacologic Treatment Assigned for Niemann Pick Type C1 Disease Partly Changes Behavioral Traits in Wild-Type Mice. Int. J. Mol. Sci. 2016, 17, 1866. https://doi.org/10.3390/ijms17111866
Schlegel V, Thieme M, Holzmann C, Witt M, Grittner U, Rolfs A, Wree A. Pharmacologic Treatment Assigned for Niemann Pick Type C1 Disease Partly Changes Behavioral Traits in Wild-Type Mice. International Journal of Molecular Sciences. 2016; 17(11):1866. https://doi.org/10.3390/ijms17111866
Chicago/Turabian StyleSchlegel, Victoria, Markus Thieme, Carsten Holzmann, Martin Witt, Ulrike Grittner, Arndt Rolfs, and Andreas Wree. 2016. "Pharmacologic Treatment Assigned for Niemann Pick Type C1 Disease Partly Changes Behavioral Traits in Wild-Type Mice" International Journal of Molecular Sciences 17, no. 11: 1866. https://doi.org/10.3390/ijms17111866






