The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antiproliferative Activities of Constituents Isolated from “Tea Flower” against Human Gastric Carcinoma HSC-2, HSC-4, MKN-45, and Caco-2 Cells
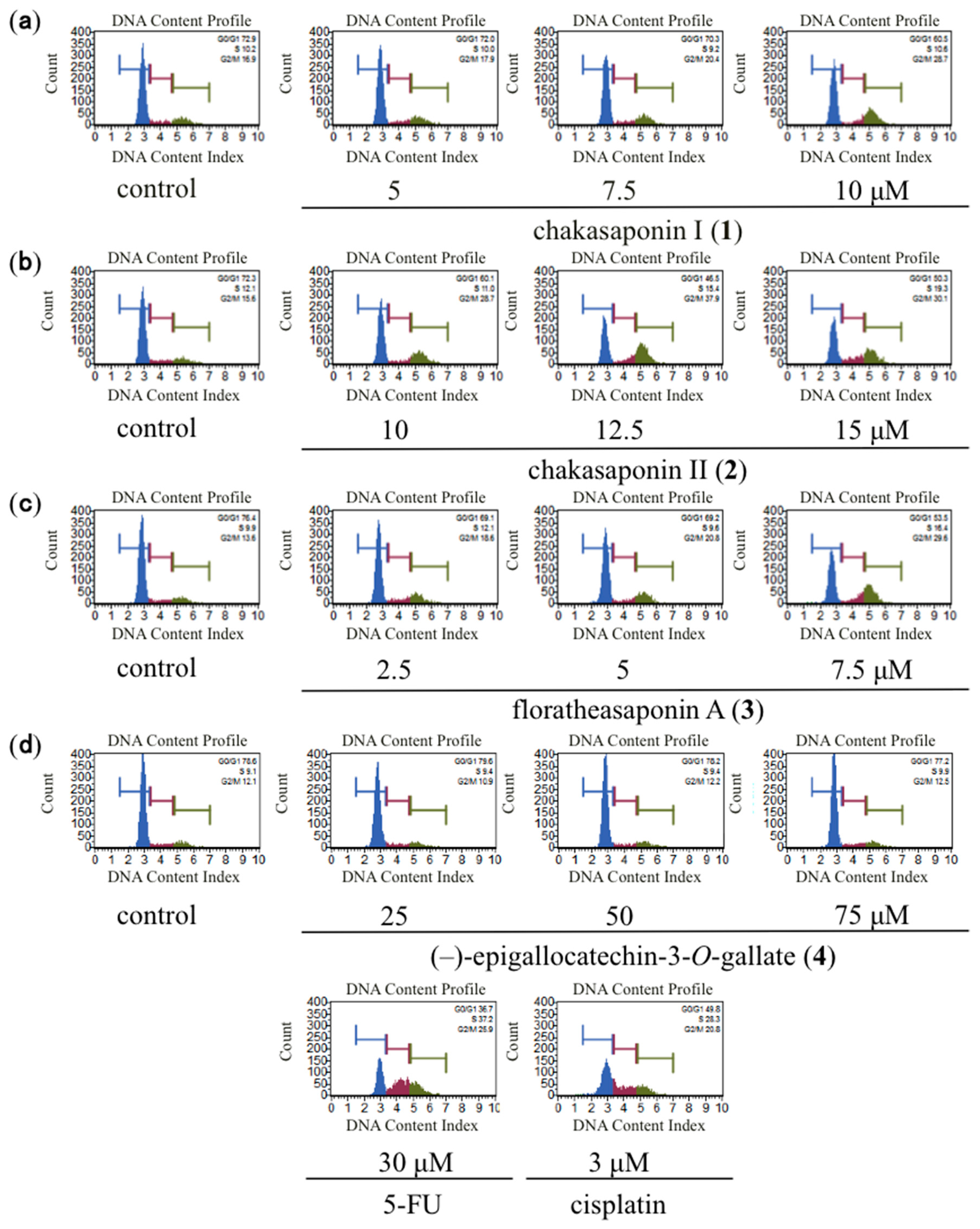
2.2. Effects of Cell Cycle Distribution in HSC-2 Cells
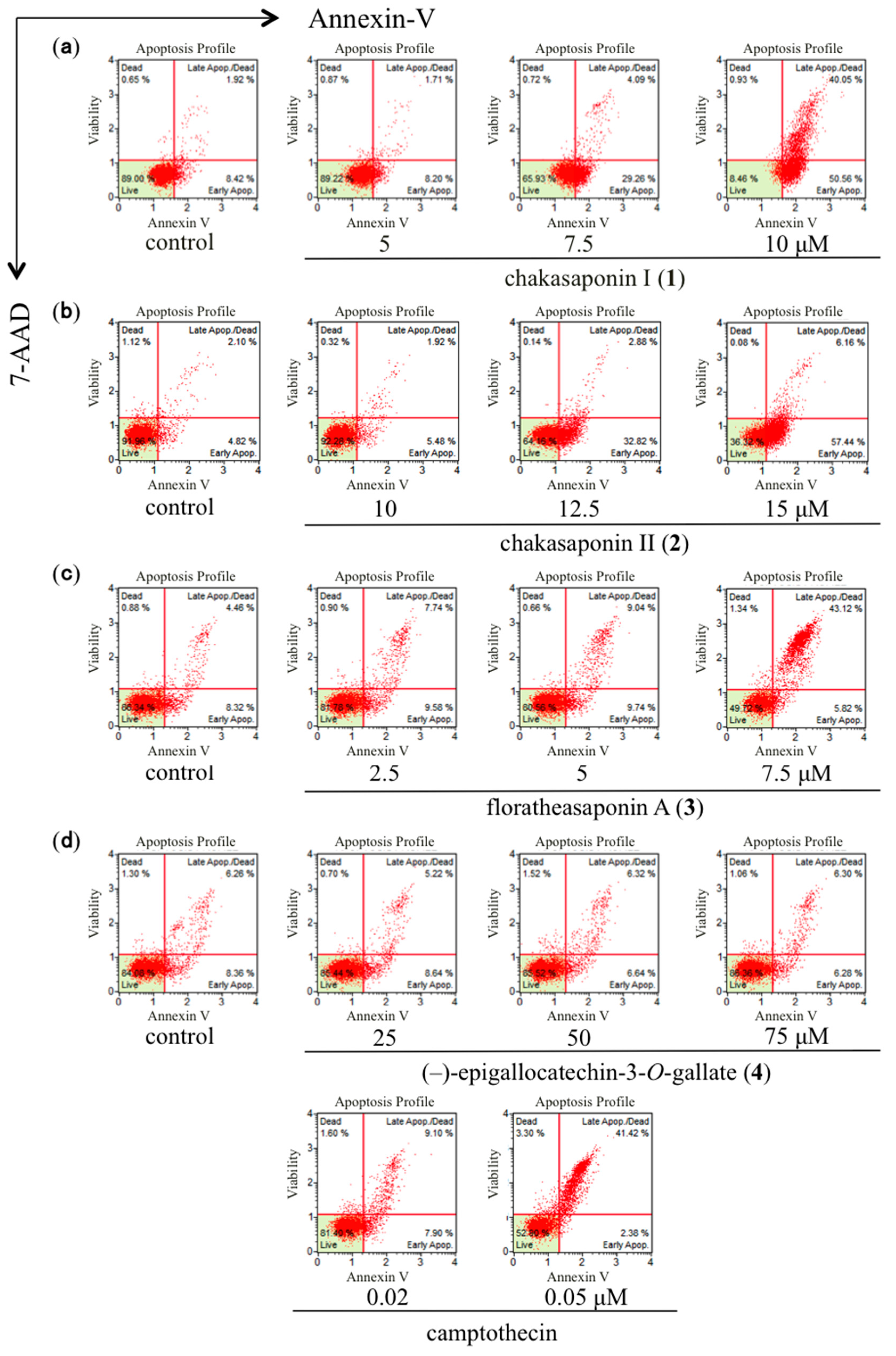
2.3. Quantification of Apoptotic Cell Death Using Annexin-V Binding Assay in HSC-2 Cells
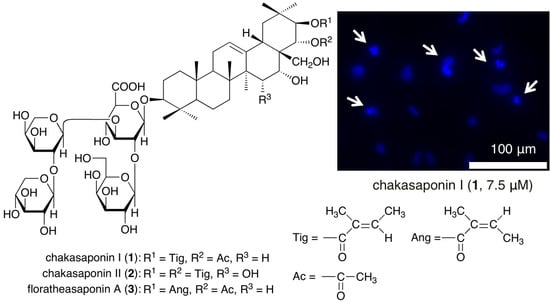
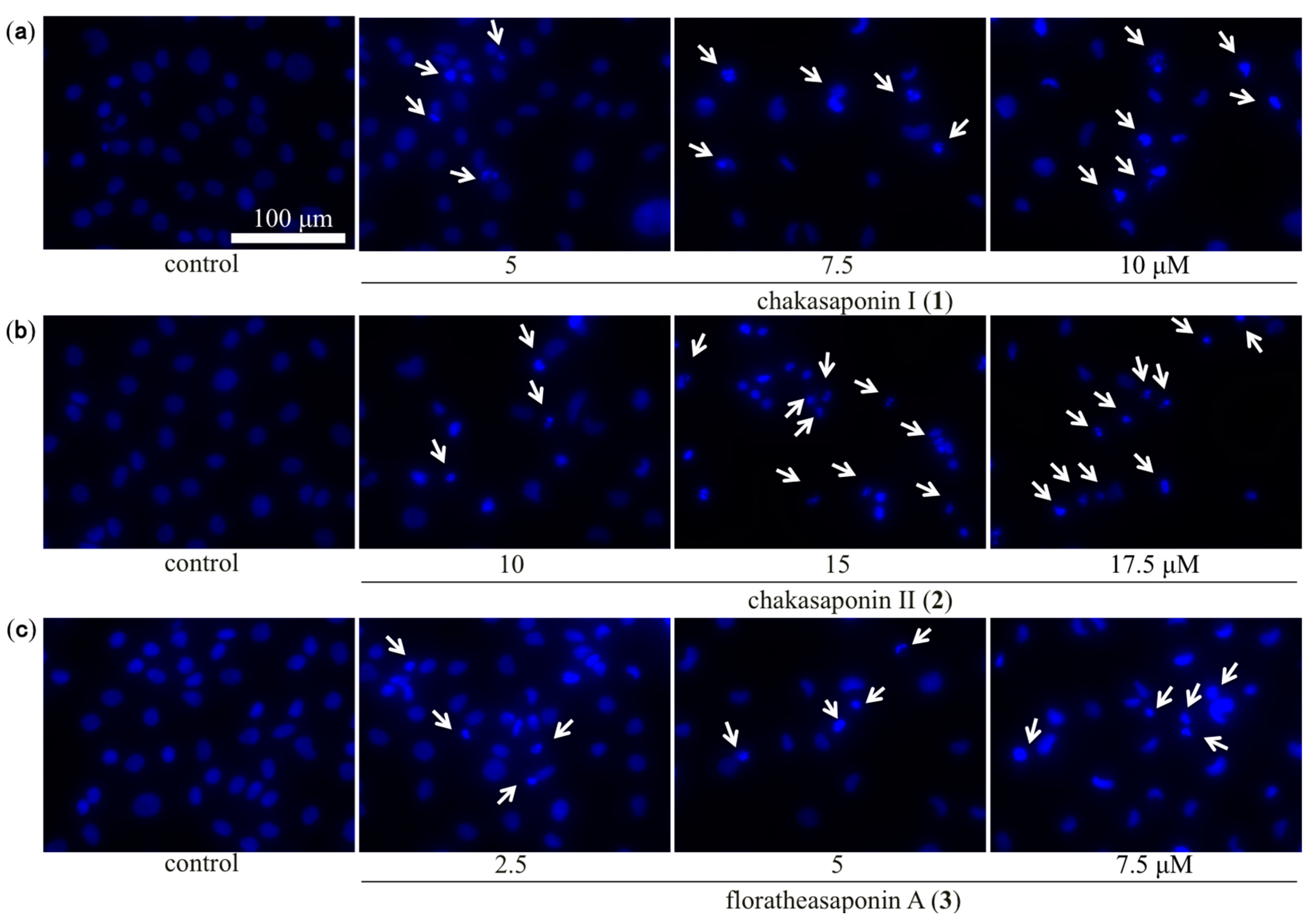
2.4. Evaluation of Apoptotic Morphological Changes in HSC-2 Cells
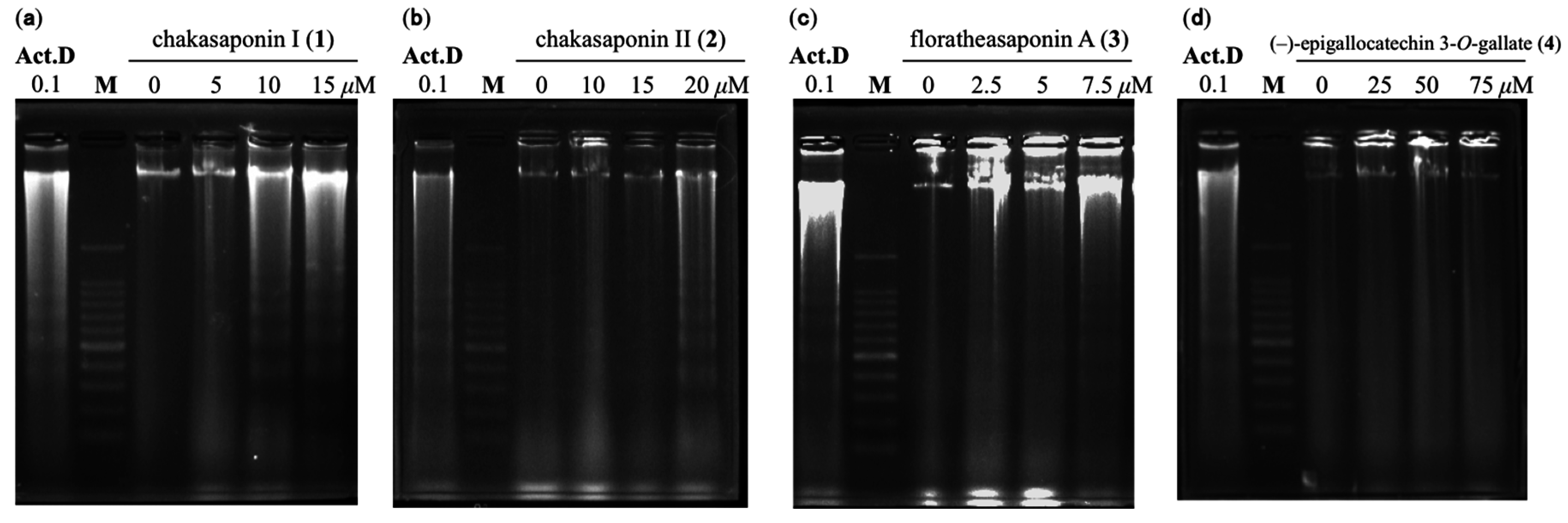
2.5. DNA Fragmentation in HSC-2 Cells
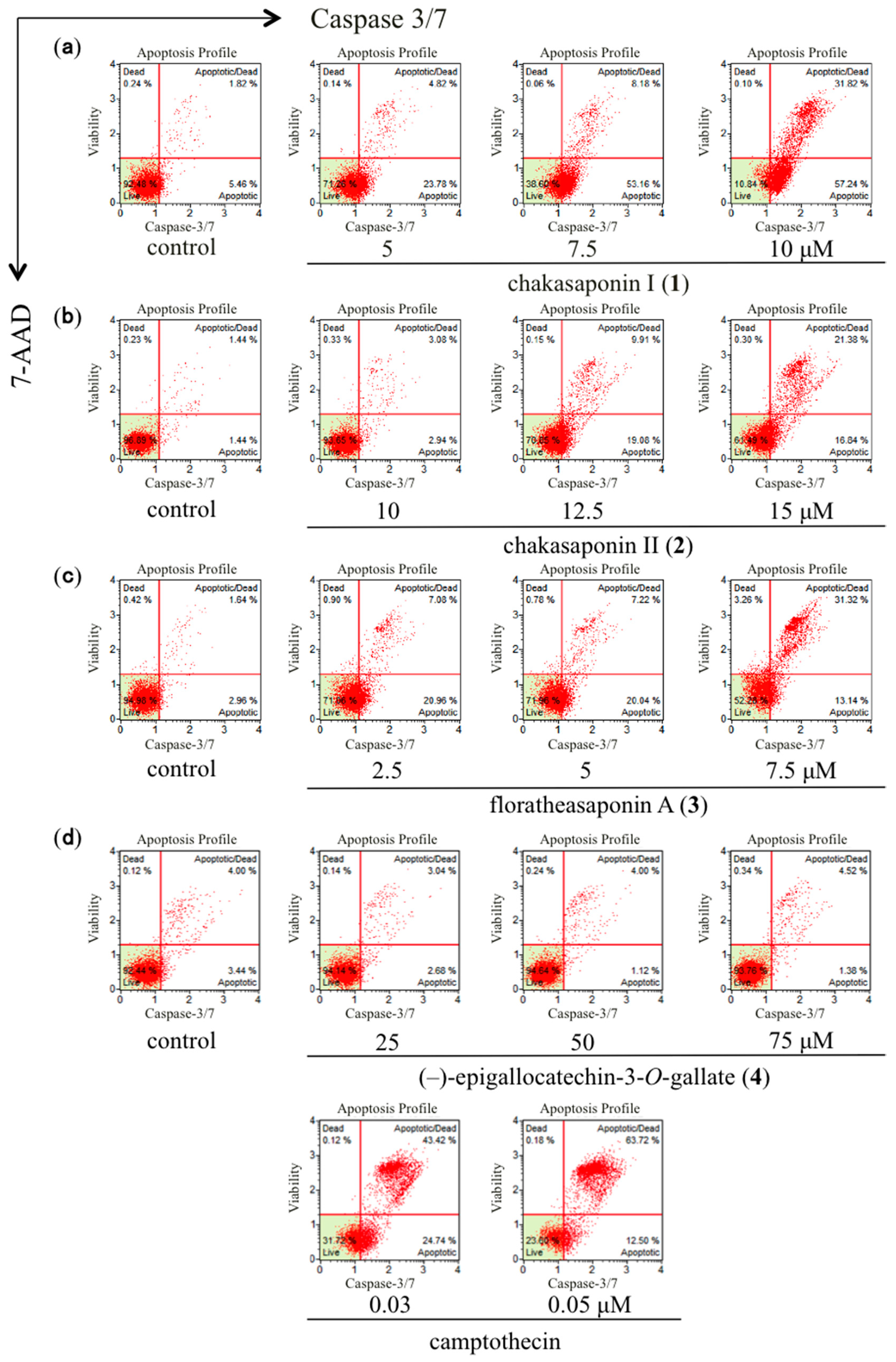
2.6. Effects of Caspase-3/7 in HSC-2 Cells
3. Materials and Methods
3.1. Chemicals Constituents from “Tea Flower”
3.2. Reagents
3.3. Cell Viability Assays
3.4. Cell Cycle Analysis
3.5. Annexin-V/7-AAD Assay
3.6. DAPI Staining for Morphological Analysis
3.7. Agarose Gel Electrophoresis for the Detection of DNA Fragmentation
3.8. Caspase-3/7 Assay
3.9. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Debnath, S.; Mohanta, B.C.; Harigaya, Y. Naturally occurring triterpenoid saponins. Chem. Biodivers. 2010, 7, 2327–2580. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins—Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Faizal, A.; Geelen, D. Saponins and their role in biological processes in plants. Phytochem. Rev. 2013, 12, 877–893. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Yamamoto, K.; Kato, Y.; Nagatomo, A.; Matsuda, H. Floratheasaponins A–C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the tea plant (Camellia sinensis). J. Nat. Prod. 2005, 68, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Miyake, S.; Miki, Y.; Ninomiya, K.; Yoshikawa, M.; Muraoka, O. Quantitative analysis of acylated oleanane-type triterpene saponins, chakasaponins I–III and floratheasaponins A–F, in the flower buds of Camellia sinensis from different regional origins. J. Nat. Med. 2012, 66, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ninomiya, K.; Miyake, S.; Miki, Y.; Okamoto, M.; Yoshikawa, M.; Muraoka, O. Flavonol glycosides with lipid accumulation inhibitory activity and simultaneous quantitative analysis of 15 polyphenols and caffeine in the flower buds of Camellia sinensis from different regions by LCMS. Food Chem. 2013, 140, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Lee, I.J.; Okugawa, S.; Miyake, S.; Miki, Y.; Ninomiya, K.; Kitagawa, N.; Yoshikawa, M.; Muraoka, O. Quantitative analysis of catechin, flavonoid, and saponin constituents in “tea flower”, the flower buds of Camellia sinensis, from different regions in Taiwan. Nat. Prod. Commun. 2013, 8, 1553–1557. [Google Scholar] [PubMed]
- Matsuda, H.; Nakamura, S.; Morikawa, T.; Muraoka, O.; Yoshikawa, M. New biofunctional effects of the flower buds of Camellia sinensis and its bioactive acylated oleanane-type triterpene oligoglycosides. J. Nat. Med. 2016, 70, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Nakamura, S.; Kato, Y.; Muraoka, O.; Matsuda, H.; Yoshikawa, M. Bioactive saponins and glycosides. XXVIII. New triterpene saponins, foliatheasaponins I, II, III, IV, and V, from tencha (the leaves of Camellia sinensis). Chem. Pharm. Bull. 2007, 55, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Gu, D.; Cao, C.; Zhang, Q.; Xu, Z.; Gong, Y.; Chen, J.; Tang, C. Increased risk of developing digestive tract cancer in subjects carrying the PLCE1 r32274223 A > G polymorphism: Evidence from a meta-analysis. PLoS ONE 2013, 8, e76425. [Google Scholar]
- Ninomiya, K.; Motai, C.; Nishida, E.; Kitagawa, N.; Yoshihara, K.; Hayakawa, T.; Muraoka, O.; Li, X.; Nakamura, S.; Yoshikawa, M.; et al. Acylated oleanane-type triterpene saponins from the flowers of Bellis perennis show anti-proliferative activities against human digestive tract carcinoma cell lines. J. Nat. Med. 2016, 70, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, J.H.; Weissman, D.B.; Sedaghat, T.; Babich, H. In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic Clin. Pharmacol. Toxicol. 2004, 95, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Krupka, M.E.; Nissim, H.A.; Zuckerbraun, H.L. Differential in vitro cytotoxicity of (–)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol. In Vitro 2005, 19, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.-H.; Xu, Q.; Tong, J.-L.; Xiao, S.-D. Apoptotic effect of epigallocatechin-3-gallate on the human gastric cancer cell line MKN45 via activation of the mitochondrial pathway. World J. Gastroenterol. 2007, 13, 4255–4259. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.; Fernandes, I.; González-Manzano, S.; de Freitas, V.; Mateus, N.; Santos-Buelga, C. Anti-proliferative effects of quercetin and catechin metabolites. Food Funct. 2014, 5, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- V G M, N.; Atmakur, H.; Katragadda, S.B.; Devabakthuni, B.; Kota, A.; S, C.K.; Kuncha, M.; M V P S, V.V.; Kulkarni, P.; Janaswamy, M.R.; et al. Antioxidant, hepatoprotective and cytotoxic effects of icetexanes isolated from stem-bark of Premna tomentosa. Phytomedicine 2014, 21, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Moskot, M.; Gabig-Ciminska, M.; Jakóbkiewicz-Banecka, J.; Wesierska, M.; Bochenska, K.; Wegrzyn, G. Cell cycle is disturbed in mucopolysaccharidosis type II fibroblasts, and can be improved by genistein. Gene 2016, 585, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Yoshida, K.; Miyagawa, K.; Nemoto, Y.; Asao, Y.; Yoshikawa, M. Nuphar alkaloids with immediately apoptosis-inducing activity from Nuphar pumilum and their structural requirements for the activity. Bioorg. Med. Chem. 2006, 16, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huo, X.-C.; Sun, F.-D.; Dong, R.-Q. Polyphenol-rich extract of Salvia chinensis exhibits anticancer activity in different cancer cell lines, and induces cell cycle arrest at the G0/G1-phase, apoptosis and loss of mitochondrial membrane potential in pancreatic cancer cells. Mol. Med. Rep. 2015, 12, 4843–4850. [Google Scholar] [PubMed]
- Adrian, C.; Martin, S.J. The mitochondrial apoptosome: A killer unleashed by the cytochrome seas. Trends Biochem. Sci. 2001, 26, 390–397. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Caspase-dependent and independent cell death pathways after DNA damage. Oncol. Rep. 2005, 14, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Akaki, J.; Nakamura, S.; Okazaki, Y.; Kojima, H.; Tamesada, M.; Yoshikawa, M. Apoptosis-inducing effects of sterols from the dried powder of cultured mycelium of Cordyceps sinensis. Chem. Pharm. Bull. 2009, 57, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.; Endo, N.; Oku, N.; Arima, Y.; Suzuki, T.; Suzuki, Y. β-Thujaplicin zinc chelate induces apoptosis in mouse high metastatic melanoma B16BL6 cells. Biol. Pharm. Bull. 1998, 21, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, L.; Xiao, H.; Xiao, C.; Wang, Y.; Liu, X. Momordin Ic induces HepG2 cell apoptosis through MAPK and PI3K/Akt-mediated mitochondrial pathways. Apoptosis 2013, 18, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Al Dhaheri, Y.; Attoub, S.; Ramadan, G.; Arafat, K.; Bajbouj, K.; Karuvantevida, N.; AbuQamar, S.; Eid, A.; Iratni, R. Carnosol induces ROS-mediated beclin 1-independent autophagy and apoptosis in triple negative breast cancer. PLoS ONE 2014, 9, e109630. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, R.; Refolo, M.G.; Lippolis, C.; Giannuzzi, G.; Carella, N.; Messa, C.; Cavallini, A.; Carr, B.I. Antagonism of Sorafenib and Regorafenib actions by platelet factors in hepatocellular carcinoma cell lines. BMC Cancer 2014, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.I.; Cavallini, A.; D’Alessandro, R.; Refolo, M.G.; Lippolis, C.; Mazzocca, A.; Messa, C. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer 2014, 14, 43–52. [Google Scholar] [CrossRef] [PubMed]
- D’Alassandro, R.; Refolo, M.G.; Lippolis, C.; Carella, N.; Messa, C.; Cavallini, A.; Carr, B.I. Modulation of regorafenib effects on HCC cell lines by epidermal growth factor. Cancer Chemother. Pharmacol. 2015, 75, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M. Green tea and cancer prevention. Proc. Jpn. Acad. 2002, 78, 263–270. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M.; Okabe, S.; Sueoka, E.; Sueoka, N.; Fujimoto, N.; Goto, Y.; Matsuyama, S.; Imai, K.; Nakachi, K. Cancer prevention with green tea and monitoring by a new biomarker, hnRNP B1. Mutat. Res. 2001, 480–481, 229–304. [Google Scholar] [CrossRef]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Primary cancer prevention by green tea, and tertiary cancer prevention by the combination of green tea catechins and anticancer compounds. J. Cancer Prev. 2015, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Saha, A.; Fujiki, H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011, 102, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 2015, 141, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Okabe, S.; Sueoka, N.; Komori, A.; Sueoka, E.; Kozu, T.; Tada, Y.; Suga, K.; Imai, K.; et al. Cancer inhibition by green tea. Mutat. Res. 1998, 402, 307–310. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013, 32, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008, 269, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Feyes, D.K.; Nieminen, A.L.; Agarwal, R.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeong, Y.-J.; Lee, S.-W.; Kim, D.; Oh, S.-J.; Lim, H.-S.; Oh, H.-K.; Kim, S.-H.; Kim, W.-J.; Jung, J.-Y. EGCG induces apoptosis in human laryngeal epidermoid carcinoma Hep2 cells via mitochondria with the release of apoptosis-inducing factor and endonuclease G. Cancer Lett. 2010, 290, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Qanungo, S.; Das, M.; Haldar, S.; Basu, A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis 2005, 26, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Saeki, K.; Sazuka, M.; Suzuki, Y.; Shoji, Y.; Nakamura, Y.; Ohta, T.; Kaji, K.; Yuo, A.; Isemura, M. Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem. Biophys. Res. Commun. 2001, 285, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Cha, Y.Y. Epigallocatechin-3-gallate induces growth inhibition and apoptosis of human anaplastic thyroid carcinoma cells through suppression of EGFR/ERK pathway and cyclin B1/CDK1 complex. J. Surg. Oncol. 2011, 104, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Onoda, C.; Kuribayashi, K.; Nirasawa, S.; Tsuji, N.; Tanaka, M.; Kobayashi, D.; Watanabe, N. (–)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer cell lines by down-regulating survivin expression. Int. J. Oncol. 2011, 38, 1403–1408. [Google Scholar] [PubMed]
- Iwasaki, R.; Ito, K.; Ishida, T.; Hamanoue, M.; Adachi, S.; Watanabe, T.; Sato, Y. Catechin, green tea component, causes caspase-independent necrosis-like cell death in chronic myelogenous leukemia. Cancer Sci. 2009, 100, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, N.-D.; Zhou, F.; Shen, T.; Duan, T.; Zhou, J.; Shi, Y.; Zhu, X.-Q.; Shen, H.-M. (–)-Epigallocatechin-3-gallate induces non-apoptotic cell death in human cancer cells via ROS-mediated lysosomal membrane permeabilization. PLoS ONE 2012, 7, e46749. [Google Scholar] [CrossRef] [PubMed]
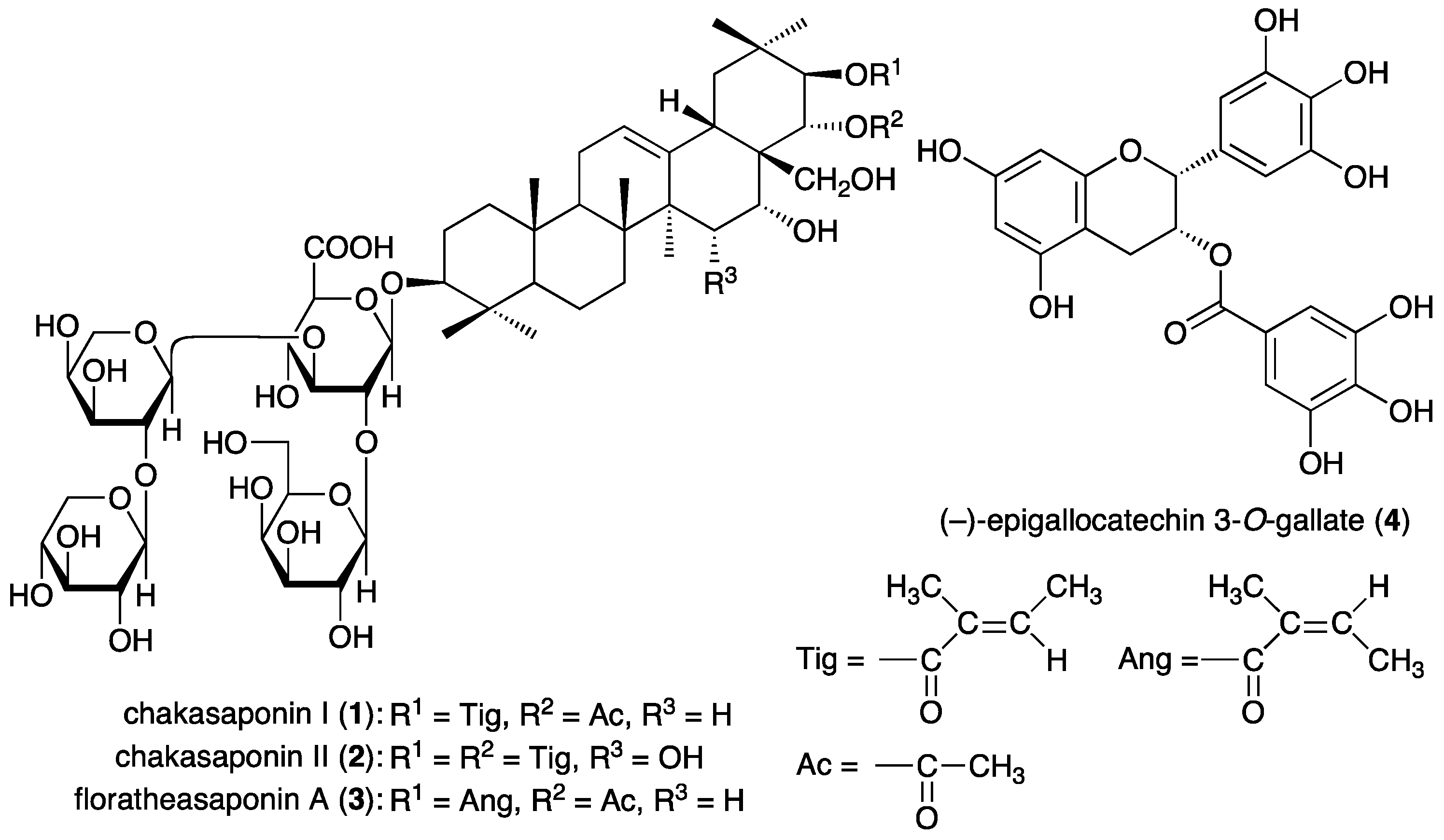

| Treatment | IC50 (µM) a | |||
|---|---|---|---|---|
| HSC-2 | HSC-4 | MKN-45 | Caco-2 | |
| Chakasaponin I (1) | 4.6 | 17.5 | 16.8 | 30.2 |
| Chakasaponin II (2) | 14.1 | 18.2 | 17.3 | 40.6 |
| Floratheasaponin A (3) | 4.4 | 6.2 | 4.5 | 19.3 |
| (–)-Epigallocatechin 3-O-gallate (4) | 28.3 | 27.2 | >100 (70.3) | >100 (71.8) |
| 5-FU | >100 (55.5) | >100 (77.3) | >100 (52.4) | >100 (80.2) |
| Cisplatin | 14.5 | 7.0 | circa 100 | >100 (69.5) |
| Doxorubicin | 0.040 | 0.18 | 0.12 | >100 (80.9) |
| Camptothecin | 0.020 | 0.089 | 0.10 | >100 (63.7) |
| Taxol | 0.0012 | 0.0059 | 0.15 | >100 (61.5) |
| Treatment | Concentration (µM) | Cell Cycle (%) a | ||
|---|---|---|---|---|
| G0/G1 Phase | S Phase | G2/M Phase | ||
| Control | – | 73.5 ± 0.8 | 10.8 ± 0.4 | 15.6 ± 0.7 |
| Chakasaponin I (1) | 5 | 71.6 ± 0.4 | 10.2 ± 0.4 | 18.0 ± 0.1 |
| – | 7.5 | 69.1 ± 1.4 * | 10.6 ± 0.8 | 20.1 ± 0.8 ** |
| – | 10 | 59.9 ± 0.2 ** | 11.2 ± 1.0 | 28.7 ± 0.9 ** |
| Control | – | 72.4 ± 1.1 | 11.6 ± 0.4 | 16.0 ± 0.7 |
| Chakasaponin II (2) | 10 | 60.3 ± 0.1 ** | 10.5 ± 0.4 | 28.9 ± 0.3 ** |
| – | 12.5 | 44.1 ± 1.3 ** | 18.8 ± 2.4 ** | 36.8 ± 1.3 ** |
| – | 15 | 49.2 ± 0.8 ** | 20.0 ± 0.3 ** | 30.5 ± 0.6 ** |
| Control | – | 76.5 ± 1.4 | 9.8 ± 0.6 | 13.5 ± 0.8 |
| Floratheasaponin A (3) | 2.5 | 69.4 ± 1.0 * | 11.8 ± 0.1 | 18.5 ± 0.9 * |
| – | 5 | 67.6 ± 1.7 ** | 10.3 ± 0.7 | 21.7 ± 0.9 ** |
| – | 7.5 | 53.1 ± 0.8 ** | 15.9 ± 1.8 ** | 30.3 ± 1.1 ** |
| Control | – | 78.5 ± 1.2 | 9.4 ± 0.5 | 11.8 ± 0.7 |
| (–)-Epigallocatechin 3-O-gallate (4) | 25 | 78.8 ± 1.1 | 9.7 ± 0.5 | 11.2 ± 0.6 |
| – | 50 | 78.6 ± 1.2 | 9.0 ± 0.5 | 12.0 ± 0.6 |
| – | 75 | 76.8 ± 0.5 | 10.1 ± 0.1 | 12.7 ± 0.4 |
| 5-FU | 30 | 36.4 ± 0.3 ** | 37.9 ± 0.9 ** | 25.4 ± 0.6 ** |
| Cisplatin | 3 | 50.7 ± 0.7 ** | 27.9 ± 0.5 ** | 20.0 ± 0.3 ** |
| Treatment | Concentration (µM) | Total Apoptotic Cells (%) a |
|---|---|---|
| Control | – | 8.9 ± 1.5 |
| Chakasaponin I (1) | 5 | 10.0 ± 0.4 |
| – | 7.5 | 35.6 ± 3.0 ** |
| – | 10 | 89.7 ± 0.4 ** |
| Control | – | 6.3 ± 0.4 |
| Chakasaponin II (2) | 10 | 7.0 ± 0.3 |
| – | 12.5 | 35.7 ± 0.4 ** |
| – | 15 | 64.2 ± 1.0 ** |
| Control | – | 12.9 ± 0.4 |
| Floratheasaponin A (3) | 2.5 | 17.5 ± 1.0 |
| – | 5 | 18.7 ± 0.3 * |
| – | 7.5 | 50.1 ± 1.9 ** |
| Control | – | 13.9 ± 0.5 |
| (–)-Epigallocatechin 3-O-gallate (4) | 25 | 14.4 ± 0.6 |
| – | 50 | 13.1 ± 0.1 |
| – | 75 | 12.7 ± 0.1 |
| Camptothecin | 0.02 | 16.9 ± 0.2 ** |
| – | 0.05 | 43.9 ± 0.7 ** |
| Treatment | Concentration (µM) | Total Apoptotic Cells (%) a |
|---|---|---|
| Control | – | 7.1 ± 0.4 |
| Chakasaponin I (1) | 5 | 25.9 ± 3.8 ** |
| – | 7.5 | 59.6 ± 6.3 ** |
| – | 10 | 90.2 ± 0.9 ** |
| Control | – | 2.8 ± 0.1 |
| Chakasaponin II (2) | 10 | 5.9 ± 0.1 |
| – | 12.5 | 35.6 ± 1.5 ** |
| – | 15 | 50.9 ± 9.7 ** |
| Control | – | 4.2 ± 0.5 |
| Floratheasaponin A (3) | 2.5 | 27.9 ± 0.6 ** |
| – | 5 | 27.9 ± 1.0 ** |
| – | 7.5 | 45.3 ± 0.6 ** |
| Control | – | 7.2 ± 0.4 |
| (–)-Epigallocatechin 3-O-gallate (4) | 25 | 5.9 ± 0.2 |
| – | 50 | 4.9 ± 0.4 |
| – | 75 | 5.9 ± 0.1 |
| Camptothecin | 0.03 | 67.2 ± 1.2 ** |
| – | 0.05 | 75.0 ± 2.1 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitagawa, N.; Morikawa, T.; Motai, C.; Ninomiya, K.; Okugawa, S.; Nishida, A.; Yoshikawa, M.; Muraoka, O. The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines. Int. J. Mol. Sci. 2016, 17, 1979. https://doi.org/10.3390/ijms17121979
Kitagawa N, Morikawa T, Motai C, Ninomiya K, Okugawa S, Nishida A, Yoshikawa M, Muraoka O. The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines. International Journal of Molecular Sciences. 2016; 17(12):1979. https://doi.org/10.3390/ijms17121979
Chicago/Turabian StyleKitagawa, Niichiro, Toshio Morikawa, Chiaki Motai, Kiyofumi Ninomiya, Shuhei Okugawa, Ayaka Nishida, Masayuki Yoshikawa, and Osamu Muraoka. 2016. "The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines" International Journal of Molecular Sciences 17, no. 12: 1979. https://doi.org/10.3390/ijms17121979